Abstract
The emergence and spread of carbapenemase-producing bacteria, that hydolyze most β-lactams, including carbapenems, are a major concern of public health system worldwide, particularly in the Middle East area. Since the plasmids harboring resistance genes could be spread across other bacterial populations, detection of carbapenemase-producing organisms has become more problematic. These organisms produce different types of enzymes including the most prevalent types including KPC, VIM, IMP, NDM, and OXA-48. Carbapenemase producers are mostly identified among Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii. This study reviewed almost all papers, which conducted in the Middle East. In order to decrease the spread of resistance, the regional cooperation has been emphasized by the Middle East countries. The highest resistance, which is mediated by KPC has been observed in Afghanistan, Saudi Arabia and Jordan followed by NDM in Pakistan and OXA in Turkey and Pakistan. It is important to mention that the spread of these types have been reported sporadically in the other countries of this area. This review described the widespread carbapenemases in the Middle East area, which have been identified in an alarming rate.
Keywords: Carbapenemase, KPC, NDM, OXA, Acinetobacter baumannii, Enterobacteriaceae, Pseudomonas aeruginosa, Middle East countries
INTRODUCTION
After methicillin resistant staphylococcus and extended spectrum beta-lactamases (ESBL), another β-lactamase causing resistance among Gram negative organisms are carbapenemase enzymes, which hydrolysis a group of antibiotics called carbapenems (1). Carbapenems are potent β-lactam antibiotics that is used to treat serious infections in hospital settings. As dipolar compounds, they rapidly enter to the Gram-negative bacterial cell wall (GNB) via outer membrane proteins (OMPs or porins) and target the bacterial penicillin-binding proteins (PBPs) (2).
In comparison to cephalosporins, penicillins or β-lactam/ β -lactamase inhibitor, carbapenems have broad antimicrobial spectrum that includes Gram-positive (e.g., imipenem, doripenem) and Gram-negative bacteria (e.g., meropenem, ertapenem) (3). In the recent few years, the emergence of carbapenem in Gram-negative bacteria either in non-fermenters (P. aeruginosa and A. baumannii) or in fermenters (Enterobacteriaceae) has been reported worldwide (4).
Transferable carbapenemases (enzymes which inactivate carbapenems together with other β -lactams and can be disseminated via horizontal gene transfer) are a major public health threat, which can increase the rate of mortality and decline the choice of appropriate antibiotic therapy (6). Currently, the spread of carbapenemase producer especially for GNB are the most important clinical problem in antibiotic resistance and it must be strongly controlled and prevented (7). The aim of this study is to provide an extensive review of carbapenemase gene dissemination in the Middle East region.
CARBAPENEMASE
Carbapenemases are a group of enzymes that are able to hydrolyze carbapenems even at low level. Carbapenems includes imipenem, meropenem, ertapenem, cephalosporins, and the broad-spectrum of penicillin (8). Fifteen years after the introduction of carbapenems penicillin became a worldwide health problem (9). Since the first detection of carbapenems in early1990s, the spread of them through all continents increased dramatically (5).
The highly mobile genetic elements of carbapenemase genes contribute to their rapid spread and frequent transfer of multiple other antibiotic resistance genes (10). However, some of them are chromosomally encoded. These mobile genetic elements frequently contain other antibiotic resistance genes, leading to a rapid evolution towards MDR bacteria (11). There are two main molecular families of carbapenemases: serine carbapenemases, which is based on presence of serine in their active site and metallo-carbapenemases, which are a subgroup of metallo- β -lactamases (MBLs) having at least one zinc atom at their active site (12). Difference in structural types has made it more strict for development of phenotypic tests that can identify MBLs, which they inhibited by chelating agents such as ethylene diamine tetra-acetic acid (EDTA) and serine-interactivating compounds such as clavulanic acid or boronic acid derivatives (13).
Based on amino acid homology carbapenemases have been identified in each of the four Ambler molecular classification, however those of class A, B, and D have major epidemiological impact (Table 1). According to the recent reports some of the class C cephalosporinases are able to hydrolyze imipenem at a measurable rate; however, these enzymes are usually not intended to be important carbapenem-hydrolyzing enzymes, because carbapenems do not represent a major substrate in their hydrolytic profile (14).
Table 1:
General classification of carbapenemases
| Molecolar Classa | A | B | D |
| Functional groupb | 2f | 3 | 2d |
| Active site | Serine | Zn2+ | Serine |
| Prominent Enzyme | GES, SME, NMC, KPC | IMP, VIM, SPM, GIM, SIM, NDM | OXA |
| ATM Hydrolysis | + | - | - |
| EDTA Inhibition | - | + | - |
| CLA Inhibition | ± | - | ± |
| APBA Inhibition | + | - | - |
| Most Common Bacteria | Enterobacteriaceae (rare reports in P. aeruginosa) | P. aeruginosa Enterobacteriaceae Acinetobacter spp. | Acinetobacter spp. |
ATM: aztreonam; APBA: 3′-Aminophenylboronic acid; EDTA: ethylene diamine tetra-acetic acid; CLA: clavulanic acid
Ambler classification.
Bush, Jacoby and Medeiros classification.
Class A
This group contains serine at their active site and are capable of hydrolyzing all β-lactams, such as aztreonam. In this group of carbapenemases, IMI (IMI-1 to IMI-3), Sme (Sme-1 to Sme-3), SFC-1 and NmcA, enzymes are mostly chromosomally encoded, while GES (GES-1 to GES-20) and K. pneumoniae carbapenemase (KPC-2 to KPC-13) are plasmid encoded (3). Moreover, all of them have an ability to hydrolyze a wide variety of β-lactam antibiotics, such as penicillins, cephalosporins, aztreonam and carbapenems; however, they are inhibited by tazobactam and clavulanate which placing them in the group 2f functional subgroup of β –lactamases (15). This specific genetic elements can carry resistance to fluoroquinolones and aminoglycosides because the encoding genes for quinolone and aminoglycoside resistance are carried on the same plasmids (10). They are inhibited by tazobactam and clavulanic acid but not sulbactam (16).
These group of carbapenemases are mostly carried and expressed by K. pneumoniae isolates, but they can be found in Escherichia coli, Salmonella enterica, Klebsiella oxytoca, Enterobacter aerogenes, Citrobacter freundii, Proteus mirabilis, Serratia marcescens, Enterobacter cloacae, as well as in non-fermenting Gram-negative bacilli like Acinetobacter spp, Pseudomonas aeruginosa and Pseudomonas putida (17).
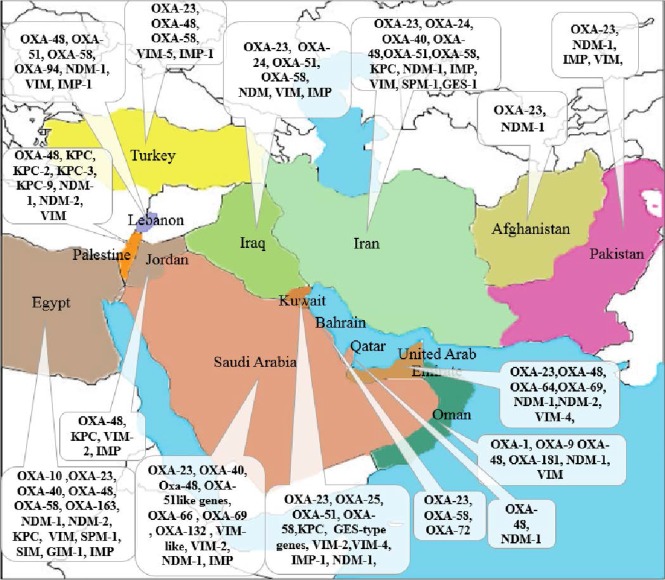
In this group, KPC-types are the most clinically common enzymes. The first KPC producer (KPC-2 in K. pneumoniae) was identified in 1996 in the eastern United States (18). Over the few years, KPC producers have been spread around the world and described across the United States (mostly in eastern coast states) and, particularly in Puerto Rico, Greece, Colombia, China, Brazil, Argentina, and Italy (19) and now is widespread in the Middle East region (Fig. 1.). Recently, GES-type carbapenemase Ambler class A has been described in A. baumannii, which is responsible for a low level of carbapenem resistance (20).
Fig. 1.
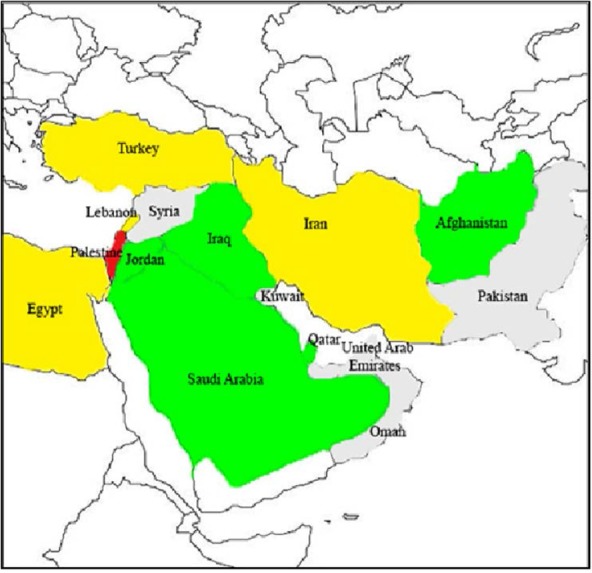
Geographic distribution of KPC enzymes in the Middle East countries. Gray, no case reported; green, single KPC-producing isolates; yellow, some outbreaks of KPC-producing isolates; red, endemicity of KPC producing isolates
Class B
Class B carbapenemases are also known as metallo- β -lactamase since they contain two zinc ions in their active site which coordinate and present polarized water ions for the oxyanion attack on the β -lactam ring. Interaction of the β -lactams with zinc ions in the active site of the enzyme is the mechanism of hydrolysis (except subgroup 3b), resulting in the distinctive trait of their inhibition by EDTA, a chelator of Zn2+ and other divalent cations (Table 1) (15). Theses enzymes are not inhibited by clavulanic acid (16). Beside those chromosomally located in environmental bacteria, acquired MBL encoding genes are often located in gene cassettes within integron, being part of a plasmid or chromosome (3).
Class B MBLs are mostly VIM (Verona integron-encoded metallo- β -lactamase) and IMP types, but the recently emerged NDM-type (New Delhi metallo- β -lactamases) is becoming the most threatening carbapenemase (17). In 1991, IMP-1 was first acquired from MBL and reported in Serratia marcescens in Japan. Since then, MBLs have been described worldwide (19). There are now more than 30 derivatives of IMP and are still dominant MBLs in Asian continent causing mainly sporadic outbreaks (21). The most commonly found class B carbapenemases are the VIM type, which has been identified in all continents (16). VIM-enzymes (there are now more than 30 derivatives) were firstly described in P. aeruginosa isolates, then emerged in Enterobacteriaceae as well. These enzymes spread over the whole Europe dramatically and causing many outbreaks in Mediterranean countries such as Greece, Italy, and Turkey (3). In addition, MBL enzymes have spread rapidly, and because of their prolific dissemination and their ability to hydrolyze all β -lactams, with the exception of aztreonam (if no ESBLs and/or AmpCs are co-produced by the isolates), presenting a serious threat (17, 22). Most MBL producers are hospital-acquired and MDR K. pneumoniae, but include Pseudomonas spp. and Acinetobacter spp. as well (17, 19).
New-Delhi MBL (NDM-1), which has more than ten variants is another metallo enzyme that arose from India in 2008 and spread rapidly over Indian subcontinent in the following few years (Fig. 2) and international travel has a significant impact on the spread of NDM-1 (3, 23). Although the diversity of the NDM-1 is low, NDM-1 is the main types among the other types of the NDM. Dissemination often involves the transfer of the blaNDM-1 gene among promiscuous plasmids and clonal outbreaks. Bacteria which contain NDM-1 are typically resistant to almost antibiotics; as a result, reliable detection and surveillance are crucial (24). Scientists claim that blaNDM-1 is widely distributed among Enterobacteriacae and has a rapid distribution around the world (25). The bacteria carrying this enzyme are Klebsiella pneumoniae, Escherichia coli, Citrobacter freundii, Enterobacter cloacae, Providencia spp. and Morganella morganii.
