Abstract
AIM: Genetic polymorphism in enzymes of carcinogen metabolism has been found to have the influence on the susceptibility to cancer. Cytochrome P450 2E1 (CYP2E1) is considered to play an important role in the metabolic activation of procarcinogens such as N-nitrosoamines and low molecular weight organic compounds. The purpose of this study is to determine whether CYP450 2E1 polymorphisms are associated with risks of gastric cancer.
METHODS: We conducted a population based case-control study in Changle county, Fujian Province, a high-risk region of gastric cancer in China. Ninety-one incident gastric cancer patients and ninety-four healthy controls were included in our study. Datas including demographic characteristcs, diet intake, and alcohol and tobacco consumption of indivduals in our study were completed by a standardized questionnaire. PCR-RFLP revealed three genotypes:heterozygote (C1/C2) and two homozygotes (C1/C1 and C2/C2) in CYP2E1.
RESULTS: The frequency of variant genotypes (C1/C2 and C2/C2) in gastric cancer cases and controls was 36.3% and 24.5%, respectively. The rare homozygous C2/C2 genotype was found in 6 indivduals in gastric cancer group (6.6%), whereas there was only one in the control group (1.1%). However, there was no statistically significan difference between the two groups (two-tailed Fisher’s exact test, P = 0.066). Indivduals in gastric cancer group were more likely to carry genotype C1/C2 (odds ratio, OR = 1.50) and C2/C2 (OR = 7.34) than indivduals in control group (χ² = 4.597, for trend P = 0.032). The frequencies of genotypes with the C2 allele (C1/C2 and C2/C2 genotypes) were compared with those of genotypes without C2 allele (C1/C1 genotype) among indivduals in gastric cancer group and control group according to the pattern of gastric cancer risk factors. The results show that indivduals who exposed to these gastric cancer risk factors and carry the C2 allele seemed to have a higher risk of developing gastric cancer.
CONCLUSION: Polymorphism of CYP2E1 gene may have some effct in the development of gastric cancer in Changle county, Fujian Province.
Keywords: gastric neoplasm/genetics, gastric neoplasm/etiology, Cytochrome P-450 2E1, CYP2E1/genetics, genotype, human, FUJIAN
INTRODUCTION
Fujian Province is one of the highest risk areas of gastric cancer in China [1,2]. Gastric cancer is the major cause of cancer mortality in Changle County, Fujian Province[3-5]. Epidemio logical studies have shown that an increased risk of developing gastric cancer is associated with diet and tobacco smoking[6-8]. One particular hypothesis, which has been paid more attention, is that N-nitroso compounds from dietary sources are involved in carcinogenesis of gastric cancer[9-10]. Recent advances in cancer research have revealed that the main etiology of human cancers are genetic changes in cancer related genes caused by carcinogens in the environment[11-14]. It is known that most of exogenous (xenobiotics) and endogenous chemical carcinogens require biotransformation to activated forms to be carcinogenic[15,16]. Most of the human metabolizing enzymes are genetically polymorphic, and those polymorphisms may affect the enzyme activity or inducibility[17-20]. The sensibitity to procarcinogen differs among individuals, which may have substantial importance in carcinogenesis[21-24]. Cytochromes P450 (CYPs) play an important role on metablism of several aspects of cancer. Cytochrome P450 2E1 (CYP2E1) is primarily responsible for the bioactivation of many low molecular weight carcinogens, and is involved in the metabolic oxidation of carcinogenic nitroso compounds, including N-nitrosoamines[25-26]. This study was designed to in vestigate the relationship between polymorphism of CYP 2E1 and gastric cancer.
MATERIALS AND METHODS
Subjects
All indivduals in our study are the residents of Changle county, Fujian Province of China. Ninety-one patients with pathologic diagnosis as primary gastric cancer between January 1996 to March 1998 and 94 age-and sex matched healthy controls were included in this study. Each indivdual was personally interviewed to obtain information on demographic characteristics, habits of cigarette smoking, alcohol drinking, and dietary consumption frequency. Blood specimens from them were also obtained.
