Abstract
AIM: To study the effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 (MMP-2) in hepatic stellate cells (HSC).
METHODS: The expressions of MMP-2, tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) and membrane type matrix metalloproteinase-1 (MT1-MMP) in cultured rat HSC were detected by immunocytochemistry (ICC) and in situ hybridization (ISH). The contents of MMP-2 and TIMP-2 in culture supernatant were detected with ELISA and the activity of MMP-2 in supernatant was revealed by zymography.
RESULTS: In the situation of hypoxia for 12 h, the expression of MMP-2 protein was enhanced (hypoxia group positive indexes: 5.7 ± 2.0, n = 10; control: 3.2 ± 1.0, n = 7; P < 0.05), while TIMP-2 protein was decreased in HSC (hypoxia group positive indexes: 2.5 ± 0.7, n = 10; control: 3.6 ± 1.0, n = 7; P < 0.05), and the activity (total A) of MMP-2 in supernatant declined obviously (hypoxia group: 7.334 ± 1.922, n = 9; control: 17.277 ± 7.424, n = 11; P < 0.01). Compared the varied duration of hypoxia, the changes of expressions including mRNA and protein level as well as activity of MMP-2 were most notable in 6 h group. The highest value (A hypoxia-A control) of the protein and the most intense signal of mRNA were in the period of hypoxia for 6 h, along with the lowest activity of MMP-2. In the situation of hyperoxia for 12 h, the contents (A450) of MMP-2 and TIMP-2 in supernatant were both higher than those in the control, especially the TIMP-2 (hyperoxia group: 0.0499 ± 0.0144, n = 16; control: 0.0219 ± 0.0098, n = 14; P < 0.01), and so was the activity of MMP-2 (hyperoxia group: 5.252 ± 0.771, n = 14; control: 4.304 ± 1.083, n = 12; P < 0.05), and the expression of MT1-MMP was increased.
CONCLUSION: HSC is sensitive to the oxygen, hypoxia enhances the expression of MMP-2 and the effect is more marked at the early stage; hyperoxia mainly raises the activity of MMP-2.
Keywords: liver/pathology, liver/metabolism, metalloproteinases/biosynthesis, metalloproteinases/metabolism, anoxia/metabolism, oxygen/pharmacology
INTRODUCTION
It is well known that the key event in the hepatic fibrogenesis is the activation of hepatic stellate cells (HSC) due to the altered circumstances and the activated cells are the main source of MMP-2 which may promote the activation of HSC owing to degradation of the basement membrane matrix rich in collagen type IV around the cells[1-23]. We also know that liver fibrosis may be induced or worsened by hypoxia and reperfusion[24-27]. However, it has not been reported that the effects of oxygen on the expression and the activity regulation of MMP-2 in HSC. In this paper, the regulation of the expression and the activity of MMP-2 in rat HSC was investigated in vitro under the conditions of hypoxia or hyperoxia.
MATERIALS AND METHODS
Isolation and culture of HSC
HSC were isolated from adult Sprague Dawley rats weighing 380 g to 420 g (bought from the Experimental Animals Center of Shanghai Medical University, China) according to the method of Di Sario et al[28,29]. The cells (105•mL-1) were inoculated in culture flasks and dishes with cover-glasses, and then cultured at 37 °C in a humidified atmosphere with 5% CO2. The medium (DMEM, Sigma Co.) was changed 24 h later and thereafter every 2 d to 3 d. After 7 d culturing, the medium was replaced with serum-free medium (DMEM/F12, V/V = 1:1). Meanwhile, some of the dishes were cultured under the condition of hypoxia or hyperoxia, as previously described [30]. Briefly, the dishes were put in a sealed container with two holes (for the gas in and out), through which 100% N2 or O2 (Shanghai Biouxi Gas Co. Ltd, China) was inflated for 30 min, and then with the holes shut the dishes were incubated in hypoxia or hyperoxia continually for 12 h. The culture supernatant was collected and centrifuged, and preserved at -20 °C. The cells on the cover-glasses were rinsed in phosphate-buffered saline (PBS) for three times, fixed in 40 g•L¯¹ paraformaldehyde/PBS, and preserved in 700 mL•L¯¹ ethanol at 4 °C[31]. In another experiment for observing the differences among the varied durations of hypoxia, the dishes were cultured with hypoxia for 6 h, 12 h and 24 h, 12 dishes for each group, along with three dishes as parallel controls for each group.