Fig. 2.
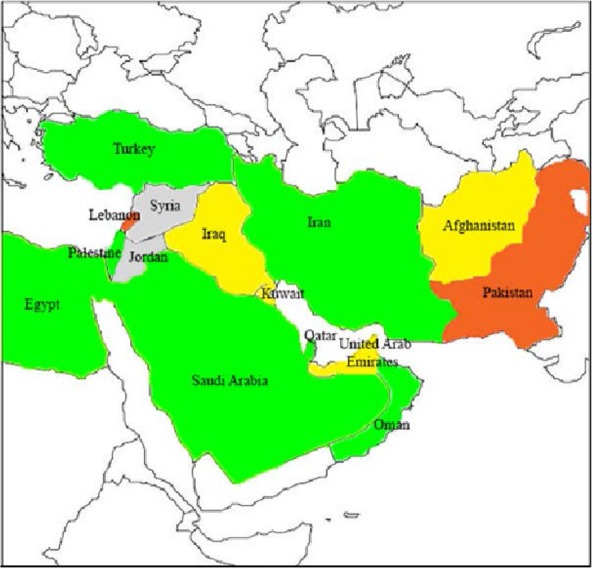
Geographic distribution of NDM type producers in Middle East countries. Gray, no case reported; green, sporadic NDM producing isolates; yellow, emerging outbreak of NDM-producing isolates; orange, single hospital outbreaks of NDM-producing isolates.
Class D
Class D β-lactamases, which named OXAs for oxacillinases, have more than 440 known variants with 232 of them showing carbapenemase activity (16) and majority of them are encoded by chromosomal genes (26).
Paton et al. described the first OXA β -lactamase with carbapenemase activity in 1993 (27). Since then multiple oxacillinases with a carbapenem-hydrolyzing activity have been reported. The enzyme was purified from a multi drug resistant A. baumannii strain that was isolated in 1985 from a patient in Edinburgh, Scotland (15).
Class D carbapenemases, based on sequence homology alone, can be divided into the following clusters: OXA-23 also named ARI-1 (an acronym of Acinetobacter resistant to imipenem) (includes OXA-27 and OXA-49); OXA-24/-40 (includes OXA-25, OXA-26) and OXA-58 (21). Some of other OXA-type carbapenemases are widely dispersed in P. aeruginosa and in A. baumannii (8) but occasionally in Enterobacteriaceae.
The most recent and worrying development is the rapid rise in OXA-48, particularly in K. pneumoniae. OXA-48-producing Enterobacteriaceae (OPE) was first identified in Turkey in 2001, then has been reported from several countries in the Middle East, North Africa and Europe (28). In order to decrease the spread of resistance, the regional cooperation has been emphasized by the Middle East countries (Fig. 3) (29). OXA-181, is a point mutant analog of OXA-48, with similar carbapenemase activity, has been detected in strains that came from India or of Indian origin (19).
Fig. 3.
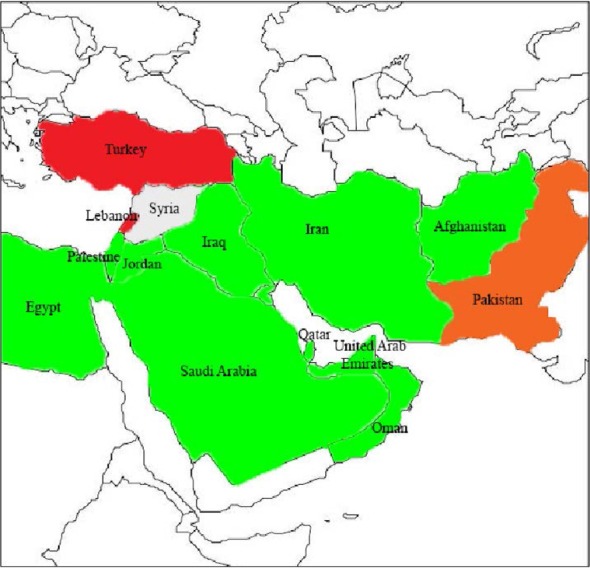
Geographic distribution of OXA-48 type producers in Middle East countries. Gray, no case reported; green, single OXA-48 producing isolates; orange, several outbreaks of OXA-48-producing isolates; red, nationwide distribution of OXA-48-producing isolates.
The fast spread of Enterobacteriaceae-producing the OXA-48 carbapenemase (mostly E. coli) is related to the dissemination of a single self-transferable plasmid, which represents another mode of resistance in healthcare-associated Gram-negative bacilli (17).
Afghanistan and Iraq
Most reports on potentially community-acquired A. baumannii from Afghanistan and Iraq have been published during the last 15 years. They acquired infections from survivors of natural disasters, community-acquired pneumonia as well as war (30). In these countries, the military medical facilities has a great evidence of providing rapid and highly advanced care to fight infection, which they have been identified a large number of MDR A. baumannii isolates as well (31). The cases of military and nonmilitary personnel of United Kingdom and U.S, which they returned from operations in Afghanistan and Iraq, harbored infections caused by MDR A. baumannii but they received an excessive attention from military medical facilities (32). Carbapenem-resistant and OXA-23–producing A. baumannii also reported in this region (33). It is notable that the blaOXA-23 gene is one of the most prevalent carbapenemase-encoding genes worldwide, which can be located on the plasmids or chromosome in various genetic structures. According to one the study from Iraq, five isolates recovered from soldiers during the Iraq conflict contained a blaOXA-23 gene that was located on a plasmid (34). Imipenem-resistant isolates also recovered from British and American soldiers repatriated from the Iraq conflict, which have been reported to be associated either with OXA-58- or OXA-23-encoding genes (34). MDR (carbapenem resistant) A. baumannii originating from injured Canadian military personnel returning from Afghanistan and Iraq have also been described (35).
Another study demonstrated that there was a difference in the prevalence of MBL production among each isolated of Gram negative bacteria. For instance, 25.9% of A. baumannii were MBL producer by PCR reaction which was higher in comparison to other bacteria in the hospital as this bacteria is more prevalent in the hospital environment and even in soil which comes from the visitors foots to the hospital. However, the main mechanism of carbapenem resistance in Acinetobacter spp. is class D blaOXA-23 carbapenemase specifically blaOXA-51, which is intrinsic to the most strains of A. baumannii (36).
The first report of NDM-1-producing P. aeruginosa in Iraq describes in 2014 (37). In recent study by Alshara et al. the detection of NDM-1-positive P. aeruginosa isolates indicates the importance of surveillance for the nosocomial infection and dissemination of NDM-1 in Najaf, Iraq.
In Iraq, little attention has been paid to β -lactamases producing isolates. However, in Najaf city, there is no information regarding the molecular studies of the occurrence of carbapenemases-producing recovered from clinical cases (38). Recent study revealed that most β -lactam resistant K. pneumoniae isolates were susceptible to both imipenem and meropenem except four (4.2%) of screened isolates were resistant to both imipenem and meropenem by standard disk diffusion method and in PCR experiments using specific primers for blaVIM, blaIMP and blaKPC genes and the results were negative among all isolates. In February 2011, a strain of Providencia stuartii submitted from a military hospital in Afghanistan was positive for blaNDM-1(39).
Egypt
In recent years, Egypt has been considered among the countries that reported high rates of antimicrobial resistance (40). While few studies have determined the resistance rates for carbapenems resistance to carbapenems in clinical A. baumannii isolates has been notable in Egypt. In 2008 Soheir Helal claimed, there was a significant increase in the number of Acinetobacter infections in teaching Cairo pediatric hospital compared with 2007 (41). In a study from Al-Agamy et al. the majority of the isolates (70%) were resistant to imipenem (MIC >8 mg/ml) (42). Phenotypic assays indicated that 82% of the carbapenem-resistant A. baumannii (CRAB) isolates had carbapenemase activity, which 2.5% of them had metallo- β -lactamase activity and the over-expression of proton gradient-dependent efflux pumps (43). In the same study A. baumannii showed the highest imipenem resistance (74%) (43).
Kaase et al. showed the first identification of a blaNDM gene in a clinical isolate arising from Egypt, without obvious link with the Indian subcontinent (44). After the recent identification of NDM producing isolates in Iraq and the Sultanate of Oman, the clinical case suggests that NDM-producing bacteria disseminated in the Middle East countries. The new NDM-2 variant was also first detected in A. baumannii from a patient who transferred from Egypt to Germany (44, 45). Recently, NDM-2-positive A. baumannii MBL isolate identified from Egypt belonged to the ST103 type (46). MBL VIM, SPM-1, and GIM-1 were also detected previously among A. baumannii isolates from Egypt (42). Nevertheless, in the recent study by Al-Agamy, none of the A. baumannii isolates harbored blaIMP, blaVIM, blaSPM, blaSIM, blaGIM, or blaNDM MBL-encoding genes (42). According to the study, which was reported carbapenemase-producers in Egypt and conducted by Poirel et al, the OXA-48 and VIM-1 which were involved in carbapenemases were not only observed in Egypt, but also they were noticed in many other countries from the Mediterranean including South Europe and North Africa (47). Furthermore, Al-Hassan et al. claimed that the prevalence of OXA-23, OXA-40, and OXA-58 were 55.88%, 2.9% and 14.7% respectively (48). In recent study by Fouad et al. results indicated that the imipenem resistance is due to the spread of OXA-23-producing clones (43).
Zafer et al. stated that the OXA-10 is the most prevalent ESBL producing P. aeruginosa in Egypt. Additionally, there were high levels of resistance to all commercially available antimicrobial agents among P. aeruginosa isolated from National Cancer Institute and Kasr El Aini Hospital; with the rate of 39.3% imipenem-resistant isolates, which reflects a threat limiting the treatment options in their hospitals (49). According to the data from Zafer et al. the rates of MBL-producing P. aeruginosa isolates from Kasr El Aini Hospital and National Cancer Institute were significant and only a limited number of antimicrobial drugs are active (49). Although the occurrence of an NDM-1-producing Acinetobacter in Egypt has been reported in recent study, there are no reports of NDM-1-producing Enterobacteriaceae in the literatures (50). However, the NDM-1 producing K. pneumoniae isolate identified in a study was shown to belong to ST11 (51, 52).
The first KPC-producing K. pneumonia, which was isolated from patients at a tertiary care hospital in Egypt was reported by Metwally et al. in 2013. Data indicates not only, the increased prevalence of Ertapenem non susceptible K. pneumoniae isolates, which partially reflects lowering of clinical breakpoints but also illustrates the spread of carbapenemases, mainly KPC types, in Suez Canal University hospital, Egypt (53).
Pakistan
Due to the few available data for CRAB in Pakistan (54) the molecular epidemiology of Acinetoacter isolates is completely unknown and the prevalence of MDRs was reported 100% among A. baumannii (55). In recent study by Shah et al. almost all A. baumannii isolates were metallo-β-lactamase and carbapenemase producer. Increased frequency of MDR isolates supports the requirement of constant surveillance to determine evolution and prevalence of these enzymes in Pakistan.
Kumarasamy et al in 2010 showed NDM-1 emergence as a novel antibiotic resistance mechanism in UK, India and the Pakistan in 37, 48 and 25 isolates respectively (56). A number of studies have included samples from Pakistan and India to evaluate the prevalence and spread of this enzyme and that’s because of its association with Indian sub-continent (57). At the beginning of the 21st century, considering the rapid population exchanges, uncontrolled NDM-1-related resistance may be expected to be identified not only in Bangladesh, India and Pakistan, but also in countries with important population exchanges with the Indian subcontinent (58). In a study which examined by multinational team the emergence and spread of 180 cases of infected patients by NDM-1 producing bacteria, including 143 cases in various sites in Pakistan and India and 37 cases in the United Kingdom, thus suggesting an extensive dissemination (56).
