Abstract
AIM: To evaluate the safety and efficacy of the bioartificial liver support system in canines with acute liver failure (ALF).
METHODS: Nine canines with acute liver failure by acetaminophen -induced received TECA- I bioartificial liver support system (BALSS) from Hong Kong TECA LTD Co. Blood was perfused through a hollow fiber tube containing (1.2) × 1010 the porcine hepatocytes. In contrast, another 10 canines with a cute liver failure by Acetaminophen received drugs. Each treatment lasted 6 h.
RESULTS: BALSS treatment resulted in beneficial effects for acetaminophen-induced ALF canines with survival and with the recovery of the live r functions and tissues, and plasma ammonia decreased from 135.9 μmol/L ± 17.5 μmol/L to 65.7 μmol/L ± 22.0 μmol/L, 32.5 μmol/L ± 8.8 μmol/L, GPT from 97.8 U/L ± 8.7 U/L to 64.8 U/L ± 11.9 U/L, 19.0 U/L ± 6.3 U/L, GOT from 103.0 U/L ± 16.7 U/L to 75.7 U/L ± 19.6 U/L, 26.5 U/L ± 5.0 U/L, and AKP from 158.3 U/L ± 12.1 U/L to 114.5 U/L ± 19.8 U/L, 43.8 U/L ± 5.6 U/L during and after the treatment. In contrast, 10 ALF canines in both the drug and control groups died 1 or 2 d after treatment.
CONCLUSION: TECA-1 artificial liver support system is safe and efficacious for canines with acute liver failure.
Keywords: liver support system, acute liver failure, canines, porcine hepatocytes, bioartificial liver, acetaminophen
INTRODUCTION
Acute liver failure (ALF) is a well-known complication in clinical diseases and the current comprehensive treatment is not satisfactory. The recent developed artificial liver support systems, especially the bioartificial liver support systems (BALSS), which perfuse the ALF blood with the exotic hepatocytes to temporarily replace the complex functions of illed liver such as synthesis, detoxication and biotransformation, provide a brand-new approach to treat the ALF[1-8]. But the difficulties in acquiring high-density culturing of hepatocytes, lack of a stable and well-reproducible big animal model of ALF impededed the development of BALSS researches. In the present study, we have introduced the basic techniques of a hollow fiber bioartificial liver system, us ed the Chinese experimental miniature swines as the donor of hepatocytes, improved the culturing conditions of porcine hepatocytes in the bioartificial liver system and developed an acetaminophen-induced ALF canine model so as to assess the efficacy, safety and feasibility of the BALSS in treating the ALF canines.
MATERIALS AND METHODS
Animal
The healthy adult hybrid canines weighing 15 kg and adult Chinese experimental miniature swines weighing 10 kg, the male or female, were provided by t he Experimental Animal Center of PLA General Hospital.
Reagent
All chemicals were obtained from Hong Kong TECA LTD Co. or Sigma Chemical Co.
The development of a canine model of ALF
The canines received multi-subcutaneous injections (sc) with acetaminophen (115 mg/kg) to induce the ALF models.
The isolation and culturing of porcine hepatocytes
Hepatocytes were isolated from Chinese experimental miniature swines by collagenase digestion. Viability of the cells was assayed by trypan blue exclusion and AO/PI fluorescence staining.
The procedure of BALS treatment
Freshly isolated hepatocytes were cultured in the TECA-I BALSS from Hong Kong TECA LTD Co. and used to treat the ALF canines. The porcine hepatocytes were circulating through the exterior space of capillary hollow fibers in the BALSS. A circuit was developed between the femoral artery and vein on one side of the ALF canine and connected to the hollow fibers of BALSS. The canine′s blood circulated through the inner space of capillary hollow fibers and the substances exchanged through the membrane of hollow fibers with the porcine hepatocytes to achieve the perfusing function.
Experimental groups
Nineteen ALF canines were randomly divided into three groups: ① BALSS perfusion group (n = 9): perfused with the hollow fiber tube BALSS; ② drug group (n = 5): intravenous injection with arginine, sodium glutamate and acidi amino chaini branchi; ③ control group (n = 5): intravenous injection of 5% dextrose solution. Each treatment lasted 6 h.