ELISA
Sandwich technique was used to detect the relative contents of MMP-2, TIMP-2 in the culture supernatant with polyclonal antibody against human MMP-2 (present of Dr. Stetler-Stevenson; 1:2000), polyclonal antibody against human TIMP-2 (1:800), goat anti rabbit IgG-HRP (Huamei Co. Shanghai, China. 1:1000) and the colorific tetramethyl benzidine (TMB) (Huamei Co. Shanghai, China). Fresh serum-free medium served as negative control. The O.D.values (A450 values) measured with the Vmax Kinetic Microplate Reader (Molecular Devices Corporation, Sunnyvale, California, USA) at 450 nm represented the relative contents of the protein.
Detection of the MMP-2 activity with zymography[10]
The activity of MMP-2 was detected by gelatin zymography using 80 g•L¯¹ polyacrylamide gels co-polymerized with 1 g•L¯¹ gelatin which served as the substrate of MMP-2. Culture supernatant (15 μL) was mixed with 2 × sample buffer (1:1) and electrophorised (80V-150V) for 4 h-5 h. Subsequently, SDS was extracted with Triton X-100 from the gels, which were then incubated for 48 h at 37 °C in 50 mmol•L¯¹ Tris/HCl, pH7.4, containing 5 mmol•L¯¹ CaCl2 and 5 mmol•L¯¹ ZnCl2. Gels were stained in 300 mL•L ¯¹ methanol/100 mL•L¯¹ acetic acid containing 5 g•L¯¹ Coomassie brilliant blue G250 and decolorized. The clear band against a blue background representing the activity of MMP-2 was measured by using Gel Image System (Image master 1D analysis software, Pharmacia)and recorded with the total A (area of clear band times mean A).
Immunocytochemistry (ICC)
Labeled streptavidin biotin method with HRP/DAB (Dako Co.) was used in ICC for detecting the expression of MMP-2, TIMP-2, MT1-MMP and desmin in the cells on the cover-glasses. The specific antibodies were: monoclonal antibody against the human MMP-2 (CalBiochem; 1:100), polyclonal antibody against human TIMP -2 (present of Dr. Stetler-Stevenson; 1:300), monoclonal antibody against the human MT1-MMP (CalBiochem; 1:10), polyclonal antibody against chicken desmin (made by the Department of Pathology, Shanghai Medical University; 1:200). According to the stain intensity, the positive result was recorded as the value of 1 (mild), 2 (middle), 3 (intense), and the positive indexes were given by the average values of the positive cells in 9 HP (× 400) fields per slide.
In situ hybridization (ISH)
Recombinant plasmids of human MMP-2, TIMP-2 cDNA were gifts from professor Marmer (Medical Center of Washington University, USA). And that of MT1-MMP was presented by Dr. Xiao. Expansion, extraction and purification of the recombinant plasmids were performed as routine. Three cRNA probes were transcripted in vitro according to the protocol of the kit (Boehringer Mannheim Co., Germany). ISH was performed as previuosly described[32,33] with immunohistochemical detection using an alkaline phosphatase (AKP)-conjugated anti-digoxigenin monoclonal antibody (Boehringer Mannheim Co., Germany). Hybridization signal was visualized through the substrate of AKP (NBT and BCIP).
Statistical METHODS
t or t’test was used for statistical analysis.
RESULTS
Morphology and growing state of HSC
The HSC isolated freshly appeared round in shape and rich in cytoplasmic lipid droplets. The cells stretched the cytoplasmic processes and numerous lipid droplets were still seen around nuclei on the third day after seeding. Thereafter the cells became stellate with several long processes, grew in clumps, and took about 7 d to 10 d to cover all over the flasks or dishes. More than 95% of the cells showed expression of desmin. It was noticed that the morphology and the number of HSC did not change obviously after treated with hypoxia or hyperoxia, as compared with control.
