Abstract
AIM: To explore the role of SF/HGF-Met autocrine and paracrine in met astasis of hepatocellular carcinoma (HCC).
METHODS: SF/HGF and c-met transcri ption and protein expression in HCC were examined by RT-PCR and Western Blot in 4 HCC cell lines, including HepG2, Hep3B, SMMC7721 and MHCC-1, the last cell line had a higher potential of metastasis. sf/hgf cDNA was transfected by the method of Lipofectin into SMMC7721. SF/HGF and c-met antibody were used to stimulate and block SF/HGF-c-met signal transduction. Cell morphology, mobility, and proliferation were respectively compared by microscopic observation, wound healing assay and cell growth curve.
RESULTS: HCC malignancy appeared to be relative to its met-SF/HGF expression. In MHCC-1, c-met expression was much stronger than that in other cell lines with lower potential of metastasis and only SF/HG F autocrine existed in MHCC-1. After sf/hgf cDNA transfection or conditioned medium of MHCC-1 stimulation, SMMC7721 changed into elongated morphology, and the abilities of proliferation (P < 0.05) and mobility increased. Such bio-activity could be blocked by c-met antibody (P < 0.05).
CONCLUSION: The system of SF/HGF-c- met autocrine and paracrine played an important role in development and metastas is potential of HCC. Inhibition of SF/HGF-c-met signal transduction system may reduce the growth and metastasis of HCC.
Keywords: hepatocyte growth factor/Scatter factor, c-met, hepatocellular carcinoma, metastasis
INTRODUCTION
The human proto-oncogene c-met encodes a Mr. 190000 heterodimeric transmembrane protein with structural features of a tyrosine kinase receptor and is expressed predominantly on epithelial cells. Its ligand HGF is a mesenchymal protein which is identical to Scatter factor (SF), a factor secreted by fibroblasts. It is well known that SF/HGF has a special binding with c-met, mainly attending mitogenic, motogenic and morphogenic effects on nor mal targeted cells[1-4]. Recently, its signal pathway has been explored a lot, including STAT in tubulogenesis[5], Gab-1 in cell growth[6,7], MAPK in morph ogenesis[8,9], and PI-3K in cell mitogenesis[10,11]. Its relationship with adhesive factors[12-16] and apoptotic factors[17,18] and the cross-talk between HGF/SF-c-Met and other growth factors[19] and proto-oncogenes[20] have also been discussed. However, in cancer research, controversial results have been found in various tumors. Some reports suggested that HGF/SF was potent stimula tor for tumor proliferation and motility[21-29], some reports made exactly opposite conclusions[30-33]. Among all the tissues, situation in liver becomes the most complicated, since SF/HGF attends all the stages of liver growth, regeneration, cirrhosis and carcinoma. SF/HGF was first found as a serum derived factor that stimulated proliferation of primary liver cells, acted as one of the initial factors in liver regeneration[34-35] and reversed cirrhosis by suppressing the increased TGF betal, fibrogenesis and hepatocyte apoptosis[36]. It has been unexpectedly reported to inhibit the growth of hepatoma cell in many reports[31]. Little has been known about the mechanism.
We studied a HGF/SF autocrine hepatocellular carcinoma (HCC) cell line which has a high potential of metastasis and compared its characteristic with several other HCC cell lines which do not have these features. Gene transfection and other interfering methods were used to further demonstrate the SF/HGF autocrine and paracrine on malignant capability of HCC.
MATERIALS AND METHODS
Cells
SMMC7721, HepG2, and Hep3B human HCC cell lines were purchased from Shanghai Institute of Cell Biology, Chinese Acadamy of Science (Shanghai, China). MHCC-1 was a cell line with high potential of metastasis from a resected lesion of a HCC patient. Cells were cultured in DMEM supplemented with 100 mL•L¯¹ fetal calf serum or AB serum (MHCC-1), penicillin (100 kU•L¯¹), streptomycin (100 kU•L¯¹).
Collection of MHCC-1 conditioned medium (MHCC-1-CM)
Cells were planted in 100 mL culture bottles. When 80% cells were subconfluent, they were washed and replaced with serum free DMEM (0.1 mL·cm-2). Three to five days later, conditioned medium was collected, centrifuged under 2000 r•min-1 for 20 min and stored at -20 °C until use.