Biochemical assays
The plasma levels of ammonia, GPT, GOT, AKP and BUN were measured before and 2, 4, 6 h, 1, 3, 5, 7, 14, 30 d after treatment.
Morphological observation
The liver, kidneys, heart, lungs were taken out when the canines died or survived for 30 d for paraffin section and HE stained and examined under light microscope.
Statistical analyses
The values of blood biochemical parameters were expressed as means ± SD. Data between the three groups and pre- and post-treatment were compared using t test. Statistical significance was considered when P < 0.05.
RESULTS
The development of a canine model for acetaminophen-induced ALF
After injected with acetaminophen, the thirty-eight healthy canines (21 male, 17 female) demonstrated a significantly increased level of NH3, GPT, GOT and AKP at 48 h (P < 0.01, Figure 1) while plasma concentration of BUN had no change. In the mean time, symptoms appeared such as vomiting, food refusal and listlessness. Histologic examination showed massive necrosis of the hepatic tissues. Sixty-three percent had ALF, in which the plasma levels of NH 3 and GPT were higher than 100 μmol/L and 60 U/L respectively.
Figure 1.
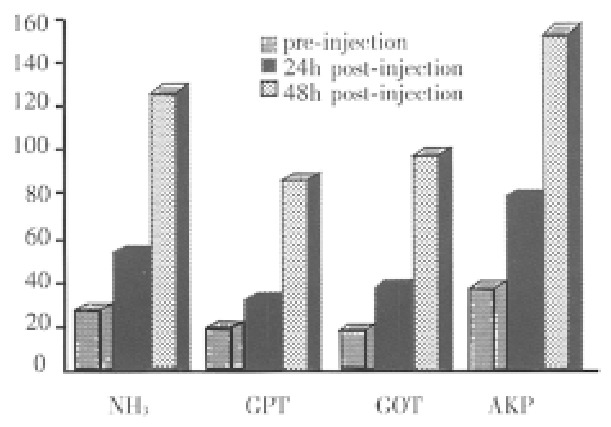
The changes of canines′ biochemistry parameters before and after injections of acetaminophen.
The porcine hepatocytes
Using the modified enzymatic digestive method, each liver yielded approximately 0.8-5 × 1010 hepatocytes. Viability of the final suspensions averaged 60%-80% by trypan blue exclusion and AO/PI fluorescence staining.
The liver function recovery of ALF canines after treatment with BALSS
All ALF canines in the control group, with increasing levels of plasma ammonia, GPT, GOP and AKP died 2-30 h after treatment. All the five ALF canines in drug group died 6-36 h after treatment. The plasma levels of ammonia, GPT, GOT and AKP declined slightly during the treatment, but it had no significant difference compared with that in the control group (P > 0.05). In BALSS perfusion group, all nine ALF canines showed remarkable liver function recovery. During BALSS treatment, the levels of plasma ammonia, GPT, GOT and AKP began to decline at the 2nd h and the declination was even more obvious compared with pre-treatment at the 4th hour and 6th h (P < 0.01) (Figure 2, Figure 3, Figure 4, Figure 5). The levels of these biochemical parameters had significant differences at the 6th h of treatment between BALS S perfusion group and the drug group (P < 0.01). Except three canines in BALSS perfusion group who died 6 and 12 h and 5 d after treatment because of narcotization and wound bleeding, the other 6 canines survived more than 30 d.
Figure 2.