DNA isolation and PCR-RFLP analysis
Genomic DNA was isolated from white blood cells by extraction using phenol/chloroform and precipitation using ethanol. PCR was performed using the primers 5’-CCAGTCGAGTCTACATTGTCA-3’ (1370-1349) and 5’-TTCATTCTGTCTTCTAACTGG-3’ (999-978). The amplification reaction was conducted in a 50 (L solution containing PCR buffer (1.5 mmol•L¯¹ MgCl2, 50 mmol•L¯¹ KCl, 10 mmol·L -1 Tris-HCl, pH8.3), 200 μmol•L¯¹ dNTP, 1 μmol•L¯¹ primer, 200 ng template DNA, and 2.5 μ Taq DNA polymerase (Promega Corp). Those reactions were performed about 35 cycles at conditions following as denaturation for 1 min at 95 °C, annealing for 1 min at 55 °C, extension for 1 min at 72 °C and a final extension for 10 min at 72 °C. The PCR products were digested with PstI or RsaI (Fermantas-MBI. Vilnius, Lithuania) for 18 h at 37 °C. The restriction sites was identified by 2.2% agarose gel electrophoresis. The genotypes of CYP2E1 were classified as following: a predominant homozygote (C1/C1), a heterozygote (C1/C2) and a rare homozygote (C2/C2). Figure 1, Figure 2.
Figure 1.
The PCR products of CYP2E1 gene. M: markers.
Figure 2.
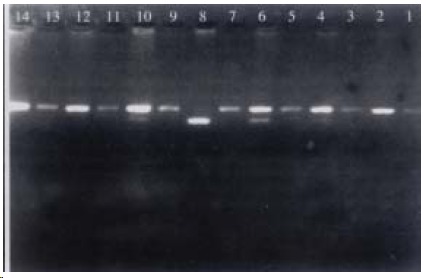
PCR-RFLP with Pst1. Lanes 2, 12, 14: C1/C1; Lanes 4, 6, 10: C1/C2; Lane 8: C2/C2; Lanes 1, 3, 5, 7, 9, 11, 13: Sample controls.
Statistical analysis
Fisher’s exact tests were determined by standard methods. The relationships between CYP 2E1 genotypes and putative risk factors were measured using the odds ratios (ORs) and their 95% confidence intervals (95%CIs).
RESULTS
The age (mean ± SD) of indivduals in gastric cancer group and control group were 58.4 ± 11 years and 58.2 ± 11 years, respectively. The distributions of age and sex were similar in cases and controls. The risks of gastric cancer were related to smoking, alcohol drinking, and fish sauce intake (a condiment commonly used by local residents), Table 1.
Table 1.
Distribution of selected variables in gastric cancer group and group
|
Gastric cancer (n = 91) |
Control (n = 94) |
P value | |
| n (%) | n (%) | ||
| Age/y | |||
| < 50 | 21 (23.1) | 22 (23.4) | |
| 50-59 | 22 (24.2) | 22 (23.4) | |
| 60-69 | 32 (35.2) | 34 (36.2) | |
| 70+ | 16 (17.6) | 16 (17.0) | 0.9983 |
| mean ± SD | 58.4 ± 10.9 | 58.2 ± 11.0 | |
| Range | 32-78 | 34-79 | |
| Gender | |||
| Male | 77 (84.62) | 82 (87.23) | |
| Female | 14 (15.38) | 12 (12.77) | 0.6094 |
| Education | |||
| College | 1 (1.1) | 1 (1.1) | |
| High school | 15 (16.5) | 63 (67.0) | |
| Elementary school | 57 (62.6) | 22 (23.4) | |
| Illiterate | 18 (19.8) | 8 (8.5) | 0.0000 |
| Smoking/y | |||
| 0 | 31 (34.1) | 60 (63.8) | |
| < 10 | 2 (2.2) | 16 (17.0) | |
| 10- | 16 (17.6) | 7 (7.5) | |
| 20- | 22 (24.2) | 4 (4.3) | |
| 30- | 20 (22.0) | 7 (7.5) | 0.0000 |
| Alcohol drinking/y | |||
| 0 | 40 (44.0) | 66 (70.2) | |
| < 10 | 3 (3.3) | 13 (13.8) | |
| 10- | 14 (15.4) | 8 (8.5) | |
| 20- | 34 (37.4) | 7 (7.5) | 0.0000 |
| Fish sauce intake | |||
| Low (< 3 times/w) | 17 (18.68) | 69 (73.40) | |
| High (≥ 3 times/w) | 76 (81.32) | 25 (26.60) | 0.0000 |
The frequency of variant genotypes (C1/C2 and C2/C2) in gastric cancer group and control group was 36.3% and 24.5%, respectively. The rare homozygous C2/C2 genotype was found in 6 of gastric cancer group (6.6%), but in 1 of the controls (1.1%). However, the result showed no statistical difference. Fisher’s exact test P = 0.066 (T able 2). The allele frequencies of CYP2E1 fit with Hardy-Weinberg equilibrium (χ² = 0.242, P > 0.05) (Table 2).
