Abstract
AIM: To evaluate the effects of varying ischemic durations on cirrhotic liver and to determine the safe upper limit of repeated intermittent hepatic inflow occlusion.
METHODS: Hepatic ischemia in cirrhotic rats was induced by clamping the common pedicle of left and median lobes after non-ischemic lobes resection. The cirrhotic rats were divided into six groups according to the duration and form of vascular clamping: sham occlusion (SO), intermittent occlusion for 10 (IO-10), 15 (IO-15), 20 (IO-20) and 30 (IO-30) minutes with 5 minutes of reflow and continuous occlusion for 60 minutes (CO-60). All animals received a total duration of 60 minutes of hepatic inflow occlusion. Liver viability was investigated in relation of hepatic adenylate energy charge (EC). Triphenyltetrazollum chloride (TTC) reduction activities were assayed to qualitatively evaluate the degree of irreversible hepatocellular injury. The biochemical and morphological changes were also assessed and a 7-day mortality was observed.
RESULTS: At 60 min after reperfusion following a total of 60 min of hepatic inflow occlusion, EC values in IO-10 (0.749 ± 0.012) and IO-15 (0.699 ± 0.002) groups were rapidly restored to that in SO group (0.748 ± 0.016), TTC reduction activities remained in high levels (0.144 ± 0.002 mg/mg protein, 0.139 ± 0.003 mg/mg protein and 0.121 ± 0.003 mg/mg protein in SO, IO-10 and IO-15 groups, respectively). But in IO-20 and IO-30 groups, EC levels were partly restored (0.457 ± 0.023 and 0.534 ± 0.027) accompanying with a significantly decreased TTC reduction activities (0.070 ± 0.005 mg/mg protein and 0.061 ± 0.003 mg/mg protein). No recovery in EC values (0.228 ± 0.004) and a progressive decrease in TTC reduction activities (0.033 ± 0.002 mg/mg protein) were shown in CO-60 group. Although not significantly different, the activities of the serum aspartate aminotransferase (AST) on the third postoperative day (POD3) and P OD7 and of the serum alanine aminotransferase (ALT) on POD3 in CO-60 group remained higher than that in intermittent occlusion groups. Moreover, a 60% animal mortality rate and more severe morphological alterations were also shown in CO-60 group.
CONCLUSION: Hepatic inflow occlusion during 60 min for liver resection in cirrhotic rats resulted in less hepatocellular injury when occlusion was intermittent rather than continuous. Each period of 15 minutes was the safe upper limit of repeated intermittent vascular occlusion that the cirrhotic liver could tolerate without undergoing irreversible hepatocellular injury.
Keywords: hepatic inflow occlusion/intermittent/continuous, liver resection, cirrhosis, rat, energy charge, triphennyltetrazollum chloride
INTRODUCTION
The effective control of intraoperative bleeding is one of the most important measures for successful hepatectomy[1]. Up to now, temporary portal triad clamping (Pringle maneuver) has been used widely as a means of reducing blood loss. In China, hepatocellular carcinoma (HCC) is commonly associated with cirrhosis[2-6], bleeding during hepatic resection is a major factor in determining the severity of postoperative liver damage and the prognosis[7-9]. The safe time limit of hepatic vascular occlusion during the cirrhotic liver resection remains the major concern of liver surgeons[10,11]. Intermittent occlusion may not only reduce intraoperative bleeding but also increase the total duration of ischemia for hepatectomy[7,12,13]. Thus it is important for the improvement of the safety of the cirrhotic liver resection that how long each time is suitable for the intermittent occlusion[14,15]. The purpose of this experiment is to study the effects of various ischemic durations on cirrhotic liver and determine the safe upper limit of intermittent hepatic inflow occlusion without irreversible damage in rats with cirrhotic liver.