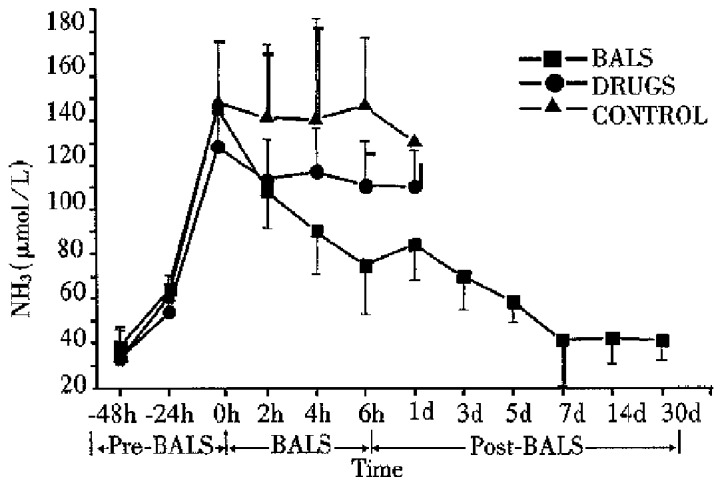
Effects of the BALS on ammonia of acetaminophen-induced ALF canines.
Figure 3.
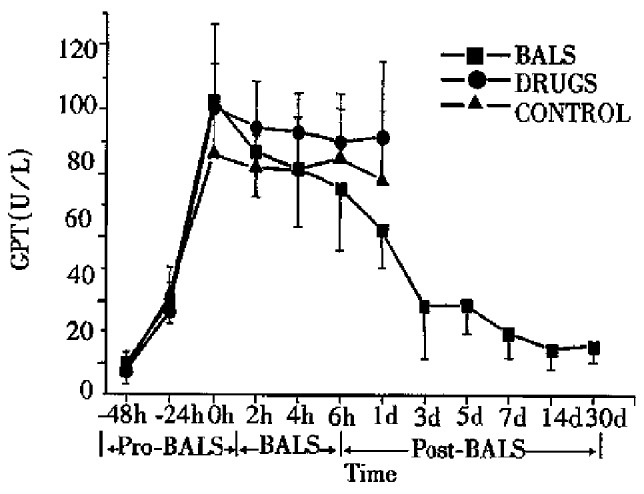
Effects of the BALS on GPT of acetaminophen -induced ALF canines.
Figure 4.
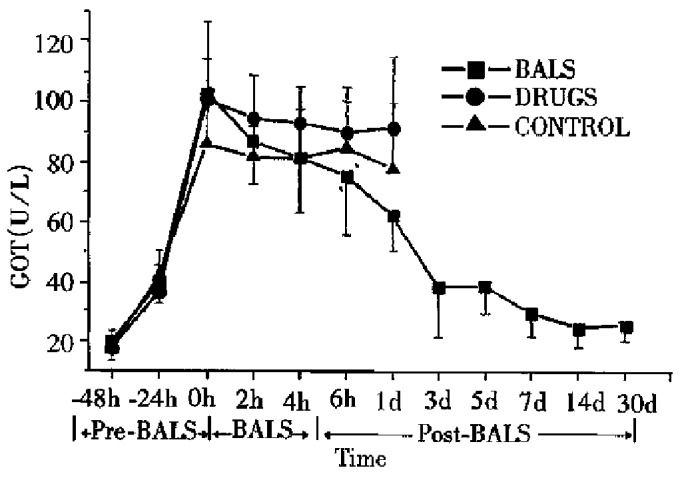
Effects of the BALS on GOT of acetaminophen -induced ALF canines.
Figure 5.
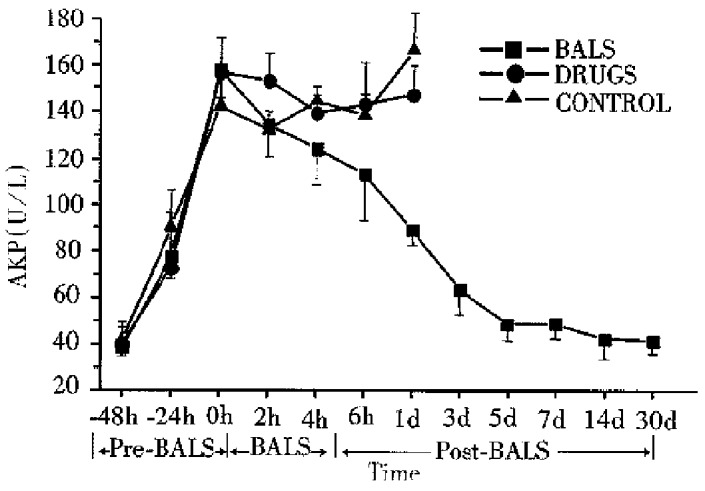
Effects of the BALS on AKP of acetaminophen-induced ALF canines.