Plasmid and DNA transfection
The plasmid pBS7.3 containing human full-length sf/hgf (kindly provided by Dr. George Vande Woode) was cloned into the BamH I-ApaI site of the pcDNA3.0(+) mammalian expression vector. SMMC7721 HCC cell line was transfected overnight with the constructed plasmid, using lipofectamine (Gibco). Cells were selected by G418. Colonies of cells were trypsinized within cloning rings, then they were transferred to 24-well dishes, and grown to confluency. After conditioned medium was changed to serum free DMEM for 3 d, both supertenant and whole lysised cells were screened for SF expression (ELISA, R&D). The highest expressing clone (SF7721) was selected for further research.
RT-PCR
Total RNA was extracted (QIAGEN) and 1 μg was reversing transcribed in a 25 μL volume using Super ScriptIITM (Gibco), according to the manufacturer’s instructions. Five μ L re verse transcription product was used for amplification with the following primer s: SF: 5’-CAGCGTTGGATTCTCAGTAT-3’, 5’-CCTATGTTTGTTCGTGTTGGA-3’. c-met: 5’-ACAGTGGCATGTCAACATCGCT-3’, 5’-GCTCGGTACTCTACAGATTC-3’. Forty cycles were performed, each consisting of 95 °C, 45 s; 60 °C, 1 mi n. There was a time delay for 7 min at 72 °C. The reaction products were visualized by 15 g•L¯¹ agarose gel electrophoresis.
Western blotting
Goat anti-human HGF antibody was purchased from R&D. Rabbit anti-human c-met anti body, anti-rabbit IgG-AP and antigoat IgG-AP were purchased from Santa Crus. Cells were washed with PBS, and lysed at 0 °C for 30 min in lysis buffer (TrisCl 50 mmol•L¯¹ pH8.0, NaCl 150 mmol•L¯¹, NaN3 0.2 g•L¯¹, PMSF 100 mg•L¯¹, Aprotinin 2 g•L¯¹, TritonX-100 10 g•L¯¹). In c-met detection, protein content was examined using BCATM Protein Assay (Pierce), and 20 μg protein per lane was electrophoreses on 80 g•L¯¹ SDS polyacrylamide gels after boiling for 5 min in 2 × loading buffer. As to SF/HGF, conditioned medium was mixed with 2 × loading buffer and added to 100 g •L¯¹ SDS polyacrymide gels. Protein was blotted onto nitrocellulose membranes. After electrobloting, the membranes were blocked in PBS-50 mg•L¯¹ non-fat dry milk, washed with PBS-Tween buffer, and incubated with the primary antibody (1:500) diluted in blocking buffer for 2 h. Membranes were then washed, incubated with the appropriate second antibody (1:500) in blocking buffer for 2 h, and re-was hed. Blotted proteins were stained with BICP-DAB (Huamei).
Cell scatter and morphology
5 × 103 cells of SMMC7721 and SF7721 were planted into the wells of 96-wells plates. Three replicated wells. Twenty-four hours later, wells were washed with PBS and fixed with ethanol and stained with Giemsa and examined under an inverted microscope. To explore the role of SF/HGF-c-met paracrine, 5 × 103 cells of SMMC7721 were planted into the wells of 96-wells plates. And 200 μL MHCC-1-CM was added into each well within diluted range 1:2, 1:4, 1:8, 0, respectively. Three replicated wells. Twenty-four hours later, cells were fixed and observed as above.
Cell migration assay
The ‘wound assay’ of Birch was used to determine the migratory capacity. 5 × 104 SMMC7721 or SF7721 cells were plan ted into the wells of 24-well plates and cultured 24-48 h until the cultures were subconfluent. A wound track was scored in each well. In paracrine system detection, 5 × 104 SMMC7721 were planted into the wells of 24-well plates and cultured 24-48 h until the cultures were subconfluent. A wound track was scored in each well. Conditioned medium was replaced in the experimental group with 1 mL MHCC-1 - CM and 1 mL control medium, control group with 1 mL surum free DMEM and 1 mL control medium. Replicated wells were terminated at 16, 24 and 48 h after wounding and examined under inverted microscope.