MATERIALS AND METHODS
Male Sprague-Dawley rats weighing 200 g ± 30 g were allocated randomly into six experimental groups of 20 animals, depending on the duration and form of ischemia: Sham occlusion (SO group), intermittent occlusion for 10 min (IO-10 group), 15 min (IO-15 group), 20 min (IO-20 group) and 30 min (IO-30 group) with 5 min of reperfusion between each period, continuous occlusion for 60 min (CO-60 group). The rat cirrhosis was induced by injecting 600 g•L¯¹ CCl4 oil subcutaneously twice weekly at a dose of 4 mL•kg¯¹ bw and drinking 50 mL•L¯¹ ethanol. A validated model[16] used by clamping the common pedicle of left and median lobes after non-ischemia lobes resection did not require splanchnic decompressing. All animals received a total duration of 60 min of hepatic inflow occlusion.
Operative procedure
Rats were anesthetized with intraperitoneal pentobarbital 0.2 mg•kg¯¹. A midline laparotomy was performed. Ligamentous attachments around the liver were divided and the total liver hilum of right and left portal vein, hepatic artery, bile duct as well as the right hepatic vein were also dissected. Ischemia was induced in the median and left hepatic lobes by clamping the corresponding arterial and portal venous branches en masse. This allowed an uninterrupted blood supply to the right lobe and caudate lobe, avoiding congestion of alimentary tract and consequent haemodynamic instability. When the assigned total ischemic duration of 60 min was completed, the clamp was removed and the non-ischemic right lobe and caudate lobe were resected (30% hepatectomy) after ligation of the corresponding pedicles en masse. This technique abrogates the effect of splanchnic congestion caused by total occlusion of the portal hilum during hepatic ischemia.
Assessment of liver viability[17]
A small portion of excised liver (about 30 mg) was immediately frozen by freeze -clamp precooled with liquid nitrogen, lyophilized overnight, and further kept at -80 °C until analysis. The dry tissue was then weighed and homogenized in 1 mL ice-cold 0.6 mol•L¯¹ HClO4. Thirty minutes later, the homogenate was centrifuged for 20 min at -4 °C, 20000 × g and the supernatant with 0.3 mL was neutralized with 1 mol•L¯¹ potassium carbonate 0.2 mL and centrifuged in the same manner. The final supernatant was filtered through a filter of 0.45 μm and used as a sample with 20 μL for determining the liver tissue concentration of ATP, ADP and AMP by high-performance liquid chromatography (Shim-pacr CLC-ODS analytical column, 150 mm × 6.0 mm i.d, λ = 254 nm) at a flow rate of 0.1 mL•min¯¹. The results were calculated as micromoles of nucleotide per gram of dry tissue. Energy charge was calculated as follows: EC = (ATP+1/2ADP)/(ATP+ADP+AMP).
Quantitation of the extent of irreversible hepatocellular injury
The degree of irreversible cellular injury was assessed by a modified technique described by Rodriguez et al[18]. A portion of each liver tissue specimen was rinsed in ice-cold Ringer’s lactate solution, and then placed on ice, weighed, and homogenized in 0.25 mol•L¯¹ sucrose to make an 8% homogenate by weight. The homogenate was filtered through a fine stainless steel mesh to remove the remaining fragments. Protein content of the homogenate was determined by the method of Lowry. A 1 mL aliquot of homogenate was then mixed with 1 mL of 10 g•L¯¹ TTC (Sigma) in 0.033 mol•L¯¹ phosphate buffer (pH7.4). The reaction was made in triplicate. The reactive mixture was incubated at 37 °C in an agitating water bath for 30 min and added with an equal volume of acetone to each reaction tube and then vortexes. The extracted mixture was centrifuged for 15 min at 2000 r•min¯¹ and the absorbance of the clear red supernatants was measured at 485 nm in a spectrophotometer. The absorbances of the reaction triplicates were averaged and unreacted reagent blanks were subtracted to arrive at a final absorption. Comparison of these absorption values to a standard curve of known reduced TTC concentrations to determine the micrograms of TTC reduced per 30 min per milligram of the liver tissue protein.