The morphological changes of liver in ALF canines after treatment with BALSS
The liver, heart, kidneys and lungs were taken out for pathological examination when the canines died and survived for 30 d after treatment. The liver tissues of the dead ALF canines showed massive necrosis in the drug and control groups. Thirty days after treatment, the liver tissue of ALF canines in BALSS perfusion group had no obvious hepatocyte necrosis but a slight cholestasis and fibrosis.
DISCUSSION
ALF is usually an integrative response caused by viruses, drugs and toxins which leads to severe hepatocyte injury and the rapid loss of synthetic, metabolic and regulative functions, followed by the release of non-inflammatory cellular factors and other indefinitive toxins into the circulation. Its mortality was as high as 50%-80% and no effective clinical therapy is available currently. Because the illed liver has a tendency of reversibility, a reproduction can occur if we replace the hepatic functions by artificial methods. Since the liver has more than 500 complex physiological functions, the current non-biological ALS such as activated charcoal absorption can not replace the liver functions completely although it can clear some middle and small molecular weight substances in the circulation. In BALSS the exotic hepatocytes were used to exchange the substances with the ALF blood, so that it can temporarily replace the complex liver functions of ALF patients directly from the exotic hepatocytes. Now there are several types of BALSS and a few small animal experiments and clinical study reports with various therapeutic effects[1-7,9-11]. The studies of BALSS in China are still in the initial stage and the BALSS techniques are not mature. This experiment studied the main problems such as the source and culturing of hepatocytes, the development of ALF canines model and assessment of the efficacy and safety of BALSS treatment based on the advanced techniques of hollow fiber tube BALSS introduced from abroad.
A large quantity of hepatocytes with a well-functioned condition and high-density in culture in the BALSS is a key factor in success. Some authors used the human liver tumor cell lines, human embryo hepatocytes and porcine hepatocytes as the sources of hepatocytes[9-11]. But the possible dangers of the tumor cells to the receptors and the scarce of the embryonic cell hampered the studies of BALSS. Our experiment selected the quarantined pure strain Chinese experimental miniature swine as the source of hepatocytes. Using the modified enzymatic digesting and culturing method, we acquired a large quantity of porcine hepatocytes that can meet the needs of BALSS. The development of a large animal ALF model, especially with high-reproductivity, stablity and a similar pathological changes to ALF patients, is still a major difficulty. Some people established the ALF canine models by surgical operation to occlude the blood supply for liver temporarily[12]. But this kind of models can not suit the studies of ALF or drugs because of the multiple organs failure induced by ischemic-reperfusion injury and the variability of death time. We used the multiple injections of acetaminophen to induce the ALF canines. Forty-eight hours after injection, the blood hepatic biochemistry parameters had obvious changes. The canines′ liver tissues manifested massive necrosis. The ALF canines would die within 56-84 h without treatment. This disease model had a good reproductivity and the changes of blood biochemical parameters were similar to ALF patients except the plasma BUN. The death time of ALF canines is relatively stable and suits the studies of BALSS and drugs.