Table 2.
Cytochrome P450 2E1 genotype and risks of gastric cancer
| CYP2E1 |
Gastric cancer |
Control (n = 94) |
OR (95%CI) | ||
| n | (%) | n | (%) | ||
| C1/C1 | 58 | (63.7) | 71 | (75.5) | 1.00 |
| C1/C2 | 27 | (29.7) | 22 | (23.4) | 1.50 (0.74-3.07) |
| C2/C2 | 6 | (6.6) | 1 | (1.1) | 7.34 (0. 84-166.60) |
Fisher’s test P = 0.066 vs controls χ² trend = 4.597 P = 0.032 vs controls Gastric cancer indivduals were more likely to carry genotype C1/C2 and C2/C2 than indivduals in control group. Individuals carried at least one C2 allele (genotypes C1/C2 or C2/C2) seemed to have an increased risk of gastric cancer (OR = 1.86, 95%CI 1.07~3.25), Table 3.
Table 3.
The allele frequencies of CYP2E1 in gastric cancer group and control group
|
C1 |
C2 |
|||
| n | Frequencies | n | Frequencies | |
| Gastric cancer | 143 | 0.7857 | 39 | 0.21 43 |
| Controls | 164 | 0.8723 | 24 | 0.1276 |
χ² = 4.91 P < 0.05 vs controls; OR = 1.86 95%CI 1.07~3.25.
The frequencies of genotypes with the C2 allele (C1/C2 and C2/C2) were compared with those of genotypes without C2 allele (C1/C1) among indivduals in gastric cancer and control group according to the pattern of gastric cancer risk factors of smoking, alcohol drinking and fish sauce intake. Indivduals who have been exposed to those risk factors of gastric cancer and carried the C2 allele seemed to have a higher risk of developing gastric cancer (Table 4).
Table 4.