Serum AST, ALT, lactic dehydrogenase (LDH), alkaline phosphatase (AKP) and total bile acid (TBA) contents were measured at 4 °C using the available kits. Animals of each group were treated as scheduled. The excised median lobe liver was fixed in 100 mL•L¯¹ formalin and embedded in paraffin. Sections 3 μm thick were stained with hematoxylin and eosin for light microscopy. A segment of the liver tissue of the median lobe, 2 mm × 2 mm × 2 mm in size, was taken and fixed in 40 g·¯¹ glutaraldehyde-phosphate buffer (0.1 mol•L¯¹, pH: 7.3) for 24 h and then post-fixed in 10•L¯¹ osmian tetroxide for two hours, dehydrated with a descending series of alcohol, and embedded with epoxied resin. The specimens were cut into ultrathin slices pigmented double with uranium acetate and lead citrate before being observed under the HITACHI-600 transmission electron microscope. Ten animals in each group were considered to have survived the procedure if they remained alive on the seventh day after operation.
Statistical analyses
Values were expressed as mean ± SD. Differences among groups were analyzed using one-way analysis of variance (ANOVA). A post hoc analysis using Fisher’s PLSD test was used to account for multiple comparisons. Differences in survival were determined using the Kaplan-Meier test. P values less than 0.05 were considered significant. All the statistical analyses were made using SPSS software package.
RESULTS
At a total of 60 min of hepatic inflow occlusion, the liver tissue levels of ATP in each ischemia group were significantly decreased, the extent of ATP resto ration at 60 min after reperfusion was markedly related with the METHODS of hepa tic inflow occlusion. The ATP levels in IO-10 and IO-15 groups were rapidly restored to that in SO group, but there was a progressive decrease in IO-20 and IO-30 groups, the recovery was significantly suppressed in CO-60 group, to only 5.8% of ATP levels in SO group (Table 1). There was significant difference among IO-10, IO-15, IO-20, IO-30 and CO-60 groups.
Table 1.
Adenine nucleotide concentrations in the dry cirrhotic livers (mean ± SD, μmol•g¯¹, n = 6)
| Groups |
Ischemia 60 min |
Reperfusion 60 min |
||||
| ATP | ADP | AMP | ATP | ADP | AMP | |
| SO | 5.00 ± 0.13 | 3.09 ± 0.38 | 0.97 ± 0.39 | 5.07 ± 0.15 | 3.19 ± 0.67 | |
| IO-10 | 0.53 ± 0.06b | 1.85 ± 0.28b | 3.40 ± 1.53a | 4.99 ± 0.14 | 3.41 ± 0.29 | 0.53 ± 0.25 |
| IO-15 | 0.45 ± 0.05b | 1.39 ± 0.55bc | 3.81 ± 2.07b | 4.99 ± 0.16 | 3.35 ± 0.16 | 1.20 ± 0.09bd |
| IO-20 | 0.43 ± 0.03b | 1.13 ± 0.43bd | 3.21 ± 1.37a | 1.01 ± 0.13bdf | 2.22 ± 0.24bdf | 1.41 ± 0.15bd |
| IO-30 | 0.45 ± 0.12b | 0.79 ± 0.05bde | 2.29 ± 0.81 | 0.90 ± 0.19bdf | 2.41 ± 0.35bdf | 0.69 ± 0.24f |
| CO-60 | 0.41 ± 0.08b | 1.00 ± 0.34bd | 3.10 ± 0.45a | 0.30 ± 0.01bdfgh | 0.85 ± 0.15bdfgh | 2.02 ± 0.19bdfgh |
P < 0.05,
P < 0.01 vs SO;
P < 0.05,
P < 0.01 vs IO-10;
P < 0.05,
P < 0.01 vs IO-15;
P < 0.01 vs IO-20;
P < 0.01 vs IO-30.
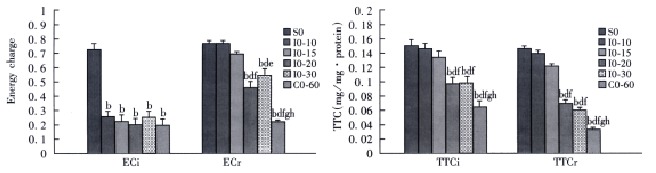
The EC levels at a total ischemic duration of 60 min significantly and immediat ely decreased in each ischemia group. At 60 min after reperfusion, there was a rapid restoration in IO-10 and IO-15 groups (0.748 ± 0.016 in SO group, 0.749 ± 0.012 in IO-10 group, 0.699 ± 0.002 in IO-15 group). The EC levels were restored partly in IO-20 and IO-30 groups (0.457 ± 0.023 and 0.534 ± 0.027) and no recovery in CO-60 group (0.228 ± 0.004) (Figure 1). A significant difference was shown among IO-10, IO-15, IO-20, IO-30 and CO-60 groups.