Cell growth curve
5 × 104 SMMC7721 or SF7721 were planted into 24-well plates, respectively. From the next day, cells of triplicate wells were digested by typsin and counted under microscope for 8 d. The average was recorded as cell number of the day. In addition, 2 × 104 SMMC 7721 cells were planted into the wells of 96-wells plates. Twenty-four hours later, 200 μL MH CC-1-CM was added into each well within diluted range 1:2, 1:4, 1:8, 0, res pectively. Three replicated wells. The effects of MHCC-1-CM on SMMC7721 were also contrasted using SMMC7721-CM. After 72 h incubation, 20 μL MTT (Methabenzthiazur on, Serva Co, USA) was added into each well to a final concentration at 50 g•L¯¹. Four hours later, the medium with 100 μL DMSO was replaced, and asorbance (OD) was read under 540 nm (Bio-rad).
Inhibition assay by c-met antibody
2 × 104 MHCC-1 or S F7721 were planted in 96-well plates, 3 replicated wells. After 24 h incubation, cells were washed by free DMEM, and incubated in 50 μL c-met polyantibody within a concentration range of 20, 10, 5, 2.5, 0 mg•L¯¹. Rabbit IgG was used as control. Two hours later, 150 μL control medium was added into each well and 72 h later, cells were examined by MTT. As to SMMC7721, 104 cells incubated with 2 μg c-met antibody for 2 h. Afterward, cells were washed by D-Hanks and added into wells of 96-well plates within 100 μL conditioned medium and 100 μL MHCC-1-CM or SM MC7721-CM, respectively. Twenty-four hours later, cells were observed under micros cope. Rabbit IgG was used as negative control.
Statistical analysis
In SMMC7721 cell proliferation experiments, Student’s t test was used to compare the difference of each corresponding dosage groups. Values were expressed as means ± standard. The cell growth curve and c-met blocking assay were tested by two-way anova.
RESULTS
SF and c-met expression in HCC cell lines (Figure 1)
Figure 1.
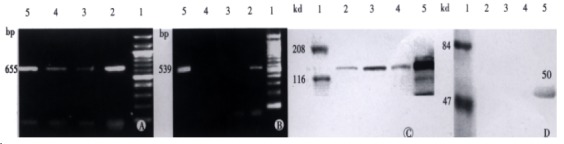
SF/HGF and c-met expression in HCC cell lines. A: c-met transcription; B: SF/HGF transcription 1: Mark; 2: MHCC-1; 3: HepG2; 4: Hep3B; 5: SMMC7721; 6: pBS7.3; C: c-met protein expression; D: SF/HGF protein expression (1: Mark; 2: SMMC7721; 3: Hep3B; 4: HepG2; 5: MHCC-1).
RT-PCR showed that all the cell lines had the transcription and protein expression of c-met, in which MHCC-1 most actively expressed. Only MHCC-1 had the transcription and protein expression of SF/HGF to the medium.
SF/HGF expression in SF7721
After sf/hgf transfection, in SF7721 cell extracts, the highest expression of SF/HGF reached 692 μg•L¯¹, compared with 0.026 μg•L¯¹ in control cells. However, no SF/HGF was detected in conditioned medium.
SF/HGF-c-met autocrine stimulate HCC malignancy
After gene transfection, SF7721 cells displayed scattered distribution and elongated morphology (Figure 2A) to get her with increased ability of proliferation. The cell growth curve showed that after 8 d, cell number of SF7721 reached almost double of that of SMMC7721 (Figure 2C, P < 0.05). Also, the transfected SF7721 acquired stronger mobility. In “wound healing assay”, SF7721 moved faster than SMMC7721 cells into cell free area (Figure 2B). However, the c-met expression in SF7721 and SMMC7721 did not show great difference.
Figure 2.