Relationships between CYP2E1 together with selected variables and risk of gastric cancer
| CYP2E1 | Variables Smoking | Gastric cancer | Controls | OR (95%CI) |
| C1/C1 | No | 21 | 48 | 1.00 |
| C1/C1 | Yes | 37 | 23 | 3.68 (1.67-8.19) |
| C1/C2 or C2/C2 | No | 10 | 12 | 1.90 (0.64-5.69) |
| C1/C2 or C2/C2 | Yes | 23 | 1 1 | 4.78 (1.82-12.78) |
| Alcohol drinking | ||||
| C1/C1 | No | 26 | 51 | 1.00 |
| C1/C1 | Yes | 32 | 20 | 3.14 (1.42-6.99) |
| C1/C2 or C2/C2 | No | 14 | 15 | 1.83 (0.70- 4.77) |
| C1/C2 or C2/C2 | Yes | 19 | 8 | 4.66 (1.65-13.53) |
| Fish sauce intakea | ||||
| C1/C1 | Low | 11 | 53 | 1.00 |
| C1/C1 | High | 47 | 18 | 12.58 (5.40-29.34) |
| C1/C2 or C2/C2 | Low | 6 | 16 | 1.81 (0.58-5.66) |
| C1/C2 or C2/C2 | High | 27 | 7 | 18.58 (6.47-53.37) |
High ≥ 3 times/w; Low < 3 times/w
DISCUSSION
Epidemiological studies have shown that up to 90% of all cancers are related to environmental factors. Most of the environmental carcinogens need to be metabolically activated to exert their carcinogenic effects[27-30]. Genetic polymorphisms in enzymes involved in carcinogen metabolism has shown to influence the susceptibility to cancer[31-33]. Cytochrome P4502E1 (CYP2E1) plays an important role in this process. It participates in the metabolic activation of carcinogenic nitrosamines. Several recent studies show that the genetic polymorphisms of metabolizing enzymes are associated with some cancers such as lung cancer[34-36], esophageal cancer[37-40] and colorectal cancer[41]. But the results of those studies of the relation between CYP2E1 and cancer susceptibility are inconsistent[42].
The possibility that N-nitrosated compounds are involved in gastric cancer has been an issue for many years. Nitrosamines occur in tobacco smoke and in some kinds of food, which are also formed endogenously in the stomach. In this study, fish sauce intake, cigarette smoke, and alcohol drinking were positively associated with gastric cancer. Fish sauce is a condiment that is particularly favored by the local residents in Fujian Province[43-45]. Many N-nitro compound precursors have been found in fish sauce [46,47]. Tobacco smoke contains many potential carcinogens, also including nitroso compounds[48]. CYP2E1 are known to vary and are induced by ethanol consumption. Several studies reported that the variant C2 was associated with enhanced enzyme activity. Hayashi et al[49] reported that enhanced activity of C2/C2 DNA was about 1 0 times than that of C1/C1 DNA. This difference in the transcriptional activities might associate with the susceptibility in human carcinogenesis. There is evidence suggesting that there may be a gene-environment interaction in the development of cancer so that cancer risk associated with a given exposure is modified by the genotype of the host[50]. In this study, a much higher risk was observed from those who exposed to the risk factors of gastric cancer and carried the C1/C2 or C2/C2 genotypes. The results suggest that polymorphic genes that code for tobacco carcinogen and alcohol metabolizing enzymes may play a role in susceptibility of gastric cancer. The intervention against cigarette smoking, alcohol drinking, bad eating habits may be important for the prevention of gastric cancer in high-incidence areas.
Because of the limited number of samples in this study, determination of precise dose-response relations with respect to a sufficient number of dose level for each of genotype groups may not be able to be con ducted. Further studies with a larger number of samples are needed to confirm the role of genetic polymorphism of human CYP2E1 in gastric cacinogenesis.
