Abstract
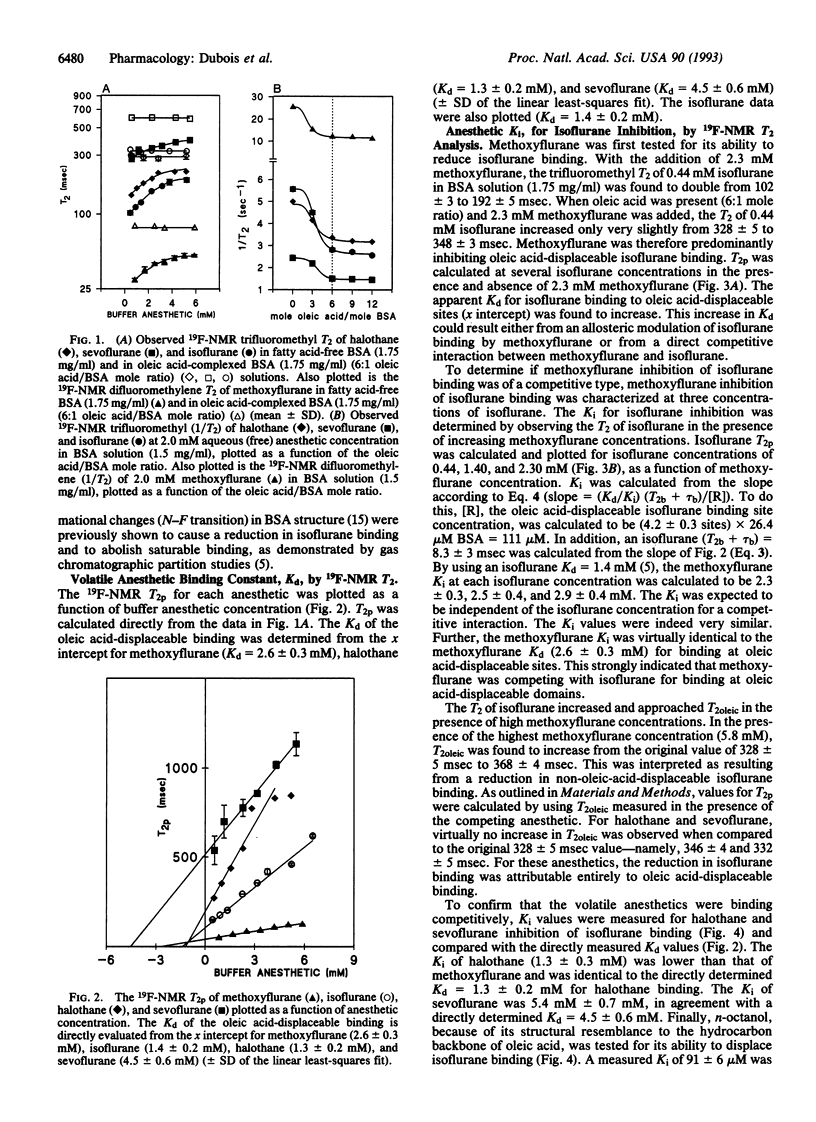
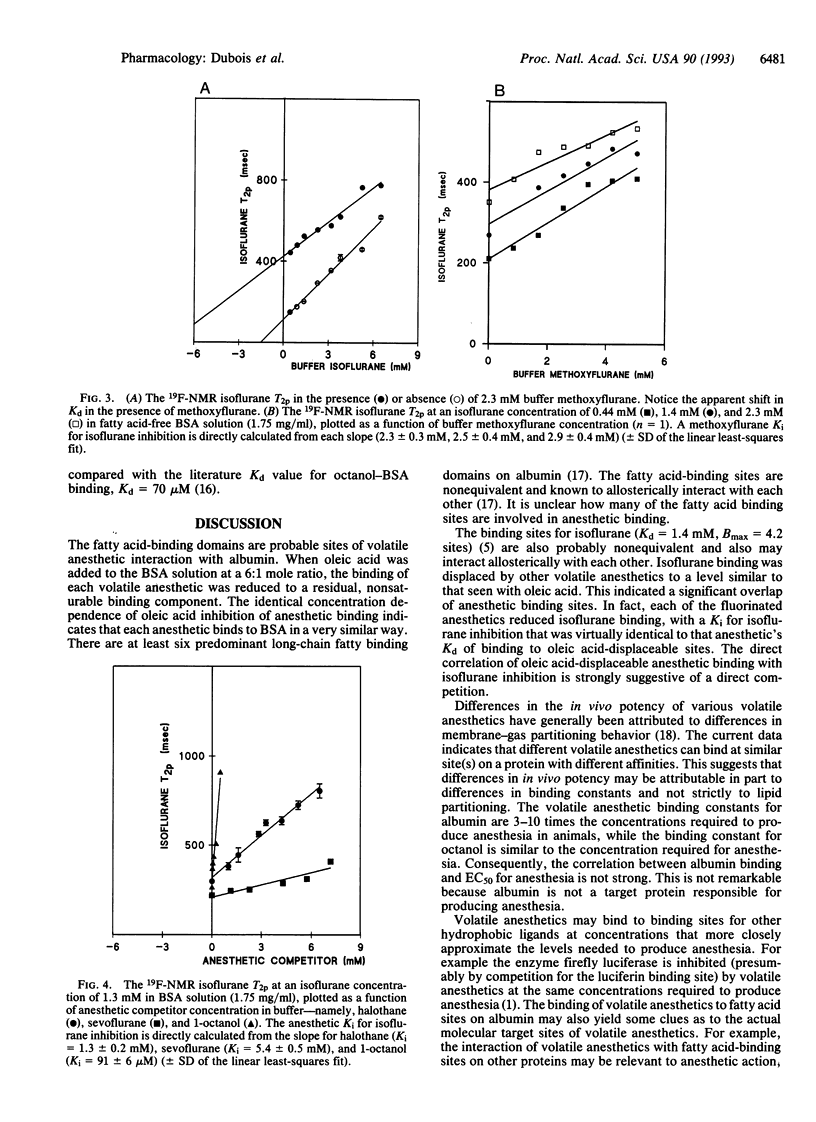
There is controversy as to the molecular nature of volatile anesthetic target sites. One proposal is that volatile anesthetics bind directly to hydrophobic binding sites on certain sensitive target proteins. Consistent with this hypothesis, we have previously shown that a fluorinated volatile anesthetic, isoflurane, binds saturably [Kd (dissociation constant) = 1.4 +/- 0.2 mM, Bmax = 4.2 +/- 0.3 sites] to fatty acid-displaceable domains on serum albumin. In the current study, we used 19F-NMR T2 relaxation to examine whether other volatile anesthetics bind to the same sites on albumin and, if so, whether they vary in their affinity for these sites. We show that three other fluorinated volatile anesthetics bind with varying affinity to fatty acid-displaceable domains on serum albumin: halothane, Kd = 1.3 +/- 0.2 mM; methoxyflurane, Kd = 2.6 +/- 0.3 mM; and sevoflurane, Kd = 4.5 +/- 0.6 mM. These three anesthetics inhibit isoflurane binding in a competitive manner: halothane, K(i) (inhibition constant) = 1.3 +/- 0.2 mM; methoxyflurane, K(i) = 2.5 +/- 0.4 mM; and sevoflurane, K(i) = 5.4 +/- 0.7 mM--similar to each anesthetic's respective Kd of binding to fatty acid displaceable sites. These results illustrate that a variety of volatile anesthetics can compete for binding to specific sites on a protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behling R. W., Yamane T., Navon G., Sammon M. J., Jelinski L. W. Measuring relative acetylcholine receptor agonist binding by selective proton nuclear magnetic resonance relaxation experiments. Biophys J. 1988 Jun;53(6):947–954. doi: 10.1016/S0006-3495(88)83175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFazio C. A., Brown R. E., Ball C. G., Heckel C. G., Kennedy S. S. Additive effects of anesthetics and theories of anesthesia. Anesthesiology. 1972 Jan;36(1):57–63. doi: 10.1097/00000542-197201000-00010. [DOI] [PubMed] [Google Scholar]
- Dubois B. W., Evers A. S. 19F-NMR spin-spin relaxation (T2) method for characterizing volatile anesthetic binding to proteins. Analysis of isoflurane binding to serum albumin. Biochemistry. 1992 Aug 11;31(31):7069–7076. doi: 10.1021/bi00146a007. [DOI] [PubMed] [Google Scholar]
