Abstract
Background
The phenotypic heterogeneity of psoriasis could be explained by the alternate activation of either T-helper (Th)-1- or Th-17-related cytokines. However, evidence directly supporting this hypothesis is scarce.
Objective
To characterize the expression of Th-1- and Th-17-related cytokines according to the morphological psoriasis phenotype: guttate vs. plaque.
Methods
In this study, we enrolled 68 patients exhibiting either guttate or plaque psoriasis, and 10 healthy controls. To avoid age-related bias, age matching was performed for each group. Circulating levels of interferon (IFN)-γ, interleukin (IL)-1RA, IL-2, IL-12p40, IL-17A, IL-22, and IL-23 were measured with an enzyme-linked immunosorbent assay (ELISA). Psoriasis-affected tissue was obtained through biopsy sampling from the eight patients who exhibited the most typical morphology. Levels of IL-1RA, IL-12p40, IL-17, IL-22, and IL-23 in the psoriasis tissue samples were measured with western blot analysis.
Results
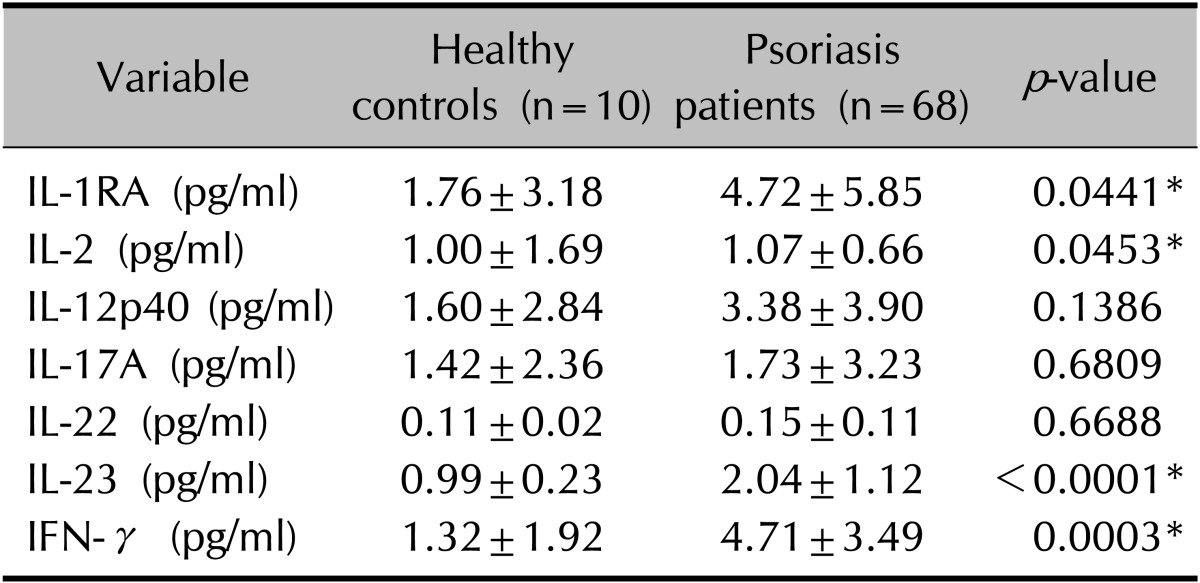
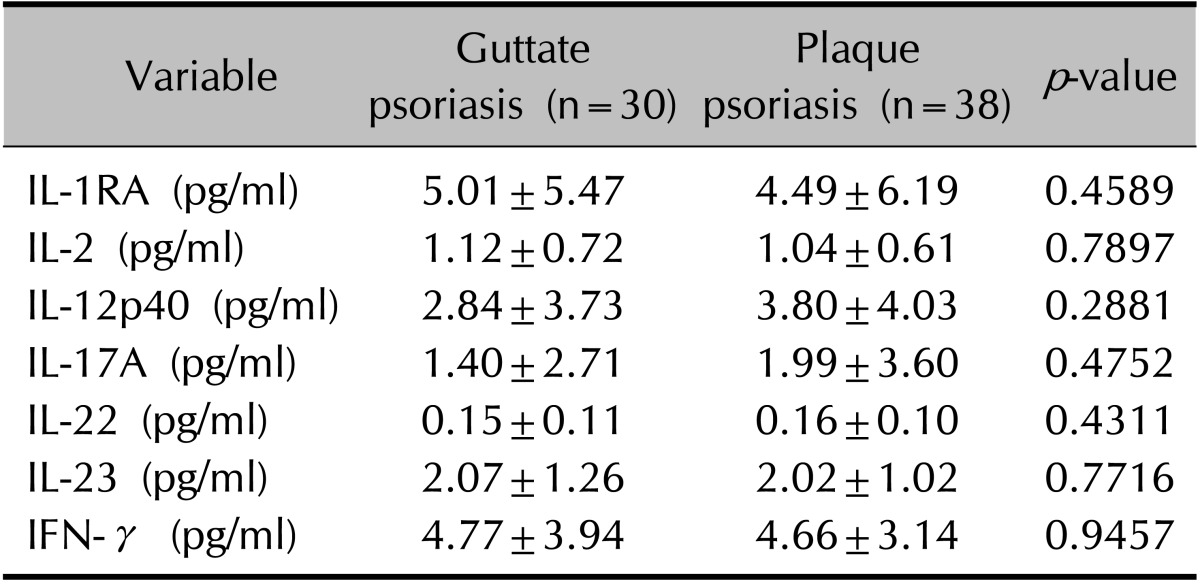
ELISAs of the serum samples showed higher levels of inflammatory cytokines such as IL-1RA, IL-2, IL-23, and IFN-γ in patients with psoriasis than in healthy controls. However, the inflammatory cytokine levels did not differ significantly between guttate and plaque psoriasis patients. Western blot analysis of psoriatic tissue revealed higher protein levels of Th-1- and Th-17-related cytokines in patients than in healthy controls. The levels of IL-12p40 and IL-23 were unexpectedly higher in plaque tissue than in guttate tissue.
Conclusion