Figure 1.
Changes of energy charge in hepatic tissues.
At a total ischemic period of 60 min, TTC reduction activities in IO-20, IO-30 and CO-60 groups (0.098 ± 0.007 mg/mg protein, 0.099 ± 0.005 mg/mg protein and 0.068 ± 0.007 mg/mg protein, respectively) markedly declined to 66.7%, 67.3% and 46.3% of SO group (0.147 ± 0.004 mg/mg protein), respectively. At 60 min after reperfusion, TTC reduction activities remained high in IO-10 and IO-15 groups (0.139 ± 0.003 mg/mg protein and 0.121 ± 0.003 mg/mg protein) and significantly decreased in IO-20 and IO-30 groups (0.070 ± 0.005 mg/mg protein and 0.061 ± 0.003 mg/mg protein), there was a progressive decrease in CO-60 group (0.033 ± 0.002 mg/mg protein) in comparison with that in each intermittent occlusion group (P < 0.01) (Figure 2).
Figure 2.
Changes of TTC reduction activities in hepatic tissues.
Although serum AST and ALT activities on POD1 markedly increased in IO-10 (986 ± 49 μkat•L¯¹ and 1356 ± 221 μkat•L¯¹), IO-15 (1431 ± 116 μkat•L¯¹ and 1611 ± 149 μkat•L¯¹), IO-20 (1558 ± 78 μkat•L¯¹ and 1186 ± 187 μkat•L¯¹), IO-30 (1743 ± 96 μkat•L¯¹ and 2466 ± 489 μkat•L¯¹) and CO-60 (1773 ± 181 μkat•L¯¹ and 2190 ± 397 μkat•L¯¹) groups as compared with SO group (294 ± 16 μkat•L¯¹ and 669 ± 26 μkat•L¯¹), there was no difference among five ischemia groups. However, serum AST activities in CO-60 group on POD3 and POD7 (203 ± 14 μkat•L¯¹, n = 5 and 183 ± 6 μkat•L¯¹, n = 4) and ALT activities on POD3 (484 ± 38 μkat•L¯¹, n = 5) remained to be higher than that in intermittent ischemia groups (P < 0.05). In addition, no significant difference was found in postoperative serum LDH, AKP and TBA levels among ischemia groups. All animals in SO and IO-10 groups survived during the 7 d period. The mortality rates in IO-15, IO-20 and IO-30 groups were 10%, 30% and 40%, respectively. In contrast, the mortality rate in CO-60 group increased to 60%, with a significant difference between IO-10 and CO-60 groups (P < 0.05).
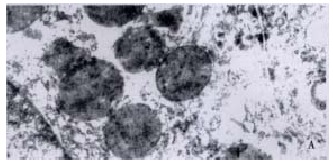
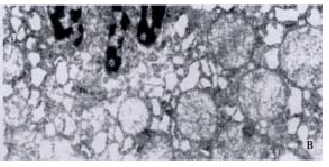
The morphologic findings revealed that hepatic cells slightly swollen in IO-10 and IO-15 groups, but the dilatation of the rough-surfaced endoplasmic reticulum (RER) and the degeneration changes in mitochondria within cytoplasm were mild, the shape of nucleus was regular. In IO-20 and IO-30 groups, hepatic cells had a moderate swelling with cytoplasmic microvacuolisation, predominantly in the midzonal areas. Ultrastructural changes were shown with a gross dilatation of RER and mitochondria, an irregular nucleus and an aggregation of the heterochromatins within the nucleus. In CO-60 group, the hepatocytes had vacuolar degeneration accompanied with infiltration of polymorphonuclear leucocytes in the sinusoids. RER and mitochondria were severely dilated, the heterochromatins within the nucleus markedly increased and nucleus concentration occurred (Figure 3).