Relative content of MMP-2, TIMP-2 proteins in supernatant
Relative contents of MMP-2 protein in each of three hypoxia periods were higher than those in the control. The highest increase in the value (Ahypoxia-Acontrol) of the protein was in the period of hypoxia for 6 h, and the lowest was in 12 h. The relative content of TIMP-2 was higher in 12 h, while it lowered in both 6 h and 24 h, compared with the control; but there were no significant differences among them (Table 1). The contents of both MMP-2 and TIMP-2 in supernatants of the hyperoxia group (12 h) were higher than those of the control, especially the TIMP-2 (P < 0.01, Table 2).
Table 1.
Increased values of MMP-2, TIMP-2 proteins and activity of MMP-2 in supernatants in three hypoxia periods
| t/h |
MMP-2 |
TIMP-2 |
MMP-2 activity |
|||
| n | ¯x ± s | n | ¯x ± s | n | ¯x ± s | |
| 6 | 12 | 0.0264 ± 0.0168b | 12 | -0.0042 ± 0.0100 | 9 | -1.110 ± 2.612 |
| 12 | 12 | 0.0026 ± 0.0111 | 12 | 0.0023 ± 0.0181 | 11 | -0.392 ± 1.543 |
| 24 | 10 | 0.0100 ± 0.0136a | 10 | -0.0012 ± 0.0140 | 10 | -1.323 ± 2.194 |
P < 0.05 vs 6 h;
P < 0.01 vs 12 h.
Table 2.
Changes of MMP-2, TIMP-2 contents and activity in supernatant in hyperoxia group
| Group |
MMP-2 |
TIMP-2 |
MMP-2 activity |
|||
| n | ¯x ± s | n | ¯x ± s | n | ¯x ± s | |
| Hyperoxia | 16 | 0.1958 ± 0.0448 | 16 | 0.0499 ± 0.0144b | 14 | 5.252 ± 0.771a |
| Control | 15 | 0.1729 ± 0.0409 | 14 | 0.0219 ± 0.0098 | 12 | 4.304 ± 1.083 |
P < 0.05,
P < 0.01 vs control.
Activity of MMP-2 in supernatant
Activity of MMP-2 in hypoxia group (7.334 ± 1.922, n = 9) was lower than that in control (17.277 ± 7.424, n = 11; P < 0.01, Figure 1). Compared the varied periods of hypoxia, the activity came down obviously in 6 h and 24 h, but slightly in 12 h (Table 1). On the contrary, the activity of MMP-2 in supernatant rose remarkably as the cells were cultured in hyperoxia for 12 h (P < 0.05, Figure 1, Table 2).
Figure 1.

Zymographic analyses. Lane1: hypoxia group; Lane 2: control; Lane 3-5: hyperoxia group; Lane 6-8: control.
Expression of MMP-2, TIMP-2, MT1-MMP proteins In cell situ
The positive stain of MMP-2, TIMP-2 and MT1-MMP appeared brown and yellow and located in cytoplasm. The expression of MMP-2 in hypoxia was more intense (positive indexes: 5.7 ± 2.0, n = 10) than that in control (3.2 ± 1.0, n = 7, P < 0.05, Figure 2, Figure 3). The expression of TIMP-2 in hypoxia (positive indexes: 2.5 ± 0.7, n = 10) was weaker (control, 3.6 ± 1.0, n = 7, P < 0.05, Figure 4, Figure 5). No significant statistical difference was found between hyperoxia and control for the expression of MMP-2. The expression of MT1-MMP was slightly more intense in hyperoxia than that in control (Figure 6, Figure 7).
Figure 2.
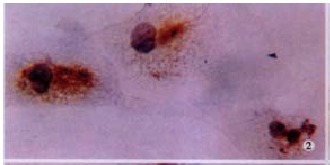
MMP-2 protein in HSC presented intense positive in hypoxia group. LSAB (DAB), × 200
Figure 3.
MMP-2 protein in HSC presented positive in hypoxia control. LSAB (DAB), × 200
Figure 4.

TIMP-2 protein in HSC presented weak positive in hypoxia group. LSAB (DAB), × 200
Figure 5.