The morphological phenotype of psoriasis does not appear to be determined by a specific activation of either the Th-1 or Th-17 pathway. Rather, the cytokine profile influences disease activity and is altered according to the status of the lesion (early or chronic).
Keywords: Cytokines, Phenotype, Psoriasis
INTRODUCTION
Psoriasis is a chronic inflammatory disorder of the skin that is known to be mediated by immune alterations. Measurements of T-cell activity after antigen challenge, therapeutic inhibition of activated T-cells, and cell transfer and explantation have been performed in a large number of studies1,2,3,4,5, with the results demonstrating that psoriasis is a T-cell-mediated disorder. It was previously assumed that T-helper (Th)-1 cells play a dominant role in the initiation and maintenance of psoriasis along with interferon (IFN)-γ, interleukin (IL)-2, and IL-126. It has recently been demonstrated that a new subset of T cells, Th-17 cells, are also important in the pathogenesis of psoriasis7,8. The secretion of IFN-α from plasmacytoid dendritic cells (DCs) is one of the earliest events driving the inflammatory eruption in psoriasis9. IFN-α stimulates DCs, which produce tumor necrosis factor (TNF)-α and inducible nitric oxide synthase, to secrete IL-237. IL-23 stimulates Th-17 cells to produce Th-17-related cytokines (henceforth referred to as Th-17 cytokines), thus increasing Th-17 cell survival and proliferation. The Th-17-derived cytokines include IL-17A, IL-17F, IL-6, TNF-α, and IL-2210,11. IL-22 has been shown to induce hyperplasia and abnormal differentiation of keratinocytes, ultimately resulting in plaque formation12.