Figure 3.
Electron micrograph of hepatocyte at a total ischemic duration of 60 min in cirrhotic rats. A: The mitochondria were regular, chroma tin in nucleus was well-distributed, nuclear membrane was clear in SO group, TEM × 17000, B: Mitochondria and RER were severely dilated, heterochromtins within the nucleus markedly increased, nucleus concentration occurred. TEM × 15000
DISCUSSION
In China, the incidence of HCC is high and almost 85% of HCC were accompanied with liver cirrhosis[4,19]. Bleeding during liver resection is known to be an important prognostic indicator of both morbidity and mortality, particularly for the cirrhotic patients[14,20,21]. Generally, blood loss has been controlled by the Pringle maneuver, which is one of the simplest and most commonly used METHODS and attention has focused on the length of time for which such ischemia to the liver can be safely tolerated[20,22-26]. Although the healthy human liver could tolerate a major hepatectomy with ischemia of more than 60 min under normothermic condition[24,25], in their studies, there were complications in 18 of 34 patients and a thirteen-fold increase in the activities of plasma transaminase on the first postoperative day. Moreover, the cirrhotic liver would be much more vulnerable to ischemia, Therefore, intermittent portal triad clamping for the control of bleeding during cirrhotic liver resection has been advocated that this would be less detrimental to the liver than continuous clamping[7,12,13,20,21]. How long the cirrhotic liver could tolerate intermittent hepatic inflow occlusion each time remains to be determined.
EC, which expressed the balance between ATP-consuming and -producing reactions and was of central importance in the regulation of metabolic sequences, had been used to assess liver viability[17]. These studies had suggested that enhanced ATP synthesis and prompt recovery of EC after revascularization were prerequisites for maintaining the liver viability. In the present study, although ATP content and EC at a total ischemic duration of 60 min were retarded during the ischemic period, a significant difference was shown at 60 min after reperfusion. Because the ability of the liver to restore EC after reperfusion played a decisive role in determining hepatic viability[19], the prompt restoration of the EC levels in IO-10 and IO-15 groups reflected the maintenance of liver viability. However, EC had only partial recovery in IO-20 and IO-30 groups, could not be restored after reperfusion in CO-60 group, indicating the irreversible mitochondrial dysfunction, subsequent ATP depletion and loss of liver viability in IO-20 and IO-30 groups, which were more severe in CO-60 group. These findings were further supported by the morphological results.
Of the variety of cell types in the liver, the hepatocytes were the most sensitive to normotheric ischemia. The liver could tolerate a temporary ischemia of short duration but prolonged ischemia caused irreversible hepatocellular injury and led to liver function failure even if the liver was reperfused. The TTC reduction activity assay reflected the ability of the intact mitochondrial reduction-oxidation enzyme systems to convert the colorless TTC to a red formazan dye. This indicator had been validated to quantitate the extent of irreversible cell damage in the study of the hepatic and cardiac ischemic injury[18]. The inability to reduce TTC indicated the irreversible loss of mitochondrial function. In the present study, the TTC reduction activities had no changes during ischemia of 60 min and remained at higher levels at 60 min after reperfusion in IO-10 and IO-15 groups, but had an aggressive decrease in IO-20, IO-30 and CO-60 groups at 60 min of ischemia or reperfusion. These results demonstrated that the irreversible hepatocellular injury firstly occurred in IO-20 group, significantly greater injury occurred in CO-60 group.
As mentioned above, when changes in EC levels were examined in conjunction with changes in TTC reduction activities and morphological features, it was possible to identify a significant injury that was completely reversible when the intermittent occlusion duration was limited to 15 min. However, the intermittent occlusion of prolonged 20 or 30 min would produce irreversible hepatocellular damage, the extent of damage became greater in continuous occlusion group.