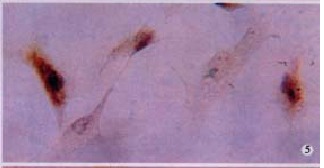
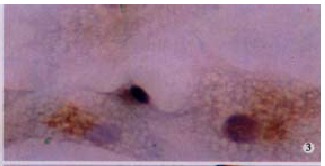
TIMP-2 protein in HSC presented intense positive in hypoxia control. LSAB (DAB), × 200
Figure 6.
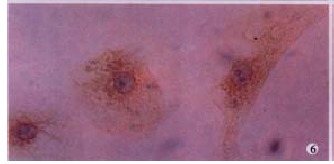
MT1-MMP protein in HSC presented positive in hyperoxia group. LSAB (DAB), × 200
Figure 7.
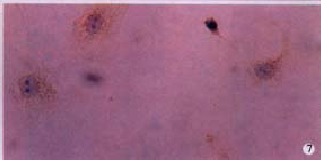
MT1-MMP protein in HSC presented weak positive in hyperoxia control. LSAB (DAB), × 200
Expression of MMP-2, TIMP-2 and MT1-MMP mRNA
The hybridization signal of MMP-2 in HSC was found in each of three periods of hypoxia. It was most intense in the 6 h group, milder in the 24 h group (Figure 8, Figure 9, Figure 10). The signal of TIMP-2 was weak and there was no difference among three periods. The expression of MT1-MMP mRNA was up regulated (Figure 11), TIMP-2 and MMP-2 were slightly elevated in HSC cultured in hyperoxia for 12 h.
Figure 8.
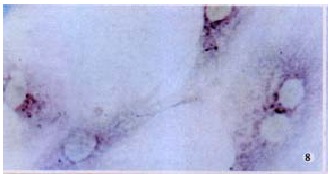
Signal of MMP-2 mRNA in HSC was intense in hypoxia for 6 h. ISH (NBT/BCIP), × 400
Figure 9.

Signal of MMP-2 mRNA in HSC was weak in hypoxia for 12 h. ISH (NBT/BCIP), × 400
Figure 10.
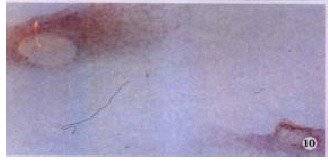
Signal of MMP-2 mRNA in HSC was intense in hypoxia for 24 h. ISH (NBT/BCIP), × 400
Figure 11.

Signal of MT1-MMP mRNA in HSC was intense in hyperoxia group. ISH (NBT/BCIP), × 100
DISCUSSION
It is known to all that oxygen is essential for cell living, and hypoxia will lead to cell dysfunction, or even death. Unfortunately, hypoxia always happens during the liver damage or inflammation, in which swelling of the hepatocytes, constriction of vessels, capillarization of sinusoids, increasing of ECMs in Space of Disse, construction of hepatocytes regenerating nodules and fibrotic septa, abnormal vessels network, increasing endothelin promoted by enterogenous endotoxin, and others may result in liver cell hypoxia[34-37]. In other words, tissue hypoxia occurs in the whole course of the liver fibrogenesis including the initiation and development. Meanwhile, ischemia-reperfusion is not rare in liver injury, and counterpulsation and hyperbaric oxygen therapy are suggested to treat some chronic liver diseases recently[38]. However, there are only a few reports about the effects of hypoxia or hyperoxia in liver fibrogenesis. Avila et al[39] reported that methionine adenosyltransferase (MAT) mRNA level was down-regulated by hypoxia, the synthesis of glutathione (GSH) decreased because of a drop of MAT, the free radicals could not be eliminated timely, and the antioxidation ability of the liver came down. Blanc et al[40] found that hypoxia-reoxygenation had direct toxic effects on sinusoidal endothelial cells with an increase in xanthine oxidase activity and lipid peroxidation. Up to now, apart from our work[11,14], we have not found any report about the effects of hypoxia or hyperoxia on the expression and the activity regulation of MMP-2 in HSC.