Psoriasis manifests with a heterogeneous phenotype and exhibits a wide range of clinical activities; it has also been suggested that one phenotype can transform into another. Christophers13 recently proposed that rerouting cutaneous inflammation from the IL-12 pathway toward the IL-23 pathway, and vice versa, has a bearing on both the diversity of phenotypes and their instability. However, very few studies have explored the heterogeneity of the psoriasis phenotype.
In this study, we measured the protein levels of the Th-1- and Th-17 cytokines in human psoriasis tissue and serum by using western blot and nzyme-linked immunosorbent assay (ELISA), respectively. In addition, differences in the levels of cytokines between the guttate and plaque types were investigated.
MATERIALS AND METHODS
Patients and controls
Sixty-eight patients with psoriasis (38 with plaque psoriasis and 30 with guttate psoriasis) were enrolled for the analysis of their serum with an ELISA. To avoid age bias, we tried to enroll age-matched patients with guttate and plaque psoriasis. Eight patients exhibiting the typical guttate or plaque morphology were enrolled to participate in the western blot analysis aimed at exploring differences according to morphological phenotype. The diagnosis of psoriasis was confirmed by using clinical and histopathological criteria. The following major inclusion criteria were implemented: no local or systemic treatment for at least 4 weeks before study entry; no significant infection or immune suppression; and no history of specific medical diagnoses with renal, hepatic, cardiovascular, pulmonary, rheumatic, or endocrine involvement. The patients were divided into two groups according to their plaque morphology: guttate or plaque. The guttate group comprised patients whose lesions were papules with diameters of <1 cm, whereas the plaque group comprised those with at least one lesion with a long axis of >5 cm. The Psoriasis Area and Severity Index (PASI) was used to evaluate the clinical severity of the disease. The control group comprised healthy volunteers with no history of specific medical diagnoses with renal, hepatic, cardiovascular, pulmonary, rheumatic, or endocrine involvement. This study was conducted in accordance with the guidelines of the Helsinki Conference and the Korean Good Clinical Practice, with the participants' rights and safety taking precedence. Approval for the study was obtained from the institutional review board of Konkuk University. All patients gave their informed consent to participate before screening.
Western blot analysis of tissue samples
Skin biopsy samples (4 mm) were collected from the lesions of patients with psoriasis (four guttate and four plaque lesional biopsies), and from the healthy residual skin of patients after lipoma excision, which was neither inflamed nor pathologic. The samples were immediately stored at -70℃ until use.
Tissue proteins were prepared in radioimmunoprecipitation assay buffer (1×phosphate-buffered saline, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS; Sigma-Aldrich, St. Louis, MO, USA], and 10 µg/ml phenylmethanesulfonyl fluoride). The proteins (50 µg/lane) were separated on NuPAGE 4% to 12% Bis-Tris polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and then transferred electrophoretically to immunoblot polyvinylidene fluoride membranes. The membranes were incubated with the following specific antibodies for 2 hours at room temperature: anti-human IL-1RA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-IL-22 (Abcam Technologies, Cambridge, MA, USA), anti-IL-23 (Abcam Technologies), anti-IL-12B/IL-12p40 (MyBiosource, San Diego, CA, USA), and anti-IL17 (R&D Systems, Minneapolis, MN, USA). The anti-IL-12B/IL-12p40 antibody used in this study detects only IL-12p40 (i.e., not IL-23p40). The membranes were then washed and incubated with horseradish-peroxidaseconjugated secondary antibodies (Santa Cruz Biotechnology). The signal was visualized with an enhanced chemiluminescence detection kit (Promega, Madison, WI, USA) by using x-ray films. The blots were stripped by using a stripping buffer (100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl [pH 6.7]) and reprobed with anti-β-actin antibody (Sigma-Aldrich) as loading controls. Multi-Gauge ver. 3.1 software from Fuji Film (Tokyo, Japan) was used to quantify the resulting bands, and the data were normalized with β-actin as a reference.