A. baumannii isolate from Pakistan carrying bla-OXA-23-like has been reported previously (59). blaOXA-23-like acquiring carbapenemase gene was found in the majority of CRABs from intensive care unit setting, indicating the importance of this gene in carbapenem resistance in Pakistan (60).
As reported by Nahid et al. in a study which is about MBLs producing organisms, PCR amplification confirmed 31 (23.6%) isolates harboring blaNDM-1 gene, 33 (25.1%) isolates having blaVIM gene and 2 (1.5%) isolates displaying blaIMP gene (57). The results demonstrated a high level of NDM-1 positive organisms from variety of samples at hospitals, implicating the spread of MBL genes in clinical isolates. In other study, Perry et al. highlighted that blaNDM-1 was detected in 64 isolates of seven species of Enterobacteriaceae and in three isolates of A. baumannii and one of Aeromonas caviae (61). In contrast to isolates with the NDM-1 enzyme from infections, the dominant producer species were E. cloacae and E. coli rather than K. pneumoniae (61). This shows that carbapenem resistance varies from region to region and hospital to hospital and this issue is emerging in Pakistan and needs more attention from all countries (62). In a recent study by Fakhuruddin et al. Enterobacteriaceae is 49.75% of the total clinical isolates and out of these amounts 12 of them (6.0%) are CRE. Moreover, the frequency of CRE is lower than other countries but it is higher in comparison to the local studies (62). Fakhuruddin mentioned that there are several possible factors: Firstly, carbapenem is not used in this country because of its cost. In this country cephalosporins are the first choice for experimental therapy and there is limited use of carbapenem group. Secondly, the rate of carbapenem resistance in Pseudomonas (14%) in Pakistan is lower than other countries. Lastly, limited work has been done on carbapenem resistance in Pakistan regarding Enterobacteriaceae (62).
Although other metallo-β-lactamases IMP and VIM have been reported in non-Enterobacteriaceae, like Acinetobacter species and Pseudomonas aeruginosa from this country (63), these were not detected in CRE isolates tested in this country (64).
In conclusion, a considerable prevalence of blaNDM-1, which carrying gram negative pathogens in patients coming to hospitals indicates the request for the countrywide screening of hospitals and community to evaluate the exact prevalence of it (57).
Lebanon
The increasing incidence of carbapenem resistant agents has been described in different countries around the world. Although in the Middle East countries such as Lebanon scattered information is found, the reliable reports are very scarce. During 1999 to 2009, A. baumannii strains harboring the bla-OXA-58 carbapenemase gene were predominant among carbapenem-resistant bacteria of this species in the hospital flora in different Mediterranean countries including Lebanon (65). Data from Saint George University Hospital in Beirut demonstrate that only 61% of the A. baumannii were susceptible to imipenem (66). Between November 2004 and October 2005, an outbreak of MDR A. baumannii was observed in the Saint George University Hospital of Beirut, Lebanon (67). It seems likely that the only mechanism for carbapenem resistance in A. baumannii isolates was the production of the carbapenem-hydrolyzing oxacillinase OXA-58 causing the outbreak at the Lebanese Hospital (67). The first detection of A. baumannii, which carrying the blaNDM-1 gene in Lebanon was described by Rafei et al. in 2014 that isolated from Syrian patients wounded during the civil war. All isolates which harbored the blaNDM-1 gene were negative for other tested carbapenemases (68). They belonged to the sequence type 85 and formed a single cluster by PFGE. Finally, blaOXA-51-like gene sequencing revealed the presence of the blaOXA-94 variant in all of these isolates (68).
The first report from Lebanon on carbapenem resistant Enterobacteriaceae isolates imported from Iraq (69). This country, like other countries, is now facing a dangerous threat with the emergence of carbapenem-resistant Enterobacteriaceae and the imported NDM-1strains to this country. In 2007, the first metallo-β-lactamase producing in Lebanon was isolated and found to harbor the blaIMP-1 and blaCTX-M genes (70). According to the two studies which were reported in 2008 and 2010 (10), OXA-48 (class D) were produced by E. coli and K. pneumoniae (10, 69). Identification of this novel and powerful resistance determinant outside of Turkey indicates that spread could be more important than expected (71). E. coli isolates represented 10% of the OXA-48 clinical producers in 2008–11 and 73% in 2012 in Lebanon (72). In addition, intestinal carriage of OXA-48-producing E. coli was observed in the community and was marked by a diversity of strains, suggesting that OXA-48 has become endemic in North Lebanon (72). Beyrouthy et al. showed that the rate of Enterobacteriaceae exhibiting a decrease in susceptibility to ertapenem in Nini Hospital, North Lebanon, increased from 0.4% in 2008–10 to 1.6% in 2012. This growth was associated with the emergence of carbapenemase OXA-48, which had been previously reported in Lebanon in 2008–2010 (72).
In 2009, a study from Lebanon revealed that 2.5% of E. coli and 7.84% of K. pneumoniae were ESBL producers and carbapenem resistant [antimicrobial brochure at the American University of Beirut Medical Center (AUB-MC)] (10) and up to 30% of isolated E. coli strains were found to be multi-drug resistant (70). Recently, IMP and VIM have been detected in most GNB isolated from nosocomial infections (73). Although the various countries discussed share regional proximity, it is interesting to note the differences in the recovered carbapenemases. For example, the similarities were noted between OXA enzymes in Turkey and Lebanon. These similarities may be attributed to interactions between different populations and communities in these countries; the exact factors that promote such differences remain to be identified (10).
Iran
Like other countries, several studies have been carried out about drug resistance in Iran as well. Increasing resistance to carbapenems by organisms producing carbapenemase enzymes, particularly Acinetobacter and Pseudomonas spp. is the main concern in this country. The use of colistin and polymyxin B as a therapeutic agent has been prompted by increasing resistant to antimicrobials including the carbapenems, which it has been used with increasing frequency to treat patients infected with MDR-GNB such as A. baumannii in the last several years (74). Carbapenem resistance has been increasingly common issue among A. baumannii isolates in Iranian hospitals in recent years (75) with the majority of isolates showing multi-drug resistance (76). Considerably high prevalence (99%) of the isolates was MBL producing which can be a main cause of high carbapenem resistance among A. baumanni isolates (77). Recent studies have shown the existence of different MBLs producing clones of A. baumannii in Iran (Fig. 4.). The first report about the existence of blaSPM-1 and blaGES-1 among the clinical strains of A. baumannii in Iran carried out by Shahcheraghi et al. Detection of GES-1 among the Iranian strains of A. baumannii is another important finding of the recent study (78). Furthermore, in the majority of studies in Iran and other countries VIM-type MBL was the most prevalent gene that reported (22). Peymani et al. found that among MBL-producing A. baumannii isolates, 61% and 29% of them carried, respectively (79).
Fig. 4.
Geographical distribution of carbapenemase produced by Gram-negative bacilli in the Middle East countries
After the recent identification of the blaKPC and the blaNDM-1 genes in a burn unit in Tehran, the identification of the blaOXA-48 carbapenemase gene has become more difficult (80, 81). Therefore, the spread of OXA-48 producers may be more widespread than expected like in any countries of the Middle East (82). The first report indicating clonal dissemination of imipenem-resistant A. baumannii which was carried out by Peymani et al. correspond to known international clones in Iran. The results of this study revealed that 94% of imipenem-resistant A.baumannii isolates belonged to IC II (IC=international clone), suggesting clonal spread and cross-transmission of this organism in different wards of Hospitals, as well as presence of IC I isolates (83). The results obtained in recent study indicated that all isolates carried the blaOXA-23-like gene with a high level of ISAba1 element (83). In the previous paper from Tehran, OXA-23 and OXA-40 were the most prevalent carbapenemases amongst imipenem-resistant isolates; however, the epidemiological background of these isolates was not investigated (76). In 2008, 50% of resistance to meropenem and 49.3% of resistance to imipenem were reported in Iran (76), followed by 52.5% of resistance to imipenem and meropenem in 2009 (84) and 49.26% of resistance to imipenem in 2011 (78). In 2012, 62% of isolates were resistant to imipenem (85). Distribution of OXA gene among Acinetobacter isolates, in Tehran in 2008 was as follows: blaOXA-23-like / blaOXA-51-like was detected in 25%, blaOXA-24-like / blaOXA-51-like in 17.9% and bla-OXA-51-like / blaOXA-58-like was detected in 9% of the isolates (76). Consequently, the high prevalence of OXA type carbapenemase-encoding genes among the clinical isolates from Iran appears responsible for low susceptibility rates for imipenem (78). According to the data the MDR strains of A. baumannii are spreading and carbapenemase resistance is increasingly common in Iran.
Data demonstrated that the majority of P. aeruginosa isolates (87.05%) were multi-drug resistant in Iran (86). These findings are also exposed that the prevalence of antibiotic resistance of the P. aeruginosa isolates was very high in comparison with the results obtained from other studies conducted in Korea, Greece, Poland, India, Italy and Turkey. Previous studies performed in burn hospitals in various cities of Iran, such as Tehran, Shiraz and Ahwaz demonstrated a high prevalence of resistance to antibiotics in P. aeruginosa strains isolated from burn infections (87–89). There are several reports on the prevalence of blaVIM and blaIMP among carbapenem resistant P. aeruginosa strains isolates from burn patients in Iran but the characteristics of other mechanisms of resistance to carbapenems are unknown (87, 90, 91). Other study which has been done in Iran reported that 20 (9%) of 212 P. aeruginosa isolate contained blaIMP, whereas 70 (33%) of them harbored the blaVIM (22). In contrast, two recent studies which carried out in Tehran and also in the south of Iran, MBL-producing P. aeruginosa isolates were only positive for blaVIM genes (12, 92).
Yazdi et al. isolated 126 P. aeruginosa strains from non-burn patients in Iran in 2007. Among 70 imipenem resistant P. aeruginosa strains, 8 showed MBLs production in E-test, all carried blaVIM, while none of them were positive for blaIMP gene (93). A paper by Saderi et al. has demonstrated that MBL-producing P. aeruginosa is an important cause of imipenem resistance in P. aeruginosa strains isolated from burn patients in Tehran, since out of 69 imipenem resistant strains, 65 strains were MBL positive using phenotypic method (94). Therefore, the production of MBLs enzyme and AmpCβ-lactamases are the major emerging mechanism of resistance carbapenem among P. aeruginosa isolates in this city (95).
There were limited data on the carbapenemase producing Enterobacteriaceae in Iran. High rates of MDR strains in isolates with positive KPC are a major challenge and it could cause an increase in both morbidity and mortality among burn patients (96). In a study which conducted on K. pneumoniae clinical isolates from a burn hospitals, imipenem-resistance was observed in 9.1% (5 from 55) isolates, which KPC production was detected in 3 isolate, showing the overall KPC production at 5.45% (unpublished data from Naseh). Azimi et al. claimed that twenty-eight (64%) out of 44 K. pneumoniae isolates were resistant to carbapenem according to CLSI breakpoints and 16 (36%) were susceptible. In addition, all isolates were negative for presence of KPC genes on gel electrophoresis (97). The first case of OXA-48-producing K. pneumoniae in Iran, was reported by same author, which the blaOXA-48 gene was detected in 27/28 isolates and one isolate was positive for the presence of the blaVIM-4 gene (98).
Jordan
A study conducted by Dhabaan et al. investigated the antimicrobial susceptibility profile of A. baumannii and the contribution of the insertion sequence upstream of ampC β-lactamase on the susceptibility profile of A. baumannii clinical isolates collected from a Jordan hospital. A total of 64 consecutive clinical isolates of A. baumannii were recovered (between March 2005 and December 2006) at the King Abdullah University Hospital (KAUH) which imipenem and meropenem showed increased resistant rates (70.1 and 71.6%, respectively) (99).