In our experiment, we developed an ALF canine model induced by acetaminophen and then treated with the clinical routine drugs, BALSS and infusion of dextrose solution for evaluating the efficacy, safety and feasibility of BALSS to treat the ALF canines. The biochemical parameters related to hepatic functions of the ALF canines in the control group were elevated except for the plasma BUN. The canines died within 2 d. The drug therapy could partly correct the changes of biochemical parameters of the ALF canines, but with a very limited efficacy, and the canines died 2 d after treatment. The perfusion treatment with the B ALSS of cultured Chinese experimental miniature porcine hepatocytes lasted 6 h. The blood biochemical parameters of hepatic functions of the ALF canines were corrected obviously at the 4th h of perfusion. The effect was significantly superior to which used in the other two groups. ALF canines can survive more than 30 d after perfusion therapy. The pathological changes of liver were also recovered obviously. The results showed that the porcine hepatocytes in this kind of BALSS could temporarily replace the hepatic functions of ALF canines by perfusion through the membrane of the hollow fiber tube, and then offered a chance for regeneration and repairment of the canine liver. It is suggested that the BALSS could temporarily replace the hepatic function of ALF patients safely and effectively, and may become a brand-new method for the treatment of ALF.
Footnotes
Dr. Yi-Long Xue, female, born on 1953-10-29 in Nanjing City, Jiangsu Province, graduated from Fourth Military Medical University in 1978, Associate Professor, majoring in artificial organs, having 22 papers published.
Supported by the Scientific Research Fund of Ministry of Education for Ouerseas Chinese Fellows who Returned Home, No. 94-001 and Hong Kong TECA LTD Co
Edited by MA Jing-Yun
References
- 1.Demetriou AA, Rozga J, Podesta L, Lepage E, Morsiani E, Moscioni AD, Hoffman A, McGrath M, Kong L, Rosen H. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995;208:111–117. doi: 10.3109/00365529509107771. [DOI] [PubMed] [Google Scholar]
- 2.Sussman NL, Gislason GT, Conlin CA, Kelly JH. The Hepatix extracorporeal liver assist device: initial clinical experience. Artif Organs. 1994;18:390–396. doi: 10.1111/j.1525-1594.1994.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 3.Rozga J, Podesta L, LePage E, Morsiani E, Moscioni AD, Hoffman A, Sher L, Villamil F, Woolf G, McGrath M. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994;219:538–544; discussion 544-546. doi: 10.1097/00000658-199405000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harland RC, Bollinger R. Extracorporeal hepatic perfusion in the treatment of patients with acute hepatic failure. Transplant Rev. 1994;8:73–79. [Google Scholar]
- 5.Dixit V. Transplantation of isolated hepatocytes and their role in extrahepatic life support systems. Scand J Gastroenterol Suppl. 1995;208:101–110. doi: 10.3109/00365529509107770. [DOI] [PubMed] [Google Scholar]
- 6.Tompkins RG, Yarmush ML. Prospects for an artificial liver. Transplant Rev. 1993;7:191–199. [Google Scholar]
- 7.Rozga J, Holzman MD, Ro MS, Griffin DW, Neuzil DF, Giorgio T, Moscioni AD, Demetriou AA. Development of a hybrid bioartificial liver. Ann Surg. 1993;217:502–509; discussion 502-509;. doi: 10.1097/00000658-199305010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue YL, Zhang ZY, Huang YC, Liu CG. The present and development of the artificial liver researches. Junyi Jinxiu Xueyuan Xuebao. 1996;17:293–297. [Google Scholar]
- 9.Nyberg SL, Remmel RP, Mann HJ, Peshwa MV, Hu WS, Cerra FB. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220:59–67. [PMC free article] [PubMed] [Google Scholar]
- 10.Sussman NL, Chong MG, Koussayer T, He DE, Shang TA, Whisennand HH, Kelly JH. Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology. 1992;16:60–65. doi: 10.1002/hep.1840160112. [DOI] [PubMed] [Google Scholar]
- 11.Sielaff TD, Hu MY, Amiot B, Rollins MD, Rao S, McGuire B, Bloomer JR, Hu WS, Cerra FB. Gel-entrapment bioartificial liver therapy in galactosamine hepatitis. J Surg Res. 1995;59:179–184. doi: 10.1006/jsre.1995.1151. [DOI] [PubMed] [Google Scholar]
- 12.Misra MK, P'eng FK, Sayhoun A, Kashii A, Derry CD, Caridis T, Slapak M. Acute hepatic coma: a canine model. Surgery. 1972;72:634–642. [PubMed] [Google Scholar]