Assays for serum samples
Venous blood samples (5~10 ml) were collected from patients with psoriasis (30 with guttate psoriasis and 38 with plaque psoriasis) into vacuum tubes in sterile conditions. The serum was extracted from the samples through centrifugal spinning, and immediately frozen at -70℃ until analysis. Multiple cytokine analyses were conducted by using xMAP technology (Luminex, Austin, TX, USA) to measure the serum levels of IFN-γ, IL-1RA, IL-2, IL-12p40, IL-17A, IL-22, and IL-23. The Milliplex MAP multiplex assay was performed by using a 96-well microplate format according to the manufacturer's instructions (Millipore, Billerica, MA, USA). Briefly, each of the bead solutions was transferred into a mixing vial and adjusted to a volume of 3 ml with bead diluents. Internal controls, in the range 0 to 10,000 pg/ml for each cytokine, were included with every assay. After the addition of sera and beads, the mixture was incubated overnight at 4℃. Detection antibodies and streptavidin-phycoerythrin were successively added at room temperature for 30 minutes, and the plate was analyzed with the Luminex 200 system.
Statistical analysis
Data analyses were performed by using SAS software (ver. 9.3; SAS Institute, Cary, NC, USA). The three groups were compared by using the Kruskal-Wallis test. In addition, the Wilcoxon rank-sum test was performed to compare the data for the two groups. The cutoff for statistical significance was set at p<0.05.
RESULTS
Patient demographics
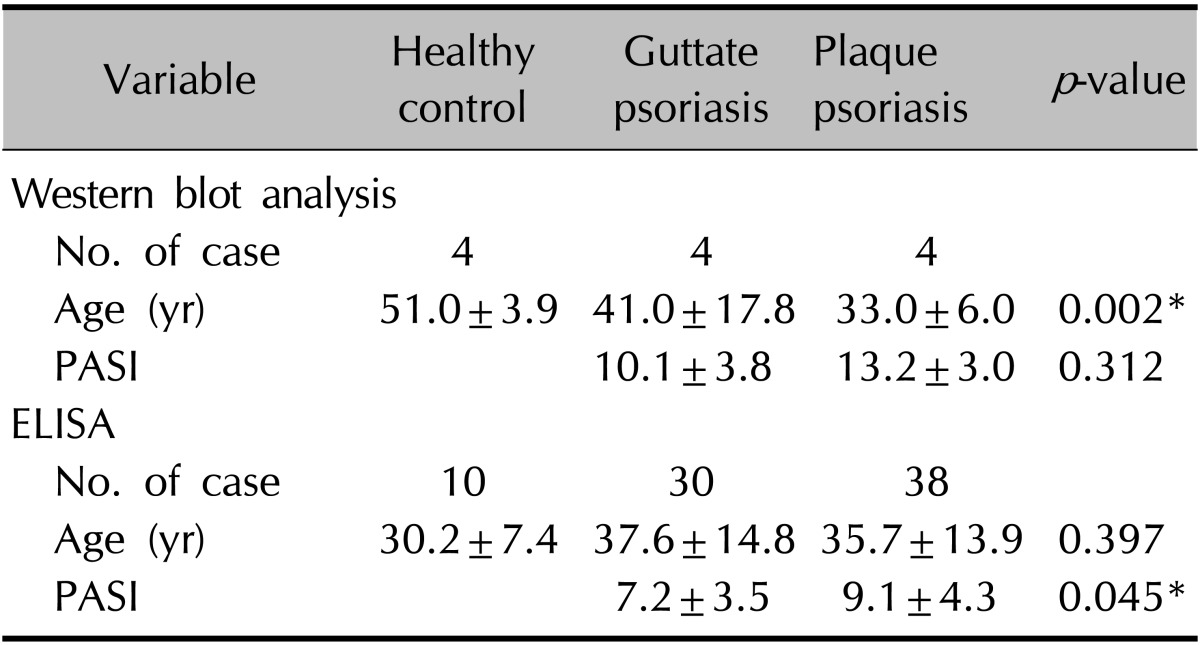
The mean age of the 68 patients was 36.6±14.2 years (mean±standard deviation [SD]), and that of controls was 32.58±10.0 years (p>0.05); among the guttate group, plaque group, and healthy controls, age showed no significant difference after age matching (37.6±14.8 years vs. 35.7±13.9 years vs. 30.2±7.4 years, p>0.05). The mean age of eight patients from whom psoriatic tissue was obtained for western blot analysis was 37.0±13.0 years, and that of controls was 51.0±3.9 years (p<0.05); there was a significant difference between the guttate group, plaque group, and healthy controls (41.0±17.8 years vs. 33.0±6.0 years vs. 51.0±3.9 years, p<0.05).