In addition, it has been emphasized in the previous literature that uncontrollable massive hemorrhage during hepatic resection would lead to a deterioration of liver function and increased postoperative morbidity and mortality, particularly in the patients with liver cirrhosis[7-9,14,20,21]. These patients could tolerate liver ischemia within certain limits better than they do the consequences of massive bleeding and blood transfusion. Wu et al[20] showed that a cirrhotic liver could tolerate intermittent ischemia for up to 200 minutes without increased postoperative complications and mortality. In another report, immediate postoperative liver function was better preserved in the intermittent occlusion group than in those patients who were operated on without using the intermittent occlusion[15]. This may be due to less hemodynamic disturbance induced by the bleeding as well as hepatovenous retrograde perfusion during the liver transection to maintain the liver viability[21]. However, the preoperative status of liver function should still be taken into account. An improved prognosis appeared to be related to a suitable selection of cirrhotic patients with well-compensated liver function and an increased proportion of limited resections of cirrhotic livers[14,27-29].
In the clinical practice, an intermittent rather than a continuous hepatic vascular occlusion was advocated during limited resection of the cirrhotic liver because it could increase the ability of the liver to tolerate the consequences of prolonged ischemia and splanchnic venous stasis[20,21]. However, the relation between the selected duration of intermittent vascular occlusion and the risk of bleeding from esophageal varices in the cirrhotic patients during the liver resection remains to be determined.
It is concluded from this experiment that intermittent hepatic inflow occlusion to the liver resulted in less hepatocellular damage when compared with continuous occlusion and suggested that intermittent occlusion of the hepatic pedicle was the preferred method to control the intraoperative hemorrhage during cirrhotic liver resections. Furthermore, each period of 15 min was the safe upper limit of repeated intermittent hepatic inflow occlusion that the cirrhotic liver could tolerate without irreversible liver damage.
Footnotes
Edited by Ma JY
Supported by the grant from the Science and Technology Committee of Zhejiang Province, No. 971103132
References
- 1.Wu MC, Zhang BH. Progress in liver surgery. Xin Xiaohuabingxue Zazhi. 1996;4:421–423. [Google Scholar]
- 2.Lin NF, Tang J, Ismael HS. Study on environmental etiology of high incidence areas of liver cancer in China. World J Gastroenterol. 2000;6:572–576. doi: 10.3748/wjg.v6.i4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang ZY. Advances in clinical research of hepatocellular carcinoma in China. Huaren Xiaohua Zazhi. 1998;6:1013–1016. [Google Scholar]
- 4.Wu MC, Shen F. Progress in research of liver surgery in China. World J Gastroenterol. 2000;6:773–776. doi: 10.3748/wjg.v6.i6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JN, Li T, Yang NW, Wei CY, Chen JS, Li H. Cryotherapy of 72 patients with liver neoplasms. Shijie Huaren Xiaohua Zazhi. 2000;8:595–596. [Google Scholar]
- 6.Liu WW. Etiological studies of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:93–95. [Google Scholar]
- 7.Chen H, Merchant NB, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality. J Gastrointest Surg. 2000;4:162–167. doi: 10.1016/s1091-255x(00)80052-9. [DOI] [PubMed] [Google Scholar]
- 8.Nakai T, Koh K, Funai S, Kawabe T, Okuno K, Yasutomi M. Comparison of controlled and Glisson's pedicle transections of hepatic hilum occlusion for hepatic resection. J Am Coll Surg. 1999;189:300–304. doi: 10.1016/s1072-7515(99)00127-1. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka N, Yamanaka J, Tanaka T, Tanaka W, Yasui C, Ando T, Okamoto E. Topical cooling assisted hepatic resection of segment 7 and 8 oriented by en-bloc interruption of the targeted portal pedicles. Hepatogastroenterology. 1999;46:417–424. [PubMed] [Google Scholar]
- 10.Yan LN. Operative treatment of primary liver cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:442. [Google Scholar]