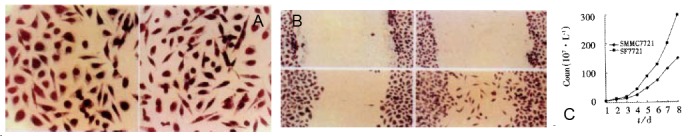
A: cell morphology comparison. × 400. Left: SMMC7721; Right: SF7721; Up: Control at “0” time ; Left down: SMMC7721 at 16 h after wound healing; Right down: SF7721 at 16 h after wound helaing; B: Cell morphology comparison × 400; C: Cell growth curve before and after gene transfection.
Role of SF/HGF-c-met paracrine
The paracrine system of SF/HGF-c-met was examined by stimulating SMMC7721 with MHCC-1-CM. Results were consistent with that in autocrine. The proliferation of SMMC7721 was stimulated by MHCC-1-CM at 1:2 dilution (Table 1, P < 0.01). In addition, MHC C-1- CM increased the mobility of SMMC7721 cells in wound healing assay and induced the elongated morphology displayed in SF7721 cells. (Data not shown).
Table 1.
MHCC-1-CM stimulated proliferation of SMMC7721 (contrasted by SMMC7721-CM, mean ± SD)
| Group | 0 |
Conditioned medium/culture medium (volume ratio) |
|||
| 1:16 | 1:8 | 1:4 | 1:2b | ||
| SMMC7721-CM | 0.596 ± 0.090 | 0.535 ± 0.315 | 0.568 ± 0.099 | 0.524 ± 0.053 | 0.541 ± 0.377 |
| MHCC-1-CM | 0.513 ± 0.043 | 0.581 ± 0.080 | 0.570 ± 0.061 | 0.620 ± 0.033 | 0.853 ± 0.031 |
P < 0.01, 0.541 ± 0.377 vs 0.853 ± 0.031.
Assay of C-met antibody blocking
Both cell proliferation and motility could be blocked by c-met antibody. After 3-day’s incubation with c-met antibody, either MHCC- 1 or SF7721 showed inhibition of growth, and was correlative with antibody concentration (Figure 3, P < 0.05). As to SMMC7721, the effect of inhibition could be seen under microscope, their shape turned round, and became shrank.
Figure 3.
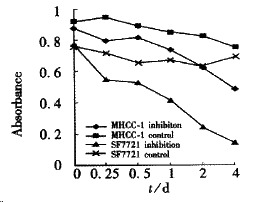
C-met inhibition assay.
DISCUSSION
Metastatic dissemination of solid tumors is a complex patho-physiological process including various factors. When we started to research on a HCC cell line (M HCC-1) with high potential of metastasis, we found that it had a high expression of c-met and with SF/HGF autocrine, which did not exist in other cell lines without or with low potential of metastasis. Thus, it became a promising approach to discuss c-met-HGF/SF signal transduction and tumor metastasis. Our result showed that cell malignancy of HCC is relative to its SF/HGF-c-met expression. The c-met expression of cell line with a higher potential of metastasis is much stronger than those with lower potential of metastasis and appeared with SF/HGF autocrine. Previous studies reported that many cancer cells expressed HGF/SF and c-met in vivo, but few of carcinoma cell lines produced HGF/SF in vitro, indicating that SF/HGF is a negative regulator in tumor progression. To further eluciate the phenomena, we transfected SF/HGF cDNA into S MMC7721 cell line, trying to demonstrate that acquired SF/HGF autocrine may increase the malignancy and improve the metastatic potential in less metastatic cells. Our results showed that the proliferation, mobility and cell morphology had greatly changed in SF7721 cells. Although the c-met expression in SF7721 cell did not increase significantly, it did improved greatly in SF7721 tumors in nude mice assay, later in the in vivo research. (Data not shown) When SF/HGF-c-met system was blocked by c-met antibldy, both MHCC-1 and SF7721 were blocked, demonstrating that SF/HGF-c-met was a positive regulator in HCC progression. Thus, we postulated that carcinoma cells may lose the ability to produce HGF/SF during in vitro passage, or the expression of HGF/SF need an activation from matrix. The high potential of metastasis of MHCC-1 may, to a great extent, contribute to its preservation of HGF/SF expression and keep the c-met activated all the way. In vivo, fibroblasts can produce HGF/SF, which may induce the expression of HGF/SF and c-met in cancer cells, thus establishing an autocrine and paracrine system and promoting cell scatter, proliferation