In our in vitro experiments, the expression of MMP-2 in HSC in hypoxia was increased, among the three different hypoxia periods, the changes of expression and activity of MMP-2 were most remarkable in the 6 h group, suggesting that the harmful effects of the hypoxia were more serious at the early stage of the liver damage. In the meanwhile, the activity of the enzyme went down, this might be related to the regulation of the enzyme activity[41-46]. As we know, the activation of MMP-2 from HSC not only requires interactions with hepatocytes[47], but also is regulated by MT1-MMP and TIMP-2 which directly mediates the binding of pro-MMP-2 to the cell surface. The cell surface-localized complex is then promoted by MT1-MMP to generate a cell surface-bound active MMP-2: TIMP-2 complex. Upon dissociation of the complex, the active site is exposed, and TIMP-2 binds MMP-2, thereby suppressing its activity[48-54]. Apparently a lack of cooperation with hepatocytes and low expression of TIMP-2 in this experiment may contribute to a drop in the enzyme activity. The change of MMP-2 was opposite to that of TIMP-2 in the different hypoxia periods, suggesting that the former might induce the expression of the latter in the negative direction. It is clear that cell function in vitro is not all the same as it is in vivo, so the activity of MMP-2 in the liver tissue under hypoxia is worthy of further study. The mechanism in detail of the MMP-2 and TIMP-2 expression variations induced by hypoxia remains to be clarified. It may be the reason why HSC under hypoxia produced transcription factors, such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1) which up-regulate the level of transforming growth factor beta 1 (TGF-β1) gene expression. TGF-β1 promotes the production of MMP-2 and inhibits that of TIMP-2[55].
The expression of MMP-2 and its regulation in HSC in the state of hyperoxia have not been reported. We found that the content of MMP-2 in supernatant was increased, and the activity of MMP-2 rose obviously in hyperoxia group. This suggests that the regulation of MMP-2 activation is stronger than that of the enzyme expression in hyperoxia. As hyperoxia promotes the expression of MT1-MMP in HSC and also that of TIMP-2, the elevated activity of MMP-2 in hyperoxia may be related to a combined action of MT1-MMP and TIMP-2.
Footnotes
Edited by Lu HM
Supported by the Scientific Research Fund for Doctorate Education, State Educational Commission, No.9837
References
- 1.Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836–847. doi: 10.1016/s0168-8278(98)80269-9. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32–38. doi: 10.1016/s0168-8278(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 3.Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–184. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- 4.Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci ( Lond) 1997;92:103–112. doi: 10.1042/cs0920103. [DOI] [PubMed] [Google Scholar]
- 5.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 6.Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13. doi: 10.1111/j.1600-0676.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu HL, Li XH, Wang DY, Yang SP. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 expression in fibrotic rat liver. World J Gastroenterol. 2000;6:881–884. doi: 10.3748/wjg.v6.i6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 10.Takahara T, Furui K, Funaki J, Nakayama Y, Itoh H, Miyabayashi C, Sato H, Seiki M, Ooshima A, Watanabe A. Increased expression of matrix metalloproteinase-II in experimental liver fibrosis in rats. Hepatology. 1995;21:787–795. [PubMed] [Google Scholar]
- 11.Chen PS, Zhai WR, Zhang YE, Zhang JS. Effect of hypoxia on production of collagens and collagenases in hepatic stellate cell. Shijie Huaren Xiaohua Zazhi. 2000;8:586–587. [Google Scholar]
- 12.Chen PS, Zhai WR, Zhang YE, Ling YQ. Effects of several kinds of facto rs on the expression and activity regulation of matrix metalloproteinase-2 in the hepatic stellate cells in vitro. J Gastroenterol Hepatol. 2000;15(Suppl I):221. [Google Scholar]