According to the Al-Khatatneh et al. the prevalence of carbapenemases among E. coli and Klebsiella spp. is due to the infected or colonized patients who are admitted to Jordan university hospital and King Hussein Cancer Center to characterize these isolates at the molecular level. Furthermore, 24 isolates from 800 isolates of E.coli and Klebsiella spp. were demonstrated to have blaKPC, blaVIM and blaIMP genes (100). Adler et al. reported a Jordanian woman referred to the hospital for oncological care and she was diagnosed with an OXA-48-producing E. coli bacteremia, and successfully treated with colistin and ceftazidime (28). The VIM-2-producing strain was isolated from a patient who may have been treated with carbapenems in Jordan (101). Although the carbapenemase gene is not well known, blaVIM-2 may be present in Jordan, as it is widespread in Asia and Eastern Europe. On the other hand, VIM-2 may have been disseminated from Jordan to the U.S. as well (101).
Persian Gulf countries
The countries of the Persian Gulf (United Arab Emirates [UAE], Qatar, Kuwait, Bahrain and Oman) represent that the international travel is a prominent problem; migrant workers are substantial proportions of the population from the Indian subcontinent and large numbers of citizens seek medical care in specialized centers in the Europe and United States(102).
United Arab Emirates
Among the Arabian Peninsula countries, the high amount of migration is a significant trade, touristic and health care links with the Indian subcontinent. For instance, a known reservoir of such isolates makes this country to encounter with strains.
Some papers demonstrated that a variety of NDM-producing isolates, have already been present in the Abu Dhabi Emirate, that representing different species and genetic support (103). Screening of 155 carbapenem non-susceptible A. baumannii strains recovered in Abu Dhabi hospitals during 2008 to 2011 identified two blaNDM MBL gene-carrying isolates. They were shown the blaNDM-2 gene recently detected in A. baumannii in Egypt. These isolates indicate that the blaNDM-2 gene have prominent similarities with those associated with blaNDM-1 in A. baumannii and Enterobacteriaceae (104).
Recent data displayed that OXA-23-positive A. baumannii strains have spread around the world especially in the UAE (105). Lu et al. mentioned in the UAE, resistance to carbapenems was associated with the OXA-23 production (106). Five CRAB isolates, collected from the UAE in 2006, were investigated to identify the responsible mechanism(s) in carbapenem resistance. Genotyping assays verified that the fifth isolates have the gene on a transferrable plasmid and the four isolates with the blaOXA-23 gene were on the chromosome within a Tn2006 composite transposon, which were clonally related (105).
In recent report by Sonnevend et al. among 28 clinically carbapenem which were non-susceptible Enterobacteriaceae isolates collected in 2009–2011 in the UAE, three K. pneumoniae, two E. coli, one C. freundi and one E. cloacae were identified to produce NDM-1 carbapenemase (103). Moreover, in Abu Dhabi hospitals NDM producing Enterobacteriaceae have been found in 9 out of 34 carbapenem non-susceptible isolates, while OXA-48-like was found in 11 isolates. Screening of these isolates have been identified an E. cloacae strain containing blaC-TX-M-15, blaCMY-4, and blaVIM-4. It was isolated from the urine of an Egyptian patient repeatedly hospitalized and treated with broad-spectrum antibiotics, including carbapenems, in the UAE (107).
Kuwait
Hospitals have long served as reservoirs for the transmission of pathogenic bacteria and this has become a problem in Kuwait (108). Recently, infections due to MDR A. baumannii strains have become a serious problem in some hospitals in Kuwait, these isolates are the second most frequently isolated pathogens, particularly in the ICUs (109).
Epidemic isolates from wounded U.S. military service members were described that a novel clone which had previously been identified in European countries, by the U.S. military healthcare facility and one hospital in Kuwait. The blaOXA-58-type carbapenemase was identified in two carbapenem resistant isolates, one from Kuwait and one from U.S. soldiers. The blaOXA-51-type gene which is usually present in A. baumannii was detected in all isolates around this country (110). The first report of a novel OXA carbapenemase, OXA-58, came from Kuwait (111, 112). Other studies reported that A. baumannii carrying OXA-type carbapenemases have emerged from Kuwait, which have been suggested that carbapenemase producers are emerging pathogens in this region (113). Besides, the sequence data were not obtained for the carbapenem-hydrolyzing OXA enzyme from the isolate in Kuwait; nevertheless, the enzyme was phenotypically similar to OXA-25 and OXA-26(111).
Recently, the emergence of GES-type Ambler class A carbapenemases in A. baumannii has been demonstrated and identified in this country (114). Bonnin et al. reported a total of 63 blaGES positive isolates were analyzed, one isolate have the blaGES-14 gene encoding a carbapenemase activity, whereas the other isolates harbored the blaGES-11 ESBL gene (114).
P. aeruginosa and A. baumannii producing VIM-2 were also reported from Kuwait (109). In 2012, Al-Sweih et al. emphasized that 40 (42.6%) of 94 isolates, were resistant to either meropenem or imipenem and in some cases to both of them (CRAB). Most CRAB isolates (29 of 40 or 72.5%) carried bla genes, which code for metallo-β-lactamase (VIM-2 and IMP-1) enzymes and two isolates was blaOXA-23 harboring (109).
Reports of emerging carbapenemases among Enterobacteriaceae have been published worldwide such as Kuwait (115). Overall, carbapenem resistance in Enterobacteriaceae was extremely rare in Kuwait, but in the last few years especially after 2009, some articles showed resistant isolates to carbapenems (116). New Delhi metallo-β-lactamase (NDM) has been recently detected among members of Enterobacteriaceae family in the Middle Eastern countries including Kuwait (113). During 2010 and 2011, two NDM-1-producing K. pneumoniae were identified in Kuwait (115). In recent study, Jamal et al. reported that 11 of 14 isolates produced VIM-4 (six K. pneumoniae, three E. coli, one E. cloacae, and one K. oxytoca) and three isolates produced the NDM-1 MBL and co-produced the plasmid-encoded AmpC CMY-4 (116). In conclusion, the emergence of carbapenem-resistant Enterobacteriaceae in Kuwait has significant prevalence like many other countries.
Qatar
Due to carbapenemase producing bacteria in Qatar, data on carbapenem resistance are extremely limited. Between January and June 2002, an outbreak of MDR A. baumannii reported by El Shafie et al. which involved 21 ICU patients, caused by carbapenem-resistant Acinetobacter occurred in a trauma intensive care unit (TICU) at the Hamad Medical Corporation, Qatar (117). It was reported that carbapenems had the best activity against A. baumannii, but in this outbreak, the strain was resistant, due to the production of carbapenemase (117). In other study, Khan et al. observed high resistance rate to carbapenems among the Acinetobacter isolates (41.5%), whereas resistance among P. aeruginosa was emerging 14.3% (3/21) and Enterobacter spp., E. coli and Klebsiella spp. were all sensitive to carbapenems (118). El Shafie et al. found that 6% of the reported isolates of both Klebsiella spp. and E. coli were resistant to imipenem but not to meropenem (117).
Bahrain
Studies reported that A. baumannii carrying OXA-type carbapenemases have emerged from Bahrain, signifying that carbapenemase producers are emerging pathogens in this region (119). Mugnier et al. have analyzed eight A. baumannii strains isolated in the Salmaniya Medical Complex from Bahrain and found that all isolates produced carbapenemase genes. Of eight isolates, two harbored blaOXA-23 and one isolate carried blaOXA-58, while the other five A. baumannii isolates produced blaOXA-72 (119). However carbapenems were the most active drug against the ESBL-producing isolates (120).
Oman
Over the past decade carbapenem resistance was not common in Omani hospitals; for example, between 2004 and 2005 all ESBL producers isolated from a hospital in Muscat were found to be susceptible to carbapenems (102). KP3 was isolated from a patient transferred to the Sultanate of Oman from India that was resistant to all β-lactams, including carbapenems (121).
In a study Dortet et al. described the dissemination of carbapenemase producers, especially OXA-48-types and NDM-1, in Sultanate of Oman. The close relationship between the India and Arab Peninsula in terms of patient exchange might be related to emergence of those carbapenemase producers in that country (122). NDM-type-producing isolates from Oman and Qatar were genetically unrelated to all other NDM-type positive strains isolated from this region (123). Piorel et al. showed that two blaNDM-1 carbapenemase genes harboring isolates including 419 and 601, which were recovered from an Omani patient who had not travelled abroad and from a patient who was transferred from India respectively. However, the two isolates were clonally unrelated, and belonged to ST340 (isolate 419) and ST14 (isolate 601) (124). In addition to NDM-1, the ST14 isolate expressed β-lactamases TEM-1, CTX-M-15, SHV-28, OXA-1, OXA-9, and the aminoglycoside resistance methylase ArmA and the ST340 isolate expressed β-lactamases OXA-1, SHV-11 and ArmA as well. In both isolates, the blaNDM-1 gene was located on plasmids with the similar size (170 kb), but in different incompatibility groups (124).
The rates of carbapenem-resistant Acinetobacter and P. aeruginosa isolates from different sites in Omani hospitals were reportedly less than observed in other GCC (Gulf Cooperation Council) states. For example, isolates from a hospital in Muscat (2007) showed that susceptibility to meropenem was 100% and 85% for Acinetobacter and P. aeruginosa respectively (102). In this study, we are aware of un-sequenced VIM-related enzymes in P. aeruginosa strains from Oman (125).
Saudi Arabia
Limited data is available on the antimicrobial susceptibility of Acinetobacter species in Saudi Arabia, and most recently; this information was restricted to the study of the epidemiology of an outbreak (126). On the other hand, studies on carbapenem resistance Acinetobacter in Saudi Arabia reported inconsistent results (102). For instant, in a tertiary hospital ICU in Riyadh, IMP susceptibility in Acinetobacter declined from 55% in 2004 to 10% in 2009 (127). In contrast, a national study on 228 Acinetobacter isolates from 24 hospitals in 2009 found that 94.6% of them were IMP susceptible. This susceptibility rate is significantly higher than other rates reported in studies from individual hospitals or from other GCC states (128). In Saudi Arabia, carbapenem resistance has high prevalence in A. baumannii isolates from hospitals, which these isolates are much more likely to be resistant than susceptible. In addition, a very high percentage of isolates (∼95% of isolates) co-harboring a VIM-type MBL whereas the high levels of carriage of the acquired carbapenemases blaOXA-40 and blaOXA-23 (129). In recent study which has been conducted by Alsultan et al. diabetic patients were significantly carried carbapenem-resistant isolates (129). The emergence of multi-drug resistant nosocomial A. baumannii has also reported in different hospitals in Saudi Arabia (130). Furthermore, in nine Acinetobacter strains isolated from three different sites, a novel OXA-51-like-encoding genes were identified (131). A high OXA-51-like genotypic diversity was revealed by sequence-based typing from inpatients at a tertiary care hospital in Riyadh, Saudi Arabia and showed that all isolates were clustered into four main groups including OXA-66 (62.3 %), followed by OXA-69 (19.1 %), OXA-132 (7.6 %) and other OXA-51-like genes (10.3 %), such as OXA-79, -82, -92, -131 and -197 (132). Moreover, a high prevalence (81.4 %) of OXA-69 and OXA-66-like genes in A. baumannii was identified (132).
The frequency of imipenem resistance among P. aeruginosa isolates in Saudi Arabia was 11% in 1998 (133) 9.3% in 1999 (134), 20% in 2004 (133) and 16.3% in 2007 (135). This resitance was increased to 38.57% in 2011 (136). Additionally, high prevalence of imipenem resistant and MBL-producing P. aeruginosa isolates in Saudi Arabia was reported by the recent studies which were conducted in this region. Imipenem resistance is increasing and MBL is responsible for 20.57% of the resistance (136). In this country, MBLs have emerged as a main mechanism of carbapenem resistance in P. aeruginosa (102). The blaVIM-2 is the dominant MBL gene in MBL-producing isolates in Saudi Arabia (136). In 2007, Al-Agamy et al. in screening of 135 P. aeruginosa clinical isolates from Riyadh has found that 16.29% of them have blaVIM-like genes (135). Another study found that 19.4% and 22.6% of 31 metallo-β-lactamase-producing P. aeruginosa isolates from Makkah (Mecca) harbored blaVIM and blaIMP, respectively (137).