and invasion[37-39]. We also studied the paracrine role of HGF/SF by stimulating SMMC7721 with MHCC-1-CM. Results were cons is tent with that from experiment of autocrine. Cell scatter, proliferation and mobility in SMMC7721 increased after they were stimulated by conditioned medium of M HCC-1. Such biological activities can be blocked by anti c-met polyclonal anti body. However, the influence of SMMC-7721 proliferation by the conditioned medium of MHCC-1 happened only when the medium was in 1:2 dilution, suggesting that the c-met receptor needs a certain amount of SF/HGF to activate. Once activated, the biological activity may depend on the quantity of c-met expression and the extent of receptor phosphorylation, thereby initiating downstream regulations. There were two reasons why we choose c-met rather than SF/HGF to be blocked. One was the conflicting reports of SF/HGF. Up to now, various of SF/HGF variants have been found, each having different structure and bioactivity in vitro and/or in vivo. This could be another reason why different results of SF/HGF are reported[40-47]. Compared with HGF/SF, c-met introduces many biological functions, but all the signal transductions start from a same ‘multifunctional docking site’[4,5,48]. The different biological functions come from different signal messages, thus making it a better choice compared with HGF/SF and other members of tyrosine kinase family. Recently, c-met inhibition has become a hot spot in anticancer research[1,2,49-51]. In our research, the tumor cells blocked by c-met antibody shrank in morphology and decreased in cell proliferation. Results suggested that the inhibition of met-SF/HGF could become one of the potential approaches to reduce tumor growth and metastasis. In conclusion, our experiment showed that the system of SF/HGF-c-met autocrine and paracrine play an important role in invasion and metastasis of hepatocellular carcinoma. Inhibition of c-met-H GF/SF system may reduce the proliferation and metastasis of hepatocellular carcinoma by lowering the expression of c-met or its downstream signal transduction.
ACKNOWLEDGEMENTS
Dr. George Vande Woude providing us PBS7.3 and Dr. Bo-Heng Zhang performing stastical analysis.
Footnotes
Edited by Xu XQ, Wang JH and Ma JY
Supported by Natural Science Foundation of China No.39970290
References
- 1.Cao B, Su Y, Oskarsson M, Zhao P, Kort EJ, Fisher RJ, Wang LM, Vande Woude GF. Neutralizing monoclonal antibodies to hepatocyte growth factor/scatter factor (HGF/SF) display antitumor activity in animal models. Proc Natl Acad Sci USA. 2001;98:7443–7448. doi: 10.1073/pnas.131200498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atabey N, Gao Y, Yao ZJ, Breckenridge D, Soon L, Soriano JV, Burke TR, Bottaro DP. Potent blockade of hepatocyte growth factor-stimulated cell motility, matrix invasion and branching morphogenesis by antagonists of Grb2 Src homology 2 domain interactions. J Biol Chem. 2001;276:14308–14314. doi: 10.1074/jbc.M010202200. [DOI] [PubMed] [Google Scholar]
- 3.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 4.Shaharabany M, Abramovitch R, Kushnir T, Tsarfaty G, Ravid-Megido M, Horev J, Ron R, Itzchak Y, Tsarfaty I. In vivo molecular imaging of met tyrosine kinase growth factor receptor activity in normal organs and breast tumors. Cancer Res. 2001;61:4873–4878. [PubMed] [Google Scholar]
- 5.Boccaccio C, Andò M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 6.Gual P, Giordano S, Anguissola S, Parker PJ, Comoglio PM. Gab1 phosphorylation: a novel mechanism for negative regulation of HGF receptor signaling. Oncogene. 2001;20:156–166. doi: 10.1038/sj.onc.1204047. [DOI] [PubMed] [Google Scholar]