Footnotes
Edited by Xu XQ and Wang JH
Supported by Natural Science Foundation of Fujian Province, China, No. C001009
References
- 1.Li L, Lu F, Zhang S. [Analyses of variation trend and short-term detection of Chinese malignant tumor mortality during twenty years] Zhonghua Zhong Liu Za Zhi. 1997;19:3–9. [PubMed] [Google Scholar]
- 2.Cai L, Yu SZ, Zhang ZF. Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control study. World J Gastroenterol. 2001;7:506–509. doi: 10.3748/wjg.v7.i4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County, Fujian Province, China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Zheng T, Chen J. [Evaluation of ten-year results of cancer prevention and treatment in Changle City with high incidence of gastric cancer] Zhonghua Zhong Liu Za Zhi. 2000;22:311–313. [PubMed] [Google Scholar]
- 5.Wang ZQ, He J, Chen W, Chen Y, Zhou TS, Lin YC. Relationship between different sources of drinking water, water quality improvement and gastric cancer mortality in Changle County-A retrospective-cohort study in high incidence area. World J Gastroenterol. 1998;4:45–47. doi: 10.3748/wjg.v4.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji BT, Chow WH, Yang G, McLaughlin JK, Zheng W, Shu XO, Jin F, Gao RN, Gao YT, Fraumeni JF. Dietary habits and stomach cancer in Shanghai, China. Int J Cancer. 1998;76:659–664. doi: 10.1002/(sici)1097-0215(19980529)76:5<659::aid-ijc8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Hill MJ. Nutritional and metabolic aspects of gastrointestinal cancer. Curr Opin Clin Nutr Metab Care. 1998;1:405–407. doi: 10.1097/00075197-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ye W, Ekström AM, Hansson LE, Bergström R, Nyrén O. Tobacco, alcohol and the risk of gastric cancer by sub-site and histologic type. Int J Cancer. 1999;83:223–229. doi: 10.1002/(sici)1097-0215(19991008)83:2<223::aid-ijc13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Deng DJ, E Z. Overview on recent studies of gastric carcinogenesis: human ex posure of N-nitrosamides. Shijie Huaren Xiaohua Zazhi. 2000;8:250–252. [Google Scholar]
- 10.Ward MH, López-Carrillo L. Dietary factors and the risk of gastric cancer in Mexico City. Am J Epidemiol. 1999;149:925–932. doi: 10.1093/oxfordjournals.aje.a009736. [DOI] [PubMed] [Google Scholar]
- 11.Katoh T, Kaneko S, Kohshi K, Munaka M, Kitagawa K, Kunugita N, Ikemura K, Kawamoto T. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and oral cavity cancer. Int J Cancer. 1999;83:606–609. doi: 10.1002/(sici)1097-0215(19991126)83:5<606::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 13.He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515–521. doi: 10.3748/wjg.v7.i4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe M. Polymorphic CYP genes and disease predisposition--what have the studies shown so far. Toxicol Lett. 1998;102-103:167–171. doi: 10.1016/s0378-4274(98)00303-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Lee CG, Park YG, Kim KS, Kim IK, Sohn YW, Min HK, Lee JM, Namkoong SE. Combined analysis of germline polymorphisms of p53, GSTM1, GSTT1, CYP1A1, and CYP2E1: relation to the incidence rate of cervical carcinoma. Cancer. 2000;88:2082–2091. doi: 10.1002/(sici)1097-0142(20000501)88:9<2082::aid-cncr14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Slattery ML, Edwards SL, Samowitz W, Potter J. Associations between family history of cancer and genes coding for metabolizing enzymes (United States) Cancer Causes Control. 2000;11:799–803. doi: 10.1023/a:1008912317909. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez FJ, Kimura S. Understanding the role of xenobiotic-metabolism in chemical carcinogenesis using gene knockout mice. Mutat Res. 2001;477:79–87. doi: 10.1016/s0027-5107(01)00109-9. [DOI] [PubMed] [Google Scholar]