- 11.Suzuki M, Fukuhara K, Unno M, Rikiyama T, Takeuchi H, Uchiyama T, Matsuno S. Simplified hepatic resection utilizing absorbable polyglycolic acid-based tape and other ligature apparatus. J Hepatobiliary Pancreat Surg. 1998;5:292–296. doi: 10.1007/s005340050048. [DOI] [PubMed] [Google Scholar]
- 12.Cherqui D, Malassagne B, Colau PI, Brunetti F, Rotman N, Fagniez PL. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg. 1999;230:24–30. doi: 10.1097/00000658-199907000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wagensveld BA, van Gulik TM, Gelderblom HC, Scheepers JJ, Bosma A, Endert E, Gouma DJ. Prolonged continuous or intermittent vascular inflow occlusion during hemihepatectomy in pigs. Ann Surg. 1999;229:376–384. doi: 10.1097/00000658-199903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagasue N, Uchida M, Kubota H, Hayashi T, Kohno H, Nakamura T. Cirrhotic livers can tolerate 30 minutes ischaemia at normal environmental temperature. Eur J Surg. 1995;161:181–186. [PubMed] [Google Scholar]
- 15.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–11; discussion 711-3. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy KJ, Tancheroen S, Shulkes A. Comparison of continuous versus intermittent ischaemia-reperfusion during liver resection in an experimental model. Br J Surg. 1995;82:833–836. doi: 10.1002/bjs.1800820636. [DOI] [PubMed] [Google Scholar]
- 17.Hickman R, Rose-Innes C, Tyler M, Bracher M, Lotz Z, Fourie J. Energy charge as an indication of liver viability. A comparison of changes in livers that remained intact with those subjected to autografting. Transplantation. 1992;53:540–545. doi: 10.1097/00007890-199203000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez AA, LaMorte WW, Hanrahan LM, Hopkins SR, O'Keane JC, Cachecho R, Hirsch EF. Liver viability after ischemia-reperfusion. Arch Surg. 1991;126:767–772. doi: 10.1001/archsurg.1991.01410300113018. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Ku JL, Park YJ, Lee KU, Kim WH, Park JG. Establishment and characterization of four human hepatocellular carcinoma cell lines containing hepatitis B virus DNA. World J Gastroenterol. 1999;5:289–295. doi: 10.3748/wjg.v5.i4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CC, Hwang CR, Liu TJ, P'eng FK. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. Br J Surg. 1996;83:121–124. doi: 10.1002/bjs.1800830139. [DOI] [PubMed] [Google Scholar]
- 21.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Yu WC, Wong J. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999;134:533–539. doi: 10.1001/archsurg.134.5.533. [DOI] [PubMed] [Google Scholar]
- 22.Buell JF, Koffron A, Yoshida A, Hanaway M, Lo A, Layman R, Cronin DC, Posner MC, Millis JM. Is any method of vascular control superior in hepatic resection of metastatic cancers问号 Longmire clamping, pringle maneuver, and total vascular isolation. Arch Surg. 2001;136:569–575. doi: 10.1001/archsurg.136.5.569. [DOI] [PubMed] [Google Scholar]
- 23.Iwanami K, Takeyoshi I, Ohwada S, Kobayashi J, Kawashima Y, Aiba M, Matsumoto K, Morishita Y. The effect of Lazaroid U-74389G on extended liver resection with ischemia in dogs. Surgery. 1999;126:908–917. [PubMed] [Google Scholar]
- 24.Huguet C, Gavelli A, Chieco PA, Bona S, Harb J, Joseph JM, Jobard J, Gramaglia M, Lasserre M. Liver ischemia for hepatic resection: where is the limit问号. Surgery. 1992;111:251–259. [PubMed] [Google Scholar]
- 25.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–458. [PubMed] [Google Scholar]
- 26.Yan JQ, Li HW, Cai WY, Zhang MJ, Yang WP. Can the rat donor liver tolerate prolonged warm ischemia问号. World J Gastroenterol. 2000;6:561–564. doi: 10.3748/wjg.v6.i4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, Zhou XD, Ye SL. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28–32. doi: 10.3748/wjg.v7.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan ST. Technique of hepatectomy. Br J Surg. 1996;83:1490–1491. doi: 10.1002/bjs.1800831103. [DOI] [PubMed] [Google Scholar]
- 29.Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208–215. doi: 10.3748/wjg.v7.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]