The mean PASI for the 68 patients was 8.2±4.0 points (mean±SD), and was significantly higher in the plaque group (9.1±4.3 points) than in the guttate group (7.2±3.5 points, p<0.05). The mean PASI of the eight participants from whom psoriatic tissue was obtained for western blot analysis was 11.6±3.6 points; the plaque group had a higher PASI than the guttate group (13.2±3.0 points vs. 10.1±3.8 points) but the difference was not significant (p>0.05; Table 1).
Table 1. Demographics of the healthy controls and psoriasis patients.
Values are presented as number only oy mean±standard deviation. Kruskal-Wallis test comparing age among healthy controls and patients with guttate and plaque psoriasis. Wilcoxon rank-sum test comparing duration and PASI between the patients with guttate and plaque psoriasis. ELISA: enzyme-linked immunosorbent assay, PASI: Psoriasis Area and Severity Index. *p<0.05.
Western blot analysis of tissue inflammatory cytokines
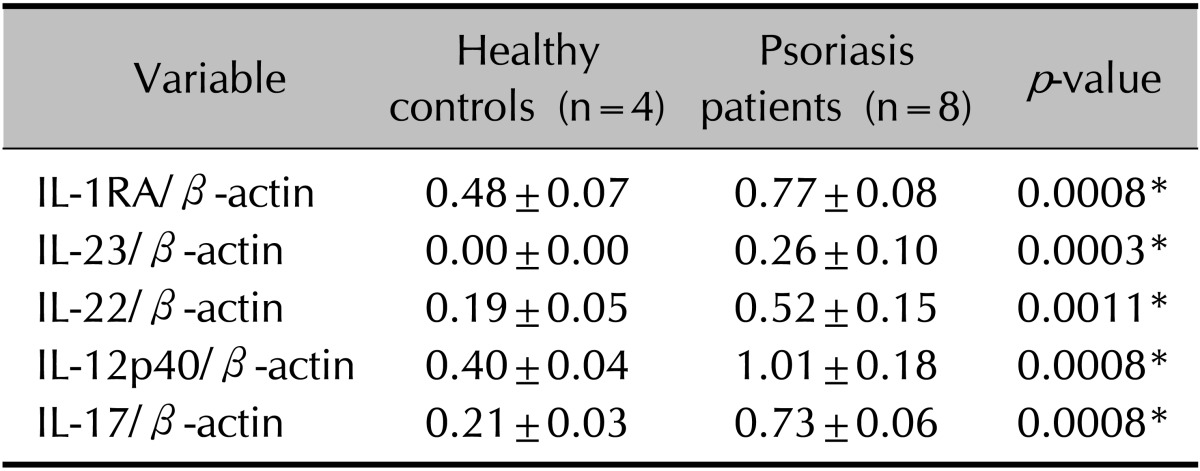
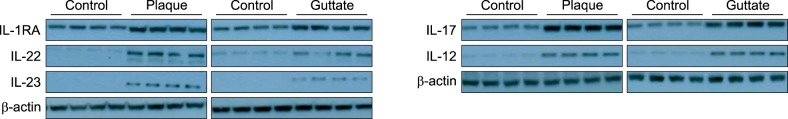
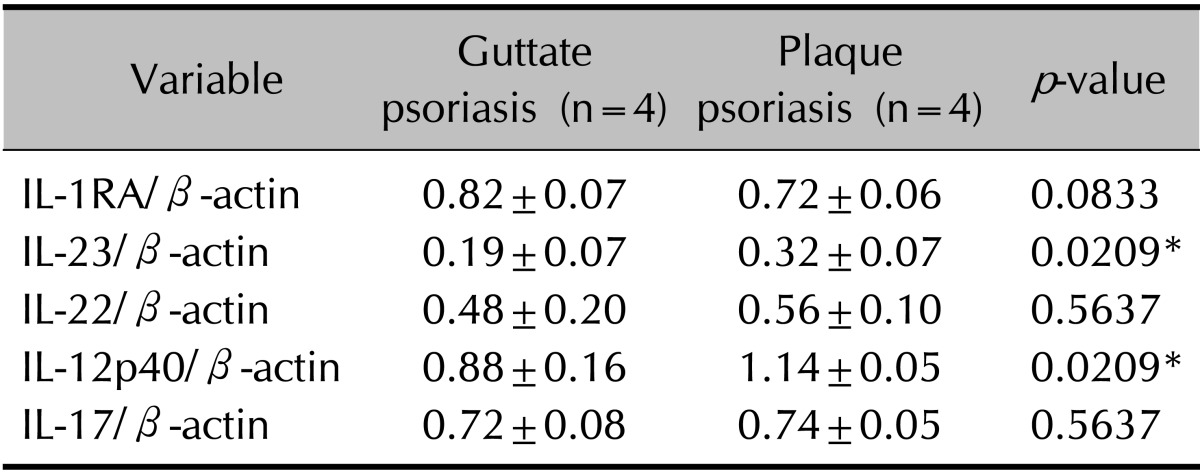
The tissue levels of five cytokines (IL-RA, IL-12p40, IL-17A, IL-22, and IL-23) were analyzed in eight patients with psoriasis and in four healthy controls. The levels of all five cytokines were higher in patients with psoriasis than in healthy controls (p<0.05; Table 2, Fig. 1). Unexpectedly, the expression levels of IL-23 and IL-12p40 were higher in the plaque group than in the guttate group (p<0.05; Table 3).
Table 2. Comparison of cytokine levels between healthy controls and psoriasis patients.