- 18.Chhabra SK, Reed CD, Anderson LM, Shiao YH. Comparison of the polymorphic regions of the cytochrome P450 CYP2E1 gene of humans and patas and cynomolgus monkeys. Carcinogenesis. 1999;20:1031–1034. doi: 10.1093/carcin/20.6.1031. [DOI] [PubMed] [Google Scholar]
- 19.Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM. Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2, and CYP2E1. Clin Cancer Res. 1999;5:2192–2197. [PubMed] [Google Scholar]
- 20.Henderson MC, Miranda CL, Stevens JF, Deinzer ML, Buhler DR. In vitro inhibition of human P450 enzymes by prenylated flavonoids from hops, Humulus lupulus. Xenobiotica. 2000;30:235–251. doi: 10.1080/004982500237631. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka E. Update: genetic polymorphism of drug metabolizing enzymes in humans. J Clin Pharm Ther. 1999;24:323–329. doi: 10.1046/j.1365-2710.1999.00236.x. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Mimura N, Fujii H, Minami H, Sasaki Y, Shimada N, Chiba K. Role of human cytochrome P450 3A4 in metabolism of medroxyprogesterone acetate. Clin Cancer Res. 2000;6:3297–3303. [PubMed] [Google Scholar]
- 23.Kamataki T, Nunoya K, Sakai Y, Kushida H, Fujita K. Genetic polymorphism of CYP2A6 in relation to cancer. Mutat Res. 1999;428:125–130. doi: 10.1016/s1383-5742(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 24.Murata M, Watanabe M, Yamanaka M, Kubota Y, Ito H, Nagao M, Katoh T, Kamataki T, Kawamura J, Yatani R, et al. Genetic polymorphisms in cytochrome P450 (CYP) 1A1, CYP1A2, CYP2E1, glutathione S-transferase (GST) M1 and GSTT1 and susceptibility to prostate cancer in the Japanese population. Cancer Lett. 2001;165:171–177. doi: 10.1016/s0304-3835(01)00398-6. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Park JY, Schantz SP, Stern JC, Lazarus P. Elucidation of CYP2E1 5' regulatory RsaI/Pstl allelic variants and their role in risk for oral cancer. Oral Oncol. 2001;37:437–445. doi: 10.1016/s1368-8375(00)00099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse MA, Lu J, Stoner GD, Murphy SE, Peterson LA. Metabolism of N-nitrosobenzylmethylamine by human cytochrome P-450 enzymes. J Toxicol Environ Health A. 1999;58:397–411. doi: 10.1080/009841099157133. [DOI] [PubMed] [Google Scholar]
- 27.Roth MJ, Dawsey SM, Wang G, Tangrea JA, Zhou B, Ratnasinghe D, Woodson KG, Olivero OA, Poirier MC, Frye BL, et al. Association between GSTM1*0 and squamous dysplasia of the esophagus in the high risk region of Linxian, China. Cancer Lett. 2000;156:73–81. doi: 10.1016/s0304-3835(00)00442-0. [DOI] [PubMed] [Google Scholar]
- 28.Chao YC, Wang LS, Hsieh TY, Chu CW, Chang FY, Chu HC. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol. 2000;95:2958–2964. doi: 10.1111/j.1572-0241.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 29.Bouchardy C, Hirvonen A, Coutelle C, Ward PJ, Dayer P, Benhamou S. Role of alcohol dehydrogenase 3 and cytochrome P-4502E1 genotypes in susceptibility to cancers of the upper aerodigestive tract. Int J Cancer. 2000;87:734–740. [PubMed] [Google Scholar]
- 30.Lechevrel M, Casson AG, Wolf CR, Hardie LJ, Flinterman MB, Montesano R, Wild CP. Characterization of cytochrome P450 expression in human oesophageal mucosa. Carcinogenesis. 1999;20:243–248. doi: 10.1093/carcin/20.2.243. [DOI] [PubMed] [Google Scholar]
- 31.Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, Cordova D, Wang MR, Guo CH, Yu SZ, et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2000;9:73–80. [PubMed] [Google Scholar]
- 32.Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:843–854. [PubMed] [Google Scholar]
- 33.Haiman CA, Hankinson SE, Spiegelman D, Colditz GA, Willett WC, Speizer FE, Kelsey KT, Hunter DJ. The relationship between a polymorphism in CYP17 with plasma hormone levels and breast cancer. Cancer Res. 1999;59:1015–1020. [PubMed] [Google Scholar]