Although there were only limited phenotypic reports, the emergence of carbapenem-resistant Enterobacteriaceae in Saudi Arabia was reported in 2000 (102). Although mechanisms of increased carbapenem MICs were not explored, during 2002 and 2003, studies claimed that 14% of ESBL-producing E. coli in the eastern province of Saudi Arabia had increased MICs to meropenem and imipenem (138). Between 2004 and 2009, studies on ICUs in Riyadh showed that out of 285 ESBL-positive isolates, just one single carbapenem-resistant was isolated (127). The first documented outbreak of carbapenem-resistant in Saudi Arabia occurred in Riyadh during 2009 and 2010 and involved 20 patients (102).
In 2013, among Enterobacteriaceae isolates, 53% showed resistance to meropenem and 36% to imipenem, which is alarming, because carbapenems have been the drug of choice. As reported by Poirel et al. in 2011, a KPC-producing isolate has also been reported in Riyadh and was found to be tigecycline resistant (139). In the Arabian Peninsula, OXA-48 and NDM have been reported in the United Arab Emirates, Oman and Kuwait (4, 104, 121, 122, 124), but regarding Saudi Arabia little has been published, which is the largest region’s and most populous country, with significant international population flows(124). In 2012, Shibl et al. provided the first insight into the widespread occurrence of isolates harboring blaOXA-48 and blaNDM-1 among patients in healthcare facilities in Riyadh, Saudi Arabia (140).
Turkey
In the last two decades, A. baumannii has become a significant nosocomial pathogen in Turkey as well as throughout the world and is a leading issue in antibiotic therapy due to its MDR (141). Carbapenemases seem to be the main cause of carbapenem-resistance in A. baumannii and thus have to be considered as the main target for development of inhibitors (142). According to the SENTRY Antimicrobial Surveil-lance Program, a significant increase in carbapenem-resistance among A. baumannii isolates (20–60 %) in two Turkish medical centers was detected in the 2000–2006 period (143). Until 2005, carbapenem resistant A. baumannii strains had only been isolated sporadically in Turkey (144). At the August 2005, a rapid increase in the isolation of CRAB was observed in the ICU setting (144). In recent years, molecular epidemiology studies have documented multiple outbreaks of MDR clones of A. baumannii in Turkish hospitals (145, 146). Moreover, the multiple data of carbapenemases have been described in this country including OXA-producing A. baumannii. From 2006, carbapenem-resistant strains were evaluated for the presence of epidemic clonally and encoding genes. OXA-23-like and OXA-58-like carbapenemase-producing strains were previously identified in Turkey. An ICU-based OXA-23-producing MDR A. baumannii outbreak was detected between 2005 and 2006 (147).
In Turkey, carbapenem resistance in Enterobacteriaceae isolates also has been reported and most often mediated by OXA-48 type carbapenemases (148). Although the spread of this type is periodically in 2001, the first OXA-48 producer was identified in 2003 from a strain isolated in Istanbul (19,149). This case was resistant to all β-lactams, including carbapenems (149). The hospital outbreaks in the main cities of this country were under controlled (150). Subsequently, dissemination of carbapenem-producing isolates not only reported in Turkey but also in the Middle East, North Africa and Europe as well (151). OXA-48 has been found in a C. freundii (152) isolate, P. rettgeri, E. cloacae(150) and an E. coli isolate as well as several isolates (153, 154). The number of OXA-48-producing isolates in the hospitals of this country from 2 and 6 isolates between 2007 and 2008 respectively, increased to 27 isolates in 2009; conversely, metallo-β-lactamase production rates were stable throughout the 3 years surveyed (155). Another study also demonstrated that although the strains were isolated from the same clinical units, the blaOXA-48 gene dissemination was not by a single clone (156). This means that in Istanbul, multiple OXA-48-producing clones are present. Clinical laboratory detection of OXA-48-producing strains may be difficult, since the blaOXA-48-carrying plasmid confers a low level of resistance to carbapenems by itself (157). As reported by Labarca et al. the first detection of the globally spread of carbapenem-resistance clone ST258 and carbapenemase-2-producing have been occurred in Turkey (158). Although, this issue have been never detected in Turkey, this country has a specific epidemiology where OXA-48 carbapenemase has been widely detected for a decade (159). But there is only one known report of imported NDM-1-producing from an Iraqi patient when admitted at the Turkish hospital (160). The OXA-23 chromosome-encoded oxacillinase was previously described for Proteus mirabilis (161).
VIM-enzymes causing outbreaks in many Mediterranean countries, like Turkey (3). VIM-5 is a variant which is closely related to VIM-1 was originally reported in an IMP-resistant isolate from this country (VIM-5 compared with VIM-1, differs by five amino acid substitutions) (162). The presence of VIM-5 enzyme, which was identified in an isolated from a different Turkish area, suggests that the regional spread of this resistance is determinant. Other MBL detected in Enterobacteriaceae was IMP-1 in 2003 and in E. cloacae in 2003 and 2004 (163).
CONCLUSION
The increasing incidence of carbapenem resistant agents has been described in different countries around the world especially in the Middle East. In recent years, the emergence of carbapenem-resistant GNB in this region is an alarming problem. Carbapenemases represent the strict threat for human health worldwide, and stand as one of the most challenging problems confronting containment of infectious diseases in the following years. The prevalence of carbapenemases is variable across the Middle East countries; a high prevalence of KPC enzymes can be found in Afghanistan, Saudi Arabia and Jordan, but little has been reported in other countries. Moreover, the highest resistance mediated by NDM carbapenemases has reported in Pakistan; however, in other countries have reported sporadically. Additionally, the resistant mediated by OXA carbapenemases in Turkey and Pakistan much has been observed and in other countries have been occasionally. Variety of carbapenemase enzymes depend on the country; may be influenced by historical and cultural relationships and may also be due to wars and moving troops. Cross border transfer of patients, medical tourism, travelers and refugees might also play a significant role. Thus, guidelines and appropriate infection control measures are needed to prevent such infections among patients.
ACKNOWLEDGEMENT
We would like thank to all staffs of educational hospitals for cooperation in Antibiotic stewardships of Tabriz University of Medical science. Also we thank Dr. Hossein Navidinia for his helpful comments on manuscript. This study was supported by a grant for evaluating carbapenemases by Drug Applied Research Center, Tabriz University of Medical Sciences.
REFERENCES
- 1. Amjad A, Mirza I, Abbasi S, Farwa U, Malik N, Zia F. Modified Hodge test: A simple and effective test for detection of carbapenemase production. Iran J Microbiol 2011;3: 189– 193. [PMC free article] [PubMed] [Google Scholar]
- 2. Patel G, Bonomo RA. Status report on carbapenemases: challenges and prospects. Expert Rev Anti Infect Ther 2011;9: 555– 570. [DOI] [PubMed] [Google Scholar]
- 3. Bedenic B, Plecko V, Sardelic S, Uzunovic S, Godic Torkar K. Carbapenemases in gram-negative bacteria: laboratory detection and clinical significance. Biomed Res Int 2014; 2014: 841951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 2012;67: 1597– 606. [DOI] [PubMed] [Google Scholar]
- 5. Meletis G, Oustas E, Bagkeri M. Carbapenemase reports from the Balkans: a systematic review. Infez Med 2014;22: 85– 106. [PubMed] [Google Scholar]
- 6. Bush K, Pannell M, Lock JL, Queenan AM, Jorgensen JH, Lee RM, et al. Detection systems for carbapenemase gene identification should include the SME serine carbapenemase. Int J Antimicrob Agents 2013;41: 1– 4. [DOI] [PubMed] [Google Scholar]
- 7. Bialvaei AZ, Samadi Kafil H, Asgharzadeh M, Memar MY, Yousefi M. Current Methods for the Identification of Carbapenemases. J Chemoter 2015. DOI: 10.1973/947815Y0000000063 [DOI] [PubMed] [Google Scholar]
- 8. De Andrade SS, Gales AC, Sader HS. Antimicrobial resistance in Gram-negative bacteria from developing countries. Antimicrobial resistance in developing countries: Springer; 2010. p. 249– 66. [Google Scholar]
- 9. Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos J, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill 2010; 15(46): pii: 19711 [DOI] [PubMed] [Google Scholar]
- 10. El-Herte RI, Kanj SS, Matar GM, Araj GF. The threat of carbapenem-resistant Enterobacteriaceae in Lebanon: an update on the regional and local epidemiology. J Infect Public Health 2012;5: 233– 43. [DOI] [PubMed] [Google Scholar]
- 11. Rolain JM, Cornaglia G. Carbapenemases in Enterobacteriaceae: the magnitude of a worldwide concern. Clin Microbiol Infect 2014; 20: 819– 20. [DOI] [PubMed] [Google Scholar]
- 12. Bahar MA, Jamali S, Samadikuchaksaraei A. Imipenem-resistant Pseudomonas aeruginosa strains carry metallo-beta-lactamase gene bla(VIM) in a level I Iranian burn hospital. Burns 2010;36: 826– 30. [DOI] [PubMed] [Google Scholar]
- 13. Bush K, Pannell M, Lock JL, Queenan AM, Jorgensen JH, Lee RM, et al. Detection systems for carbapenemase gene identification should include the SME serine carbapenemase. Int J Antimicrob Agents 2013;41: 1– 4. [DOI] [PubMed] [Google Scholar]
- 14. Rasmussen BA, Bush K. Carbapenem-hydrolyzing beta-lactamases. Antimicrob Agents Chemother 1997;41: 223– 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007;20: 440– 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Djahmi N, Dunyach-Remy C, Pantel A, Dekhil M, Sotto A, Lavigne JP. Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean countries. Biomed Res Int 2014; 2014: 305784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levy Hara G, Gould I, Endimiani A, Pardo PR, Daikos G, Hsueh PR, et al. Detection, treatment, and prevention of carbapenemase-producing Enterobacteriaceae: recommendations from an International Working Group. J Chemother 2013;25: 129– 140. [DOI] [PubMed] [Google Scholar]
- 18. Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001;45: 1151– 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011;17: 1791– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, Kholy AE. Molecular characterization of carbapenem-insensitive “Acinetobacter baumannii” in Egypt. Intern J Infect Dis 2014;22: 49– 54. [DOI] [PubMed] [Google Scholar]