- 7.Sachs M, Brohmann H, Zechner D, Müller T, Hülsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriuchi A, Hirono S, Ido A, Ochiai T, Nakama T, Uto H, Hori T, Hayashi K, Tsubouchi H. Additive and inhibitory effects of simultaneous treatment with growth factors on DNA synthesis through MAPK pathway and G1 cyclins in rat hepatocytes. Biochem Biophys Res Commun. 2001;280:368–373. doi: 10.1006/bbrc.2000.4063. [DOI] [PubMed] [Google Scholar]
- 9.Karihaloo A, O'Rourke DA, Nickel C, Spokes K, Cantley LG. Differential MAPK pathways utilized for HGF- and EGF-dependent renal epithelial morphogenesis. J Biol Chem. 2001;276:9166–9173. doi: 10.1074/jbc.M009963200. [DOI] [PubMed] [Google Scholar]
- 10.Day RM, Cioce V, Breckenridge D, Castagnino P, Bottaro DP. Differential signaling by alternative HGF isoforms through c-Met: activation of both MAP kinase and PI 3-kinase pathways is insufficient for mitogenesis. Oncogene. 1999;18:3399–3406. doi: 10.1038/sj.onc.1202683. [DOI] [PubMed] [Google Scholar]
- 11.Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- 12.Wielenga VJ, van der Voort R, Taher TE, Smit L, Beuling EA, van Krimpen C, Spaargaren M, Pals ST. Expression of c-Met and heparan-sulfate proteoglycan forms of CD44 in colorectal cancer. Am J Pathol. 2000;157:1563–1573. doi: 10.1016/S0002-9440(10)64793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trusolino L, Serini G, Cecchini G, Besati C, Ambesi-Impiombato FS, Marchisio PC, De Filippi R. Growth factor-dependent activation of alphavbeta3 integrin in normal epithelial cells: implications for tumor invasion. J Cell Biol. 1998;142:1145–1156. doi: 10.1083/jcb.142.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiscox S, Jiang WG. Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells. Biochem Biophys Res Commun. 1999;261:406–411. doi: 10.1006/bbrc.1999.1002. [DOI] [PubMed] [Google Scholar]
- 15.Ried S, Jäger C, Jeffers M, Vande Woude GF, Graeff H, Schmitt M, Lengyel E. Activation mechanisms of the urokinase-type plasminogen activator promoter by hepatocyte growth factor/scatter factor. J Biol Chem. 1999;274:16377–16386. doi: 10.1074/jbc.274.23.16377. [DOI] [PubMed] [Google Scholar]
- 16.van der Voort R, Taher TE, Wielenga VJ, Spaargaren M, Prevo R, Smit L, David G, Hartmann G, Gherardi E, Pals ST. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem. 1999;274:6499–6506. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura S, Kondo S, Shinomura Y, Kanayama S, Miyazaki Y, Kiyohara T, Hiraoka S, Matsuzawa Y. Met/HGF receptor modulates bcl-w expression and inhibits apoptosis in human colorectal cancers. Br J Cancer. 2000;83:668–673. doi: 10.1054/bjoc.2000.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 20.Follenzi A, Bakovic S, Gual P, Stella MC, Longati P, Comoglio PM. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 21.Wong AS, Pelech SL, Woo MM, Yim G, Rosen B, Ehlen T, Leung PC, Auersperg N. Coexpression of hepatocyte growth factor-Met: an early step in ovarian carcinogenesis. Oncogene. 2001;20:1318–1328. doi: 10.1038/sj.onc.1204253. [DOI] [PubMed] [Google Scholar]
- 22.Sowter HM, Corps AN, Smith SK. Hepatocyte growth factor (HGF) in ovarian epithelial tumour fluids stimulates the migration of ovarian carcinoma cells. Int J Cancer. 1999;83:476–480. doi: 10.1002/(sici)1097-0215(19991112)83:4<476::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Teofili L, Di Febo AL, Pierconti F, Maggiano N, Bendandi M, Rutella S, Cingolani A, Di Renzo N, Musto P, Pileri S, et al. Expression of the c-met proto-oncogene and its ligand, hepatocyte growth factor, in Hodgkin disease. Blood. 2001;97:1063–1069. doi: 10.1182/blood.v97.4.1063. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka H, Hamasuna R, Itoh H, Kitamura N, Koono M. Activation of hepatocyte growth factor/scatter factor in colorectal carcinoma. Cancer Res. 2000;60:6148–6159. [PubMed] [Google Scholar]