- 13.Chen PS, Zhai WR, Zhang YE, Zhou XM, Zhang JS. Investigation of the ma trix metalloproteinase-2 expression in two kinds of liver fibrosis animal models. Zhongguo Zuzhihuaxue Yu Xibaohuaxue Zazhi. 2000;9(Suppl):133–134. [Google Scholar]
- 14.Chen P, Zhai W, Zhang Y, Zhang J, Gu Y. [Effects of hypoxia on expression and activity of matrix metalloproteinase-2 in hepatic stellate cell] Zhonghua Gan Zang Bing Za Zhi. 2000;8:276–278. [PubMed] [Google Scholar]
- 15.Loréal O, Levavasseur F, Fromaget C, Gros D, Guillouzo A, Clément B. Cooperation of Ito cells and hepatocytes in the deposition of an extracellular matrix in vitro. Am J Pathol. 1993;143:538–544. [PMC free article] [PubMed] [Google Scholar]
- 16.Ebata M, Fukuda Y, Nakano I, Katano Y, Fujimoto N, Hayakawa T. Serum levels of tissue inhibitor of metalloproteinases-2 and of precursor form of matrix metalloproteinase-2 in patients with liver disease. Liver. 1997;17:293–299. doi: 10.1111/j.1600-0676.1997.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 17.Arthur MJ. Collagenases and liver fibrosis. J Hepatol. 1995;22:43–48. [PubMed] [Google Scholar]
- 18.Wang Y, Gao Y, Huang YQ, Yu JL, Fang SG. Gelatinase A proenzyme expres sion in the process of experimental liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:165–167. [Google Scholar]
- 19.Takahara T, Furui K, Yata Y, Zhang LP, Jin B, Nambu S, Watanabe A. Dual expression of matrix metalloproteinase-2 and membrane-type-1-matrix met alloproteinase in fibrotic human livers. Hepatology. 1997;26(4 Pt2):450A. doi: 10.1002/hep.510260620. [DOI] [PubMed] [Google Scholar]
- 20.Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabró A, Ciancio G, Stefanini F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528–537. [PMC free article] [PubMed] [Google Scholar]
- 21.Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895–1902. doi: 10.1016/S0002-9440(10)65703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benyon RC, Hovell CJ, Arthur MJP. Gelatinase A (72KD Type IV colla genase) is an autocrine proliferation factor for rat hepatic stellate cells. Hepatology. 1997;26(4 Pt2):186A. [Google Scholar]
- 23.Takahara T, Furui K, Yata Y, Jin B, Zhang LP, Nambu S, Sato H, Seiki M, Watanabe A. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology. 1997;26:1521–1529. doi: 10.1002/hep.510260620. [DOI] [PubMed] [Google Scholar]
- 24.Zar HA, Tanigawa K, Kim YM, Lancaster JR. Rat liver postischemic lipid peroxidation and vasoconstriction depend on ischemia time. Free Radic Biol Med. 1998;25:255–264. doi: 10.1016/s0891-5849(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 25.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology. 2000;31:141–148. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- 26.Nagatomi A, Sakaida I, Matsumura Y, Okita K. Cytoprotection by glycine against hypoxia-induced injury in cultured hepatocytes. Liver. 1997;17:57–62. doi: 10.1111/j.1600-0676.1997.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 27.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 28.Di Sario A, Baroni GS, Bendia E, D'Ambrosio L, Ridolfi F, Marileo JR, Jezequel AM, Benedetti A. Characterization of ion transport mechanisms regulating intracellular pH in hepatic stellate cells. Am J Physiol. 1997;273:G39–G48. doi: 10.1152/ajpgi.1997.273.1.G39. [DOI] [PubMed] [Google Scholar]
- 29.Farrell GC. Research workshop: Techniques for primary culture of liver cells. J Gastroenterol Hepatol. 1998;13:842–858. [Google Scholar]
- 30.Vender RL, Clemmons DR, Kwock L, Friedman M. Reduced oxygen tension induces pulmonary endothelium to release a pulmonary smooth muscle cell mitogen (s) Am Rev Respir Dis. 1987;135:622–627. doi: 10.1164/arrd.1987.135.3.622. [DOI] [PubMed] [Google Scholar]
- 31.Tbakhi A, Totos G, Hauser-Kronberger C, Pettay J, Baunoch D, Hacker GW, Tubbs RR. Fixation conditions for DNA and RNA in situ hybridization: a reassessment of molecular morphology dogma. Am J Pathol. 1998;152:35–41. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang GC, Zhang JS, Zhang YE. Effects of retinoic acid on proliferation, phenotype and expression of cyclin-dependent kinase inhibitors in TGF-beta1-stimulated rat hepatic stellate cells. World J Gastroenterol. 2000;6:819–823. doi: 10.3748/wjg.v6.i6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie YM, Nie QH, Zhou YX, Cheng YQ, Kang WZ. Detection of TIMP-1 and TIMP-2 RNA expressions in cirrhotic liver tissue using digoxigenin labelled probe by in situ hybridization. Shijie Huaren Xiaohua Zazhi. 2001;9:251–254. [Google Scholar]