- 21. Walsh TR. Emerging carbapenemases: a global perspective. Intern J Antimicrob Agents 2010;36 Suppl 3: S8– 14. [DOI] [PubMed] [Google Scholar]
- 22. Aghamiri S, Amirmozafari N, Fallah Mehrabadi J, Fouladtan B, Samadi Kafil H. Antibiotic Resistance Pattern and Evaluation of Metallo-Beta Lactamase Genes Including bla-IMP and bla- VIM Types in Pseudomonas aeruginosa Isolated from Patients in Tehran Hospitals. ISRN Microbiol 2014; 2014: 941507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poirel L, Fortineau N, Nordmann P. International transfer of NDM-1-producing Klebsiella pneumoniae from Iraq to France. Antimicrob Agents Chemother 2011;55: 1821– 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol 2011;19(12): 588– 95. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto T, Takano T, Iwao Y, Hishinuma A. Emergence of NDM-1-positive capsulated Escherichia coli with high resistance to serum killing in Japan. J Infect Chemother 2011;17: 435– 9. [DOI] [PubMed] [Google Scholar]
- 26. Walther-Rasmussen J, Hoiby N. OXA-type carbapenemases. J Antimicrob Chemother 2006;57: 373– 83. [DOI] [PubMed] [Google Scholar]
- 27. Karmostaji A, Peerayeh SN, Salmanian AH. Distribution of OXA-Type Class D β-Lactamase Genes Among Nosocomial Multi Drug Resistant Acinetobacter baumannii Isolated in Tehran Hospitals. Jundishapur J Microbiol 2013; 6: e8219 [Google Scholar]
- 28. Adler A, Shklyar M, Schwaber MJ, Navon-Venezia S, Dhaher Y, Edgar R, et al. Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. J Antimicrob Chemother 2011;66: 2763– 6. [DOI] [PubMed] [Google Scholar]
- 29. Goren MG, Chmelnitsky I, Carmeli Y, Navon-Venezia S. Plasmid-encoded OXA-48 carbapenemase in Escherichia coli from Israel. J Antimicrob Chemother 2010: dkq467. [DOI] [PubMed] [Google Scholar]
- 30. Eveillard M, Kempf M, Belmonte O, Pailhories H, Joly-Guillou ML. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Intern J Infect Dis 2013;17: e802– 5. [DOI] [PubMed] [Google Scholar]
- 31. Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Aagents Chemother 2006;50: 4114– 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2007; 51: 3471– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg 189: 395– 404. [DOI] [PubMed] [Google Scholar]
- 34. Kusradze I, Diene SM, Goderdzishvili M, Rolain JM. Molecular detection of OXA carbapenemase genes in multidrug-resistant Acinetobacter baumannii isolates from Iraq and Georgia. Intern J Antimicrob Agents 2011;38: 164– 8. [DOI] [PubMed] [Google Scholar]
- 35. Shali A. Identification of Multidrug-Resistant Genes in Acinetobacter baumannii in Sulaimani City-Kurdistan Regional Government of Iraq. Asian J Med Sci 2012;4: 179– 83. [Google Scholar]
- 36. Anoar KA, Ali FA, Omer SA. Detection of metallo β-lactamase enzyme in some gram negative bacteria isolated from burn patients in sulaimani city, iraq. European Sci J 2014;10: 458– 496. [Google Scholar]
- 37. Alshara JMR, Alsehlawi ZSR, Aljameel DSA. First Report of New Delhi Metallo-beta-Lactamase (NDM-1) Producing Pseudomonas aeruginosa in Iraq. J Biol Agricul Healthcare 2014;4: 40– 7. [Google Scholar]
- 38. Al Sehlawi ZS, Almohana AM, AlThahab AA. Occurrence and Detection of Carbapenemase-Producing Klebsiella pneumoniae Clinical Isolates in Najaf Hospitals. Al-Kufa J Biol 2013; 5: e 321. [Google Scholar]
- 39. McGann P, Hang J, Clifford RJ, Yang Y, Kwak YI, Kuschner RA, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56: 1673– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahmoud A, Zahran W, Hindawi G, Labib A, Galal R. Prevalence of Multidrug-Resistant Pseudomonas aeruginosa in Patients with Nosocomial Infections at a University Hospital in Egypt, with Special Reference to Typing Methods. J Virol Microbiol 2013: 1– 13. [Google Scholar]
- 41. Soheir Helal, Haleim MMA, Gaafar aM. Detection of the Relationship between Imipenem Susceptible and Non-Susceptible Clinical Isolates of Acinetobacter Baumannii by Repetitive Element PCR-Mediated DNA Fingerprinting in an Egyptian Hospital. J Life Sci 2011;5: 59– 65. [Google Scholar]
- 42. Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, El Kholy A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Intern J Infect Dis 2014; 22: 49– 54. [DOI] [PubMed] [Google Scholar]
- 43. Fouad M, Attia AS, Tawakkol WM, Hashem AM. Emergence of carbapenem-resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. Intern J Infect Dis 2013;17: e1252– 4. [DOI] [PubMed] [Google Scholar]
- 44. Kaase M, Nordmann P, Wichelhaus TA, Gatermann SG, Bonnin RA, Poirel L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J Antimicrob Chemother 2011: dkr135. [DOI] [PubMed] [Google Scholar]
- 45. Hrabák J, Stolbová M, Studentová V, Fridrichová M, Chudackova E, Zemlickova H. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011. Euro Surveill 2012; 17: pii: 20085 [PubMed] [Google Scholar]
- 46. Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother 2012; 56: 1087– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poirel L, Abdelaziz MO, Bernabeu S, Nordmann P. Occurrence of OXA-48 and VIM-1 carbapenemase-producing Enterobacteriaceae in Egypt. Intern J of Anti-microb Agents 2013;41(1): 90– 1. [DOI] [PubMed] [Google Scholar]
- 48. Al-Hassan L, El Mehallawy H, A. SG. Diversity in Acinetobacter baumannii isolates from paediatric cancer patients in Egypt. Clin Microbiol Infect 2013;19: 1082– 8. [DOI] [PubMed] [Google Scholar]
- 49. Zafer Mai M., Al-Agamy Mohamed H., El-Mahallawy Hadir A., Amin Magdy A., Ashour aMSE-D. Antimicrobial Resistance Pattern and Their Beta-Lactamase Encoding Genes among Pseudomonas aeruginosa Strains Isolated from Cancer Patients. BioMed Res Intern 2014; Article ID 101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hrabák J, Štolbová M, Študentová V, Fridrichová M, Chudáčková E, H Z. NDM-1 producing Acinetobacter baumannii isolated from a patient repatriated to the Czech Republic from Egypt, July 2011. Euro Surveill 2012; 17(7): pii: 20085 [PubMed] [Google Scholar]
- 51. Abdelaziz MO, Bonura C, Aleo A, El-Domany RA, Fasciana T, Mammina C. OXA-163-producing Klebsiella pneumoniae in Cairo, Egypt, in 2009 and 2010. J Clin Microbiol 2012;50: 2489– 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abdelaziz MO, Bonura C, Aleo A, Fasciana T, Mammina C. NDM-1-and OXA-163-producing “ Klebsiella pneumoniae” isolates in Cairo, Egypt, 2012. J Glob Antimicrob Resist 2013;1: 213– 5. [DOI] [PubMed] [Google Scholar]
- 53. Metwally L., Gomaa N., Attallah M, Kamel aN. High prevalence of Klebsiella pneumoniae carbapenemase-mediated resistance in K. pneumoniae isolates from Egypt. Eastern Mediterranean Health J 2013;19: 947– 52. [PubMed] [Google Scholar]
- 54. Khan MS, Siddiqui SZ, Haider S, Zafar A, Zafar F, Khan RN, et al. Infection control education: impact on ventilator-associated pneumonia rates in a public sector intensive care unit in Pakistan. Trans R Soc Trop Med Hyg 2009;103: 807– 11. [DOI] [PubMed] [Google Scholar]
- 55. Begum S, Hasan F, Hussain S, Ali Shah A. Prevalence of multi drug resistant Acinetobacter baumannii in the clinical samples from Tertiary Care Hospital in Islamabad, Pakistan. Pak J Med Sci 2013;29: 1253– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 2010;10: 597– 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nahid F, Khan AA, Rehman S, Zahra R. Prevalence of metallo-beta-lactamase NDM-1-producing multi-drug resistant bacteria at two Pakistani hospitals and implications for public health. J Infect public health 2013;6: 487– 93. [DOI] [PubMed] [Google Scholar]
- 58. Nordmann P, Poirel L, Toleman MA, Walsh TR. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J Antimicrob Chemother 2011; 66: 689– 92. [DOI] [PubMed] [Google Scholar]
- 59. Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Intern J Antimicrob Agents 2006; 27: 351– 3. [DOI] [PubMed] [Google Scholar]
- 60. Irfan S, Turton JF, Mehraj J, Siddiqui SZ, Haider S, Zafar A, et al. Molecular and epidemiological characterisation of clinical isolates of carbapenem-resistant Acinetobacter baumannii from public and private sector intensive care units in Karachi, Pakistan. J hospit Infect 2011;78: 143– 8. [DOI] [PubMed] [Google Scholar]
- 61. Perry JD, Naqvi SH, Mirza IA, Alizai SA, Hussain A, Ghirardi S, et al. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J Antimicrob Chemother 2011;66: 2288– 94. [DOI] [PubMed] [Google Scholar]
- 62. Fakhuruddin MAD, Bakar I, Ahmed R, Kumar M. Frequency of Class B Carbapenemases (MbL) in enterobacteriacae. J Pak Med Assoc 2014;64: 519– 23. [PubMed] [Google Scholar]
- 63. Woodford N. Rapid characterization of beta-lactamases by multiplex PCR. Methods Mol Biol 2010;642: 181– 92. [DOI] [PubMed] [Google Scholar]
- 64. Sultan BA, Irfan S. Detection of metallo-beta lactamases (IMP, VIM, NDM) and KPC carbapenemases in Carbapenem Resistant Enterobacteriaceae: Report from Clinical Laboratory Aga Khan University Karachi, Pakistan. Infect Dis J Pakistan 2014: 584. [Google Scholar]
- 65. Liakopoulos A, Miriagou V, Katsifas E A, Karagouni A D, Daikos G L, Tzouvelekis L S, et al. Identification of OXA-23-producing Acinetobacter baumannii in Greece, 2010 to 2011. Euro Surveill 2012; 17(11): 20117. [PubMed] [Google Scholar]
- 66. Daoud Z., Hobeika E., Choucair A., Rohban R. Isolation of the first metallo-β-lactamase producing Klebsiella pneumoniae in Lebanon. Rev Esp Quimioter 2008;21(2): 123– 6. [PubMed] [Google Scholar]
- 67. Zarrilli R, Vitale D, Di Popolo A, Bagattini M, Daoud Z, Khan AU, et al. A plasmid-borne blaOXA-58 gene confers imipenem resistance to Acinetobacter baumannii isolates from a Lebanese hospital. Antimicrob Agents Chemother 2008;52: 4115– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rafei R, Dabboussi F, Hamze M, Eveillard M, Lemarie C, Mallat H, et al. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Intern J Infect Dis 2014;21: 21– 3. [DOI] [PubMed] [Google Scholar]
- 69. El-Herte Rima I., Araj George F., Matar Ghassan M., Baroud Maysa, Kanafani Zeina A., Kanj Souha S. Detection of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae producing NDM-1 in Lebanon. Infect Dev Ctries 2012;6: 457– 61. [DOI] [PubMed] [Google Scholar]
- 70. Salem SE, Dahdouh E, Daoud Z. Resistance of Gram-Negative Bacilli in Lebanon. ISRN Infect Dis 2013;2013: 1– 6. [Google Scholar]
- 71. Matar G. M., Cuzon G., Araj G. F., Naas T., Corkill J., Nordmann MMKaP. Oxacillinase-mediated resistance tocarbapenems in Klebsiella pneumoniae from-Lebanon. Clin Microbiol Infect 2008;14: 887– 8. [DOI] [PubMed] [Google Scholar]
- 72. Beyrouthy R, Robin F, Dabboussi F, Mallat H, Hamze M, Bonnet R. Carbapenemase and virulence factors of Enterobacteriaceae in North Lebanon between 2008 and 2012: evolution via endemic spread of OXA-48. J Antimicrob Chemother 2014;69: 2699– 705. [DOI] [PubMed] [Google Scholar]