Values are presented as mean±standard deviation. The experimental Western blot data are normalized relative to the β-actin reference. Wilcoxon rank-sum test comparing the protein level of tissue inflammatory cytokines between the healthy controls and psoriasis patients. IL: interleukin, IL-1RA: IL-1 receptor antagonist. *p<0.05.
Fig. 1. Western blot results for T-helper (Th)-1- and Th-2-related cytokines in the lesions of psoriasis patients and healthy controls. Note that the protein levels of the interleukins (ILs) and IL-1 receptor antagonist (IL-1RA) are higher in both the plaque and guttate psoriasis groups than in the healthy controls.
Table 3. Comparison of cytokine levels between guttate and plaque psoriasis patients.
Values are presented as mean±standard deviation. The experimental Western blot data are normalized relative to the β-actin reference. Wilcoxon rank-sum test comparing the protein level of tissue inflammatory cytokines between the patients with guttate and plaque psoriasis. IL: interleukin, IL-1RA: IL-1 receptor antagonist. *p<0.05.
ELISA of serum inflammatory cytokines
The serum levels of seven cytokines were analyzed in 68 patients with psoriasis and in 10 healthy controls (Table 4). As expected, the comparison between psoriatic and healthy control samples revealed that serum IL-1RA, IL-2, IL-23, and IFN-γ levels were significantly higher in the patients with psoriasis (p<0.05). However, the levels of these seven cytokines did not differ significantly between the plaque and guttate groups (p>0.05; Table 5).