- 34.Nishida T, Huang TP, Seiyama A, Hamada E, Kamiike W, Ueshima S, Kazuo H, Matsuda H. Endothelin A-receptor blockade worsens endotoxin-induced hepatic microcirculatory changes and necrosis. Gastroenterology. 1998;115:412–420. doi: 10.1016/s0016-5085(98)70208-2. [DOI] [PubMed] [Google Scholar]
- 35.Yan JC, Ma Y, Chen WB, Xu CJ. Dynamic observation on vascular diseases of liver tissues of rats induced by CCl4. Shijie Huaren Xiaohua Zazhi. 2000;8:42–45. [Google Scholar]
- 36.Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- 37.Biasi F, Chiarpotto E, Lanfranco G, Capra A, Zummo U, Chiappino I, Scavazza A, Albano E, Poli G. Oxidative stress in the development of human ischemic hepatitis during circulatory shock. Free Radic Biol Med. 1994;17:225–233. doi: 10.1016/0891-5849(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 38.Chen MF, Chen HM, Ueng SW, Shyr MH. Hyperbaric oxygen pretreatment attenuates hepatic reperfusion injury. Liver. 1998;18:110–116. doi: 10.1111/j.1600-0676.1998.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 39.Avila MA, Carretero MV, Rodriguez EN, Mato JM. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology. 1998;114:364–371. doi: 10.1016/s0016-5085(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 40.Blanc MC, Housset C, Lasnier E, Rey C, Capeau J, Giboudeau J, Poupon R, Vaubourdolle M. Direct cytotoxicity of hypoxia-reoxygenation towards sinusoidal endothelial cells in the rat. Liver. 1999;19:42–49. doi: 10.1111/j.1478-3231.1999.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 41.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 42.Corcoran ML, Hewitt RE, Kleiner DE, Stetler-Stevenson WG. MMP-2: expression, activation and inhibition. Enzyme Protein. 1996;49:7–19. doi: 10.1159/000468613. [DOI] [PubMed] [Google Scholar]
- 43.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- 44.Théret N, Lehti K, Musso O, Clément B. MMP2 activation by collagen I and concanavalin A in cultured human hepatic stellate cells. Hepatology. 1999;30:462–468. doi: 10.1002/hep.510300236. [DOI] [PubMed] [Google Scholar]
- 45.Préaux AM, Mallat A, Nhieu JT, D'Ortho MP, Hembry RM, Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999;30:944–950. doi: 10.1002/hep.510300432. [DOI] [PubMed] [Google Scholar]
- 46.Belkhiri A, Richards C, Whaley M, McQueen SA, Orr FW. Increased expression of activated matrix metalloproteinase-2 by human endothelial cells after sublethal H2O2 exposure. Lab Invest. 1997;77:533–539. [PubMed] [Google Scholar]
- 47.Théret N, Musso O, L'Helgoualc'h A, Clément B. Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interactions with hepatocytes. Am J Pathol. 1997;150:51–58. [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy G, Stanton H, Cowell S, Butler G, Knäuper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 49.Seiki M. Membrane-type matrix metalloproteinases. APMIS. 1999;107:137–143. doi: 10.1111/j.1699-0463.1999.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 50.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–166. [PubMed] [Google Scholar]
- 51.Yu M, Sato H, Seiki M, Spiegel S, Thompson EW. Calcium influx inhibits MT1-MMP processing and blocks MMP-2 activation. FEBS Lett. 1997;412:568–572. doi: 10.1016/s0014-5793(97)00849-1. [DOI] [PubMed] [Google Scholar]
- 52.Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- 53.Maquoi E, Frankenne F, Baramova E, Munaut C, Sounni NE, Remacle A, Noël A, Murphy G, Foidart JM. Membrane type 1 matrix metalloproteinase-associated degradation of tissue inhibitor of metalloproteinase 2 in human tumor cell lines. J Biol Chem. 2000;275:11368–11378. doi: 10.1074/jbc.275.15.11368. [DOI] [PubMed] [Google Scholar]
- 54.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997;122:264–71; discussion 271-2. doi: 10.1016/s0039-6060(97)90017-9. [DOI] [PubMed] [Google Scholar]
- 55.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]