- 73. Al Sehlawi Zuhair Sadiq, Almohana Ali Muhsin, Al-Thahab. AA. Occurrence and Detection of Carbapenemase-Producing Klebsiella pneumoniae Clinical Isolates in Najaf Hosp Mag Al-Kufa Uni Biol 2013; 5: 12. [Google Scholar]
- 74. Bialvaei AZ, Samadi Kafil H. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin 2015;31: 707– 21. [DOI] [PubMed] [Google Scholar]
- 75. Mohajeri Parviz, Farahani Abbas, Feizabadi Mohammad Mehdi, Ketabi Hosnieh, Abiri Ramin, Najafi F. Antimicrobial susceptibility profiling and genomic diversity of Acinetobacter baumannii isolates: A study in western Iran. Iran J Microbiol 2013;5: 295– 02. [PMC free article] [PubMed] [Google Scholar]
- 76. Feizabadi MM, Fathollahzadeh B, Taherikalani M, Rasoolinejad M, Sadeghifard N, Aligholi M, et al. Antimicrobial Susceptibility Patterns and Distribution of blaOXA Genes among Acinetobacter spp. Isolated from Patients at Tehran Hospitals. Jpn J Infect Dis 2008;61: 274– 8. [PubMed] [Google Scholar]
- 77. Safari M, Saidijam M, Bahador A, Jafari R, MY A. High Prevalence of Multidrug Resistance and Metallo-beta-lactamase (MβL) producing Acinetobacter Baumannii Isolated from Patients in ICU Wards, Hamadan, Iran. J Res Health Sci 2013;13: 162– 7. [PubMed] [Google Scholar]
- 78. Shahcheraghi F, Abbasalipour M, Feizabadi M, Ebrahimipour G, N A. Isolation and genetic characterization of metallo-β-lactamase and carbapenamase producing strains of Acinetobacter baumannii from patients at Tehran hospitals. Iran J Microbiol 2011;3: 68– 74. [PMC free article] [PubMed] [Google Scholar]
- 79. Peymani Amir, Nahaei Mohammad-Reza, Farajnia1 Safar, H A, Mirsalehian Akbar, Sohrabi Nasrollah, Abbasi aL. High Prevalence of Metallo-b-Lactamase-Producing Acinetobacter baumannii in a Teaching Hospital in Tabriz, Iran. Jpn J Infect Dis 2011;64: 69– 71. [PubMed] [Google Scholar]
- 80. Sidjabat HE, Silveira FP, Potoski BA, Abu-Elmagd KM, Adams-Haduch JM, Paterson DL, et al. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin Infect Dis 2009;49: 1736– 8. [DOI] [PubMed] [Google Scholar]
- 81. Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP, et al. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob Agents Chemother 2007;51: 1553– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lari Abdolaziz Rastegar, Azimi Leila, Rahbar Mohammad, Alaghehbandan Reza, Sattarzadeh-Tabrizi M. First report of Klebsiella pneumonia carbapenemase producing Pseudomonas aeruginosa isolated from burn patients in Iran: phenotypic and genotypic methods. GMS Hyg Infect Control 2014. 2014; 8( 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Peymania Amir, Paul G, Higgin sb, Nahaeic Mohammad-Reza, Farajniad Safar, Seifert H. Characterisation and clonal dissemination of OXA-23-producing Acinetobacter baumannii in Tabriz, northwest Iran. Intern J Antimicrob Agents 2012;39(2012): 526– 8. [DOI] [PubMed] [Google Scholar]
- 84. Taherikalani M., Fatolahzadeh B., Emaneini M., Soroush S., FM. M. Distribution of different carbapenem resistant clones of Acinetobacter baumannii in Tehran Hospitals. New Microbiologica 2009;32: 265– 71. [PubMed] [Google Scholar]
- 85. Sohrabi Nasrollah, Farajnia Safar, Akhi Mohammad Taghi, Nahaei Mohammad Reza, Naghili Behrooz, Amir Peymani, et al. Prevalence of OXA-Type b-Lactamases Among Acinetobacter baumannii Isolates from Northwest of Iran. Microb Drug Resist 2010;18: 385– 9. [DOI] [PubMed] [Google Scholar]
- 86. Mirsalehian A, Feizabadi M, Nakhjavani FA, Jabalameli F, Goli H, Kalantari N. Detection of VEB-1, OXA-10 and PER-1 genotypes in extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients. Burns 2010;36: 70– 4. [DOI] [PubMed] [Google Scholar]
- 87. Khosravi AD, Mihani F. Detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis 2008;60: 125– 8. [DOI] [PubMed] [Google Scholar]
- 88. Japoni A, Alborzi A, Kalani M, Nasiri J, Hayati M, Farshad S. Susceptibility patterns and cross-resistance of antibiotics against Pseudomonas aeruginosa isolated from burn patients in the South of Iran. Burns 2006;32: 343– 7. [DOI] [PubMed] [Google Scholar]
- 89. Estahbanati HK, Kashani PP, Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns 2002;28: 340– 8. [DOI] [PubMed] [Google Scholar]
- 90. Jabalameli F, Mirsalehian A, Sotoudeh N, Jabalameli L, Aligholi M, Khoramian B, et al. Multiple-locus variable number of tandem repeats (VNTR) fingerprinting (MLVF) and antibacterial resistance profiles of extended spectrum beta lactamase (ESBL) producing Pseudomonas aeruginosa among burnt patients in Tehran. Burns 2011;37: 1202– 7. [DOI] [PubMed] [Google Scholar]
- 91. Feizabadi MM, Delfani S, Raji N, Majnooni A, Aligholi M, Shahcheraghi F, et al. Distribution of bla(TEM), bla(SHV), bla(CTX-M) genes among clinical isolates of Klebsiella pneumoniae at Labbafinejad Hospital, Tehran, Iran. Microb Drug Resist 2010;16: 49– 53. [DOI] [PubMed] [Google Scholar]
- 92. Shahcheraghi F, Nikbin VS., Feizabadi MM. Identification and genetic characterization of metallo-beta-lactamase-producing strains of Pseudomonas aeruginosa in Tehran, Iran. New Microbiologica 2010;33: 243– 8. [PubMed] [Google Scholar]
- 93. Yazdi HR, Nejad GB, Peerayeh SN, Mostafaei M. Prevalence and detection of metallo-β-lactamase (MBL)-producing Pseudomonas aeruginosa strains from clinical isolates in Iran. Ann Microbiol 2007;57: 293– 5. [Google Scholar]
- 94. Saderi H, Lotfalipour H, Owlia P, Salimi H. Detection of Metallo- -Lactamase Producing Pseudomonas aeruginosa Isolated From Burn Patients in Tehran, Iran. Lab Med 2010;41: 609– 12. [Google Scholar]
- 95. Neyestanaki DK, Mirsalehian A, Rezagholizadeh F, Jabalameli F, Taherikalani M, Emaneini M. Determination of extended spectrum beta-lactamases, metallo-beta-lactamases and AmpC-beta-lactamases among carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. Burns 2014. 40: 1556– 61 [DOI] [PubMed] [Google Scholar]
- 96. Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of “Klebsiella pneumoniae” carbapenemase among burns patients: First report from Iran. Burns 2013;39: 174– 6. [DOI] [PubMed] [Google Scholar]
- 97. Azimi L, Lari AR, Talebi M, Namvar AE, Soleymanzadeh-Moghadam S. Evaluation of Phenotypic Methods for Detection of Klebsiella Pneumoniae Carbapenemase-Producing K. Pneumoniae in Tehran. J Med Bacteriol 2013;2: 26– 31. [Google Scholar]
- 98. Azimi L, Nordmann P, Lari AR, Bonnin RA. First report of OXA-48-producing Klebsiella pneumoniae strains in Iran. GMS hygien Infect Cont 2014; 9:Doc07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dhabaan GN, Hamimah H, Shorman M. Emergence of extensive drug-resistant Acinetobacter baumannii in North of Jordan. Afr J Microbiol Res 2011;5: 1070– 4. [Google Scholar]
- 100. Al-Khatatneh MJ, Mahafzaa A, Sa’ad M. Detection and molecular characterization of carbapenemase-producing Esherichia coli and Klebseilla spp . Isolates at the Jordan University Hospital (JUH) and king Hussein Cancer Center (KHCC): The University of Jordan; 2010.
- 101. Aboufaycal H, Sader HS, Rolston K, Deshpande LM, Toleman M, Bodey G, et al. blaVIM-2 and blaVIM-7 carbapenemase-producing Pseudomonas aeruginosa isolates detected in a tertiary care medical center in the United States: report from the MYSTIC program. J Clin Microbiol 2007;45: 614– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zowawi HM, Balkhy HH, Walsh TR, Paterson DL. beta-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clinical Microbiol Rev 2013;26: 361– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sonnevend A, Al Baloushi A, Ghazawi A, Hashmey R, Girgis S, Hamadeh MB, et al. Emergence and spread of NDM-1 producer Enterobacteriaceae with contribution of IncX3 plasmids in the United Arab Emirates. J Med Microbiol 2013;62: 1044– 50. [DOI] [PubMed] [Google Scholar]
- 104. Ghazawi A, Sonnevend A, Bonnin RA, Poirel L, Nordmann P, Hashmey R, et al. NDM-2 carbapenemase-producing Acinetobacter baumannii in the United Arab Emirates. Clin Microbiol Infect 2012;18: E34– 6. [DOI] [PubMed] [Google Scholar]
- 105. Mugnier P, Poirel L, Pitout M, Nordmann P. Carbapenem-resistant and OXA-23-producing Acinetobacter baumannii isolates in the United Arab Emirates. Clin Microbiol Infect 2008;14(9): 879– 82. [DOI] [PubMed] [Google Scholar]
- 106. Lu PL, Doumith M, Livermore DM, Chen TP, Woodford N. Diversity of carbapenem resistance mechanisms in Acinetobacter baumannii from a Taiwan hospital: spread of plasmid-borne OXA-72 carbapenemase. J Antimicrob Chemother 2009;63: 641– 7. [DOI] [PubMed] [Google Scholar]
- 107. Sonnevend A, Ghazawi A, Yahfoufi N, Al-Baloushi A, Hashmey R, Mathew M, et al. VIM-4 carbapenemase-producing Enterobacter cloacae in the United Arab Emirates. Clin Microbiol Infect 2012;18: E494– 6. [DOI] [PubMed] [Google Scholar]
- 108. Al-Hasan AR. Study of carbapenem resistance in Acinetobacter baumannii isolates from Kuwait. University of Edinburgh; 2013; 2012. [Google Scholar]
- 109. Al-Sweih NA, Al-Hubail M, Rotimi VO. Three distinct clones of carbapenem-resistant Acinetobacter baumannii with high diversity of carbapenemases isolated from patients in two hospitals in Kuwait. J Infect Public Health 2012;5: 102– 8. [DOI] [PubMed] [Google Scholar]
- 110. Da Silva GJ, Van Der Reijden T, Domingues S, Mendonca N, Petersen K, Dijkshoorn L. Characterization of a novel international clonal complex (CC32) of Acinetobacter baumannii with epidemic potential. Epidemiol Infect 2014;142: 1554– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Afzal-Shah M, Woodford N, Livermore DM. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D beta-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 2001;45: 583– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Coelho J, Woodford N, Afzal-Shah M, Livermore D. Occurrence of OXA-58-like carbapenemases in Acinetobacter spp. collected over 10 years in three continents. Antimicrob Agents Chemother 2006;50: 756– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bakour S, Alsharapy SA, Touati A, Rolain JM. Characterization of Acinetobacter baumannii Clinical Isolates Carrying bla Carbapenemase and 16S rRNA Methylase armA genes in Yemen. Microb Drug Resist 2014; 20: 604– 9 [DOI] [PubMed] [Google Scholar]
- 114. Bonnin RA, Rotimi VO, Al Hubail M, Gasiorowski E, Al Sweih N, Nordmann P, et al. Wide dissemination of GES-type carbapenemases in Acinetobacter baumannii isolates in Kuwait. Antimicrob Agents Chemother 2013;57: 183– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jamal W, Rotimi VO, Albert MJ, Khodakhast F, Udo EE, Poirel L. Emergence of nosocomial New Delhi metallo-beta-lactamase-1 (NDM-1)-producing Klebsiella pneumoniae in patients admitted to a tertiary care hospital in Kuwait. Intern J Antimicrob Agents 2012;39: 183– 4. [DOI] [PubMed] [Google Scholar]