Table 4. Comparison of serum cytokine levels between healthy controls and psoriasis patients.
Values are presented as mean±standard deviation. Wilcoxon rank-sum test comparing the serum level of inflammatory cytokines between healthy controls and psoriasis patients. IL: interleukin, IL-1RA: IL-1 receptor antagonist, IFN: interferon. *p<0.05.
Table 5. Comparison of serum inflammatory cytokine levels between patients with guttate and plaque psoriasis.
Values are presented as mean±standard deviation. IL: interleukin, IL-1RA: IL-1 receptor antagonist, IFN: interferon. Wilcoxon rank-sum test comparing the serum levels of inflammatory cytokines between patients with guttate and plaque psoriasis.
DISCUSSION
Psoriasis manifests with various morphological phenotypes, and in each form the morphology may alter with the disease activity. The question of what explains the difference between these morphological phenotypes is a long-standing one. It was assumed that the morphological diversity of psoriasis is related to differences in inflammatory cytokines, particularly Th-1- and Th-17-related cytokines. The accumulated evidence13,14,15,16,17,18,19 concerning inflammatory cytokines and the histopathology among different psoriatic phenotypes suggests that a combination of inflammatory pathways routing from IL-12/IFN-γ to IL-23/IL-17, and vice versa, could determine the psoriasis phenotype. Christophers13 proposed that Th-1-predominant activation represents stable plaque morphology and that Th-17 activation explains the inflammatory psoriasis types such as guttate or pustular psoriasis. However, studies of cytokine profiles relative to morphological phenotypes have yielded conflicting results. In this study, we used western blot analysis to directly compare Th1-/Th17-related cytokines between the guttate and plaque psoriasis types. We also evaluated the differences in serum inflammatory cytokines between the two groups by using ELISA.
Consistent with prior research results16,20,21,22,23,24 with reverse transcription polymerase chain reaction (RT-PCR) and ELISA, our western blot results showed that both Th-1- and Th-17-related cytokine levels were significantly elevated in patient tissue compared with those in healthy control tissue, confirming that both the Th-1 and Th-17 pathways are associated with the pathogenesis of psoriasis regardless of morphology.
In contrast to previous studies13, our serum inflammatory cytokine data showed no difference in the levels of inflammatory Th-1 and Th-17 cytokines between the guttate and plaque groups. There has been discrepancy in the findings of analyses of inflammatory cytokines relative to morphological phenotype16,17,24. Yan et al.16 and Choe et al.24 compared serum inflammatory cytokine levels between guttate and plaque psoriasis or early inflammatory and stable psoriasis by using ELISA, and found that IL-17 levels were higher in the former. By contrast, Yilmaz et al17. performed cytokine analysis on inflammatory psoriatic tissue by using RT-PCR, and reported that the expression of tissue IL-17 mRNA was not significantly elevated in guttate psoriasis compared with that in plaque psoriasis.
In the present study, we also performed western blot analysis with human psoriasis tissue. Our results showed a discrepancy between the results of the tissue and serum samples when comparing the guttate and plaque groups; in the serum, there were no significant differences in the levels of inflammatory Th-1 and Th-17 cytokines between the two groups, whereas, unexpectedly, the expression of Th-17 cytokines such as IL-23 was higher in plaque tissue than in guttate tissue. In a recent review by Christophers et al.25, it was postulated that during the progression of stable psoriasis, two types of inflammation may coexist in the same patient: an IL-1-/Th17-dominated autoinflammation in highly inflammatory early lesions, and a Th1-dominated late-phase inflammation that prevails in stable psoriasis plaques. In other words, it means that each lesion even in the same patient shows a different disease activity, which leads to a different immune pathway predominance in each lesion. Thus, the results of the present study conclusively support the hypothesis that the cytokine profile is not associated with morphological phenotype, but rather with whether the lesions are early or chronic.