- Fraenkel Y., Navon G., Aronheim A., Gershoni J. M. Direct measurement of agonist binding to genetically engineered peptides of the acetylcholine receptor by selective T1 NMR relaxation. Biochemistry. 1990 Mar 13;29(10):2617–2622. doi: 10.1021/bi00462a027. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985 Jul 25;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Stereospecific effects of inhalational general anesthetic optical isomers on nerve ion channels. Science. 1991 Oct 18;254(5030):427–430. doi: 10.1126/science.1925602. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Guggino S. E., Guggino W. B. Direct modulation of secretory chloride channels by arachidonic and other cis unsaturated fatty acids. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5706–5709. doi: 10.1073/pnas.87.15.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Witzemann V., Quast U., Raftery M. A. Proton magnetic resonance studies of cholinergic ligand binding to the acetylcholine receptor in its membrane environment. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3580–3584. doi: 10.1073/pnas.76.8.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. W., Pang K. Y. General anaesthetics can selectively perturb lipid bilayer membranes. Nature. 1976 Sep 16;263(5574):253–255. doi: 10.1038/263253a0. [DOI] [PubMed] [Google Scholar]
- Morgan P. G., Sedensky M., Meneely P. M. Multiple sites of action of volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2965–2969. doi: 10.1073/pnas.87.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Ray A., Reynolds J. A., Polet H., Steinhardt J. Binding of large organic anions and neutral molecules by native bovine serum albumin. Biochemistry. 1966 Aug;5(8):2606–2616. doi: 10.1021/bi00872a019. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Martin K., Gregory S., Keightley C. A., Hesketh T. R., Smith G. A., Warren G. B., Metcalfe J. C. Degenerate perturbations of protein structure as the mechanism of anaesthetic action. Nature. 1978 Dec 21;276(5690):775–779. doi: 10.1038/276775a0. [DOI] [PubMed] [Google Scholar]
- Shimada T., Somlyo A. P. Modulation of voltage-dependent Ca channel current by arachidonic acid and other long-chain fatty acids in rabbit intestinal smooth muscle. J Gen Physiol. 1992 Jul;100(1):27–44. doi: 10.1085/jgp.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. C., Towler S. C., White P. F., Evers A. S. Elimination kinetics of sevoflurane and halothane from blood, brain, and adipose tissue in the rat. Anesth Analg. 1990 Dec;71(6):658–664. doi: 10.1213/00000539-199012000-00014. [DOI] [PubMed] [Google Scholar]
- WISHNIA A., PINDER T. HYDROPHOBIC INTERACTIONS IN PROTEINS: CONFORMATION CHANGES IN BOVINE SERUM ALBUMIN BELOW PH5. Biochemistry. 1964 Sep;3:1377–1384. doi: 10.1021/bi00897a031. [DOI] [PubMed] [Google Scholar]