- 116. Jamal W, Rotimi VO, Albert MJ, Khodakhast F, Nordmann P, Poirel L. High prevalence of VIM-4 and NDM-1 metallo-beta-lactamase among carbapenem-resistant Enterobacteriaceae. J Med Microbiol 2013;62: 1239– 44. [DOI] [PubMed] [Google Scholar]
- 117. El Shafie SS, Alishaq M, Leni Garcia M. Investigation of an outbreak of multidrug-resistant Acinetobacter baumannii in trauma intensive care unit. J hospit Infect 2004;56: 101– 5. [DOI] [PubMed] [Google Scholar]
- 118. Khan FY, Elshafie SS, Almaslamani M, Abu-Khattab M, El Hiday AH, Errayes M, et al. Epidemiology of bacteraemia in Hamad general hospital, Qatar: a one year hospital-based study. Travel Med Infect Dis 2010;8: 377– 87. [DOI] [PubMed] [Google Scholar]
- 119. Mugnier PD, Bindayna KM, Poirel L, Nordmann P. Diversity of plasmid-mediated carbapenem-hydrolysing oxacillinases among carbapenem-resistant Acinetobacter baumannii isolates from Kingdom of Bahrain. J Antimicrob Chemother 2009;63: 1071– 3. [DOI] [PubMed] [Google Scholar]
- 120. Bindayna KM, Senok AC, Jamsheer AE. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Bahrain. J Infect public health 2009;2: 129– 35. [DOI] [PubMed] [Google Scholar]
- 121. Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, Poirel L. Characterization of OXA-181, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55: 4896– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect 2012;18: E144– 8. [DOI] [PubMed] [Google Scholar]
- 123. Zowawi HM, Sartor AL, Balkhy HH, Walsh TR, Al Johani SM, AlJindan RY, et al. Molecular Characterization of Carbapenemase-Producing Escherichia coli and Klebsiella pneumoniae in the Countries of the Gulf Cooperation Council: Dominance of OXA-48 and NDM Producers. Antimicrob Agents Chemother 2014;58: 3085– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Poirel L, Al Maskari Z, Al Rashdi F, Bernabeu S, Nordmann P. NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J Antimicrob Chemother 2011;66: 304– 6. [DOI] [PubMed] [Google Scholar]
- 125. Livermore DM, Woodford N. Carbapenemases: a problem in waiting? Curr Opin Microbiol 2000; 3: 489– 95. [DOI] [PubMed] [Google Scholar]
- 126. Jaffar A., Al-Tawfiq, Mohandhas TX. Prevalence of Antimicrobial Resistance in Acinetobacter calcoaceticus-baumannii Complex in a Saudi Arabian Hospital. Infect Control hosp Epidemiol 2007;28: 870– 2. [DOI] [PubMed] [Google Scholar]
- 127. Al Johani SM, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med 2010;30: 364– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Memish ZA, Shibl AM, Kambal AM, Ohaly YA, Ishaq A, Livermore DM. Antimicrobial resistance among non-fermenting Gram-negative bacteria in Saudi Arabia. J Antimicrob Chemother 2012;67: 1701– 5. [DOI] [PubMed] [Google Scholar]
- 129. Alsultan AA, Evans BA, Elsayed EA, Al-Thawadi SI, Al-Taher AY, Amyes SG, et al. High frequency of carbapenem-resistant Acinetobacter baumannii in patients with diabetes mellitus in Saudi Arabia. J Med Microbiol 2013;62: 885– 8. [DOI] [PubMed] [Google Scholar]
- 130. Abdullah A., Al-Arfaj, Abdelnasser S.S., Ibrahim, Somily AM, Al-Salamah aAA. Genetic basis of carbapenem resistance in Acinetobacter clinical isolates in Saudi Arabia. Afr J Biotechnol 2011;10: 14186– 96. [Google Scholar]
- 131. Alsultan AA, Hamouda A, Evans BA, Amyes SG. Acinetobacter baumannii: emergence of four strains with novel bla(OXA-51-like) genes in patients with diabetes mellitus. J Chemother 2009;21: 290– 5. [DOI] [PubMed] [Google Scholar]
- 132. Aly M, Tayeb HT, Al Johani SM, Alyamani EJ, Aldughaishem F, Alabdulkarim I, et al. Genetic diversity of OXA-51-like genes among multidrug-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. European J Clin Microbiol Infect Dis 2014;33: 1223– 8. [DOI] [PubMed] [Google Scholar]
- 133. Bukharie A, Mowafi HA. Antimicrobial susceptibility pattern of Pseudomonas aeruginosa and antibiotic use in King Fahd hospital of the University in Khobar, Saudi Arabia. Sci J King Faisal Unive (Basic and Applied Sciences) 2010;11: 185– 91. [Google Scholar]
- 134. Al-Jasser AM, Elkhizzi NA. Antimicrobial susceptibility pattern of clinical isolates of Pseudomonas aeruginosa. Saudi Med J 2004;25: 780– 4. [PubMed] [Google Scholar]
- 135. Al-Agamy MH, Shibl AM, Tawfik AF, Radwan HH. High prevalence of metallo-beta-lactamase-producing Pseudomonas aeruginosa from Saudi Arabia. J Chemother 2009;21: 461– 2. [DOI] [PubMed] [Google Scholar]
- 136. Mohamed HA-A. Antimicrobial resistance pattern and prevalence of metallo-β-lactamases in Pseudomonas aeruginosa from Saudi Arabia. Afr J Microbiol Res 2011;5: 5528– 5533. [Google Scholar]
- 137. Asghar AH, Faidah HS. Frequency and antimicrobial susceptibility of gram-negative bacteria isolated from 2 hospitals in Makkah, Saudi Arabia. Saudi Med J 2009;30: 1017– 23. [PubMed] [Google Scholar]
- 138. Kader AA, Kumar AK. Prevalence of extended spectrum beta-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med J 2004;25: 570– 4. [PubMed] [Google Scholar]
- 139. Al-Qadheeb NS, Althawadi S, Alkhalaf A, Hosaini S, Alrajhi AA. Evolution of tigecycline resistance in Klebsiella pneumoniae in a single patient. Ann Saudi Med 2010;30(5): 404– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shibl A, Al-Agamy M, Memish Z, Senok A, Khader SA, Assiri A. The emergence of OXA-48- and NDM-1-positive Klebsiella pneumoniae in Riyadh, Saudi Arabia. Intern J Infect Dis 2013;17: e1130– 3. [DOI] [PubMed] [Google Scholar]
- 141. Aygencel Gülbin, Dizbay Murat, Çiftçi Arzu, Türkoğlu M. Colistin-Resistant Acinetobacter baumannii Isolated from Ventilator-Associated Pneumonia: A Case Report from Turkey. Yoğun Bakιm Dergisi 2010;9: 164– 7. [Google Scholar]
- 142. Ozen Nevgun, Ergani Ayla, Thierry Naas Ö D. Outbreak of Carbapenem-Resistant Acinetobacter baumannii Producing the Carbapenemase OXA-58 in Turkey. Open Antimicrob Agents J 2009;1: 1– 8. [Google Scholar]
- 143. Gur D, Korten V, Unal S, Deshpande LM, Castanheira M. Increasing carbapenem resistance due to the clonal dissemination of oxacillinase (OXA-23 and OXA-58)-producing Acinetobacter baumannii: report from the Turkish SENTRY Program sites. J Med Microbiol 2008;57: 1529– 32. [DOI] [PubMed] [Google Scholar]
- 144. Kulah Canan, Mooij Marlies J., Comert Fusun, Aktas Elif, Celebi Guven, Ozlu Nagihan, et al. Characterisation of carbapenem-resistant Acinetobacter baumannii outbreak strains producing OXA-58 in Turkey. Intern J Antimicrob agents 2010;36: 114– 8. [DOI] [PubMed] [Google Scholar]
- 145. Vahaboglu H, Budak F, Kasap M, Gacar G, Torol S, Karadenizli A, et al. High prevalence of OXA-51-type class D beta-lactamases among ceftazidime-resistant clinical isolates of Acinetobacter spp.: co-existence with OXA-58 in multiple centres. J Antimicrob Chemother 2006;58: 537– 42. [DOI] [PubMed] [Google Scholar]
- 146. Alp E, Esel D, Yildiz O, Voss A, Melchers W, Doganay M. Genotypic analysis of Acinetobacter bloodstream infection isolates in a Turkish university hospital. Scandinavian J Infect Dis 2006;38: 335– 40. [DOI] [PubMed] [Google Scholar]
- 147. Meric M, Kasap M, Gacar G, Budak F, Dundar D, Kolayli F, et al. Emergence and spread of carbapenem-resistant Acinetobacter baumannii in a tertiary care hospital in Turkey. FEMS Microbiol let 2008;282: 214– 8. [DOI] [PubMed] [Google Scholar]
- 148. Kilic Abdullah, Aktas Zerin, Bedir Orhan, Gumral Ramazan, Bulut Yasemin, Charles Stratton, et al. Identification and Characterization of OXA-48 Producing,-Carbapenem-Resistant Enterobacteriaceae Isolates in Turkey. Ann Clin Lab Sci 2011;41: 161– 6. [PubMed] [Google Scholar]
- 149. Poirel L, Heritier C, Tolun V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 2004; 48: 15– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Carrer A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother 2008;52: 2950– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Thomas CP, Moore LS, Elamin N, Doumith M, Zhang J, Maharjan S, et al. Early (2008–2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Intern J Antimicrob agents 2013;42: 531– 6. [DOI] [PubMed] [Google Scholar]
- 152. Nazic H, Poirel L, Nordmann P. Further identification of plasmid-mediated quinolone resistance determinant in Enterobacteriaceae in Turkey. Antimicrob agents Chemother 2005;49: 2146– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Aktas Z, Kayacan CB, Schneider I, Can B, Midilli K, Bauernfeind A. Carbapenem-hydrolyzing oxacillinase, OXA-48, persists in Klebsiella pneumoniae in Istanbul, Turkey. Chemother 2008;54(2): 101– 6. [DOI] [PubMed] [Google Scholar]
- 154. Gulmez D, Woodford N, Palepou MF, Mushtaq S, Metan G, Yakupogullari Y, et al. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. Intern J Antimicrob agents 2008;31: 523– 6. [DOI] [PubMed] [Google Scholar]
- 155. Castanheira M, Mendes RE, Woosley LN, Jones RN. Trends in carbapenemase-producing Escherichia coli and Klebsiella spp. from Europe and the Americas: report from the SENTRY antimicrobial surveillance programme (2007–09). J Antimicrob Chemother 2011;66: 1409– 11. [DOI] [PubMed] [Google Scholar]
- 156. Nazik H, Ongen B, Mete B, Aydin S, Yemisen M, Kelesoglu F, et al. Coexistence of blaOXA48 and aac(6′)-Ib-cr Genes in Klebsiella Pneumoniae Isolates from Istanbul, Turkey. J Intern Med Res 2011; 39: 1932– 40. [DOI] [PubMed] [Google Scholar]
- 157. Carrër Amélie, Poirel Laurent, Y M, Akan Özay Arikan, Feriha Cilli, Cuzon Gaëlle, Ghassan Matar, et al. Spread of OXA-48-Encoding Plasmid in Turkey and Beyond. Antimicrob agents Chemother 2010;54: 1369– 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Labarca J., Poirel L., Ozdamar M., Turkoglu S., Nordmann EHaP. KPC-producing Klebsiella pneumoniae, finally targeting Turkey. New Microbe New Infect 2014. 2014; 2: 50– 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm!. Trends Mol Med 2012;18: 263– 72. [DOI] [PubMed] [Google Scholar]
- 160. Poirel L, Ozdamar M, Ocampo-Sosa AA, Turkoglu S, Ozer UG, Nordmann P. NDM-1-producing Klebsiella pneumoniae now in Turkey. Antimicrob agents Chemother 2012;56: 2784– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bonnet R, Marchandin H, Chanal C, Sirot D, Labia R, De Champs C, et al. Chromosome-Encoded Class D -Lactamase OXA-23 in Proteus mirabilis. Antimicrob agents Chemother 2002;46: 2004– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bahar G, Mazzariol A, Koncan R, Mert A, Fontana R, Rossolini GM, et al. Detection of VIM-5 metallo-beta-lactamase in a Pseudomonas aeruginosa clinical isolate from Turkey. J Antimicrob Chemother 2004;54: 282– 3. [DOI] [PubMed] [Google Scholar]
- 163. Cantoń R., Akóva M., Carmeli Y., Giske C. G., Glupczynski Y., Gniadkowski M., et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 2012;18: 413– 31. [DOI] [PubMed] [Google Scholar]