Overall, the IL-23/IL-17 pathway did not predominate in guttate psoriasis relative to plaque psoriasis. However, a limitation of this study is the relatively small number of patients involved, and thus this finding needs to be confirmed in future studies involving larger samples.
In conclusion, the findings of this study suggest that both the Th-1 and Th-17 pathways are associated with the pathogenesis of psoriasis regardless of the morphological phenotype. Our results support the theory25 that the predominating pathway, either IL-23/IL-17 or IL-12/IFN-γ, is determined by the status of the lesion as either early or chronic.
ACKNOWLEDGMENT
This paper was supported by Konkuk University in 2015.
References
- 1.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinz JC. Disease mimicry--a pathogenetic concept for T cell-mediated autoimmune disorders triggered by molecular mimicry. Autoimmun Rev. 2004;3:10–15. doi: 10.1016/S1568-9972(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 3.Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin Exp Immunol. 2004;138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos JD. The pathomechanisms of psoriasis; the skin immune system and cyclosporin. Br J Dermatol. 1988;118:141–155. doi: 10.1111/j.1365-2133.1988.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 5.Rustin MH. Long-term safety of biologics in the treatment of moderate-to-severe plaque psoriasis: review of current data. Br J Dermatol. 2012;167(Suppl 3):3–11. doi: 10.1111/j.1365-2133.2012.11208.x. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 7.Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008;128:1064–1067. doi: 10.1038/jid.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 11.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 13.Christophers E. Explaining phenotype heterogeneity in patients with psoriasis. Br J Dermatol. 2008;158:437–441. doi: 10.1111/j.1365-2133.2007.08307.x. [DOI] [PubMed] [Google Scholar]
- 14.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 16.Yan KX, Fang X, Han L, Zhang ZH, Kang KF, Zheng ZZ, et al. Foxp3+ regulatory T cells and related cytokines differentially expressed in plaque vs. guttate psoriasis vulgaris. Br J Dermatol. 2010;163:48–56. doi: 10.1111/j.1365-2133.2010.09742.x. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch Dermatol Res. 2012;304:465–469. doi: 10.1007/s00403-012-1229-1. [DOI] [PubMed] [Google Scholar]
- 18.Terui T, Ozawa M, Tagami H. Role of neutrophils in induction of acute inflammation in T-cell-mediated immune dermatosis, psoriasis: a neutrophil-associated inflammationboosting loop. Exp Dermatol. 2000;9:1–10. doi: 10.1034/j.1600-0625.2000.009001001.x. [DOI] [PubMed] [Google Scholar]
- 19.Brody I. Dermal and epidermal involvement in the evolution of acute eruptive guttate psoriasis vulgaris. J Invest Dermatol. 1984;82:465–470. doi: 10.1111/1523-1747.ep12260965. [DOI] [PubMed] [Google Scholar]
- 20.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima H, Nakajima K, Tarutani M, Morishige R, Sano S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res. 2011;303:451–455. doi: 10.1007/s00403-011-1159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo YH, Torii K, Saito C, Furuhashi T, Maeda A, Morita A. Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J Dermatol Sci. 2010;58:225–227. doi: 10.1016/j.jdermsci.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H, Tsuji H, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol. 2010;35:645–649. doi: 10.1111/j.1365-2230.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 24.Choe YB, Hwang YJ, Hahn HJ, Jung JW, Jung HJ, Lee YW, et al. A comparison of serum inflammatory cytokines according to phenotype in patients with psoriasis. Br J Dermatol. 2012;167:762–767. doi: 10.1111/j.1365-2133.2012.11038.x. [DOI] [PubMed] [Google Scholar]
- 25.Christophers E, Metzler G, Röcken M. Bimodal immune activation in psoriasis. Br J Dermatol. 2014;170:59–65. doi: 10.1111/bjd.12631. [DOI] [PubMed] [Google Scholar]