- 34.Le Marchand L, Sivaraman L, Pierce L, Seifried A, Lum A, Wilkens LR, Lau AF. Associations of CYP1A1, GSTM1, and CYP2E1 polymorphisms with lung cancer suggest cell type specificities to tobacco carcinogens. Cancer Res. 1998;58:4858–4863. [PubMed] [Google Scholar]
- 35.Persson I, Johansson I, Lou YC, Yue QY, Duan LS, Bertilsson L, Ingelman-Sundberg M. Genetic polymorphism of xenobiotic metabolizing enzymes among Chinese lung cancer patients. Int J Cancer. 1999;81:325–329. doi: 10.1002/(sici)1097-0215(19990505)81:3<325::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Tan W, Shao K. [Susceptibility to lung cancer in Chinese is associated with genetic polymorphism in cytochrome P4502E1] Zhonghua Zhong Liu Za Zhi. 2000;22:5–7. [PubMed] [Google Scholar]
- 37.Lin DX, Tang YM, Peng Q, Lu SX, Ambrosone CB, Kadlubar FF. Susceptibility to esophageal cancer and genetic polymorphisms in glutathione S-transferases T1, P1, and M1 and cytochrome P450 2E1. Cancer Epidemiol Biomarkers Prev. 1998;7:1013–1018. [PubMed] [Google Scholar]
- 38.Lin D, Tang Y, Peng Q. [Genetic polymorphisms of cytochrome P450 2E1 and glutathione S-transferase P1 and susceptibility to esophageal cancer] Zhonghua Zhong Liu Za Zhi. 1998;20:94–97. [PubMed] [Google Scholar]
- 39.Zhang HY, Shun CS, Li LS, Yan MX. Cytochrome P450 and the genetic susceptibility to esophageal carcinoma. Zhonghua Yufang Yixue Zazhi. 2000;34:69–71. [PubMed] [Google Scholar]
- 40.Tan W, Song N, Wang GQ, Liu Q, Tang HJ, Kadlubar FF, Lin DX. Impact of genetic polymorphisms in cytochrome P450 2E1 and glutathione S-transferases M1, T1, and P1 on susceptibility to esophageal cancer among high-risk individuals in China. Cancer Epidemiol Biomarkers Prev. 2000;9:551–556. [PubMed] [Google Scholar]
- 41.Kiss I, Sándor J, Pajkos G, Bogner B, Hegedüs G, Ember I. Colorectal cancer risk in relation to genetic polymorphism of cytochrome P450 1A1, 2E1, and glutathione-S-transferase M1 enzymes. Anticancer Res. 2000;20:519–522. [PubMed] [Google Scholar]
- 42.Nishimoto IN, Hanaoka T, Sugimura H, Nagura K, Ihara M, Li XJ, Arai T, Hamada GS, Kowalski LP, Tsugane S. Cytochrome P450 2E1 polymorphism in gastric cancer in Brazil: case-control studies of Japanese Brazilians and non-Japanese Brazilians. Cancer Epidemiol Biomarkers Prev. 2000;9:675–680. [PubMed] [Google Scholar]
- 43.Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province, China. World J Gastroenterol. 2000;6:671–675. doi: 10.3748/wjg.v6.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye WM, Yi YN, Luo RX, Zhou TS, Lin RT, Chen GD. Diet and gastric cancer: a casecontrol study in Fujian Province, China. World J Gastroenterol. 1998;4:516–518. doi: 10.3748/wjg.v4.i6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai L, Yu SZ. A molecular epidemiologic st udy on gastric cancer in Changle, Fujian Province. Shijie Huaren Xiaohua Zazhi. 1999;7:652–655. [Google Scholar]
- 46.Deng DJ, Yang SM, Li T, Xin HJ. Confirmation of N-(nitrosomethyl) urea as a nitrosourea derived by nitrosation of fish sauce. Biomed Environ Sci. 1999;12:54–61. [PubMed] [Google Scholar]
- 47.Deng DJ, Xin HJ. Formation of N-(nitrosomethyl) urea in stomachs of experimental pigs and human volunteers taken fish sauce. in vivo. In: 3rd Int gastric Cancer Congress. Monduzzi Editore S.p. A-Bologna (Italy) 1999:215–219. [Google Scholar]
- 48.Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi S, Watanabe J, Kawajiri K. Genetic polymorphisms in the 5'-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991;110:559–565. doi: 10.1093/oxfordjournals.jbchem.a123619. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Wang RT, Xu XP. Application of the interaction models between the polymorph ism (s) of metabolic gene (s) and environmental exposure. Zhonghua Liuxingbingxue Zazhi. 2001;22:61–64. [PubMed] [Google Scholar]