- 25.Bredin CG, Liu Z, Hauzenberger D, Klominek J. Growth-factor-dependent migration of human lung-cancer cells. Int J Cancer. 1999;82:338–345. doi: 10.1002/(sici)1097-0215(19990730)82:3<338::aid-ijc6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.Camp RL, Rimm EB, Rimm DL. Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer. 1999;86:2259–2265. doi: 10.1002/(sici)1097-0142(19991201)86:11<2259::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Presnell SC, Hooth MJ, Borchert KM, Coleman WB, Grisham JW, Smith GJ. Establishment of a functional HGF/C-MET autocrine loop in spontaneous transformants of WB-F344 rat liver stem-like cells. Hepatology. 1998;28:1253–1259. doi: 10.1002/hep.510280513. [DOI] [PubMed] [Google Scholar]
- 28.Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, et al. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58:5157–5167. [PubMed] [Google Scholar]
- 29.Nakashiro K, Okamoto M, Hayashi Y, Oyasu R. Hepatocyte growth factor secreted by prostate-derived stromal cells stimulates growth of androgen-independent human prostatic carcinoma cells. Am J Pathol. 2000;157:795–803. doi: 10.1016/s0002-9440(10)64593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronen D, Altstock RT, Firon M, Mittelman L, Sobe T, Resau JH, Vande Woude GF, Tsarfaty I. Met-HGF/SF mediates growth arrest and differentiation in T47D breast cancer cells. Cell Growth Differ. 1999;10:131–140. [PubMed] [Google Scholar]
- 31.von Schweinitz D, Faundez A, Teichmann B, Birnbaum T, Koch A, Hecker H, Glüer S, Fuchs J, Pietsch T. Hepatocyte growth-factor-scatter factor can stimulate post-operative tumor-cell proliferation in childhood hepatoblastoma. Int J Cancer. 2000;85:151–159. doi: 10.1002/(SICI)1097-0215(20000115)85:2<151::AID-IJC1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Matteucci E, Castoldi R, Desiderio MA. Hepatocyte growth factor induces pro-apoptotic genes in HepG2 hepatoma but not in B16-F1 melanoma cells. J Cell Physiol. 2001;186:387–396. doi: 10.1002/1097-4652(2000)9999:9999<000::AID-JCP1033>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Qadan LR, Perez-Stable CM, Schwall RH, Burnstein KL, Ostenson RC, Howard GA, Roos BA. Hepatocyte growth factor and vitamin D cooperatively inhibit androgen-unresponsive prostate cancer cell lines. Endocrinology. 2000;141:2567–2573. doi: 10.1210/endo.141.7.7546. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu M, Hara A, Okuno M, Matsuno H, Okada K, Ueshima S, Matsuo O, Niwa M, Akita K, Yamada Y, et al. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology. 2001;33:569–576. doi: 10.1053/jhep.2001.22650. [DOI] [PubMed] [Google Scholar]
- 35.Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954–3960. [PubMed] [Google Scholar]
- 36.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 37.Tokunou M, Niki T, Eguchi K, Iba S, Tsuda H, Yamada T, Matsuno Y, Kondo H, Saitoh Y, Imamura H, et al. c-MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol. 2001;158:1451–1463. doi: 10.1016/S0002-9440(10)64096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess S, Gulati R, Peluso JJ. Hepatocyte growth factor induces rat ovarian surface epithelial cell mitosis or apoptosis depending on the presence or absence of an extracellular matrix. Endocrinology. 1999;140:2908–2916. doi: 10.1210/endo.140.6.6773. [DOI] [PubMed] [Google Scholar]
- 39.Blanquaert F, Delany AM, Canalis E. Fibroblast growth factor-2 induces hepatocyte growth factor/scatter factor expression in osteoblasts. Endocrinology. 1999;140:1069–1074. doi: 10.1210/endo.140.3.6553. [DOI] [PubMed] [Google Scholar]
- 40.Jakubczak JL, LaRochelle WJ, Merlino G. NK1, a natural splice variant of hepatocyte growth factor/scatter factor, is a partial agonist in vivo. Mol Cell Biol. 1998;18:1275–1283. doi: 10.1128/mcb.18.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsuka T, Jakubczak J, Vieira W, Bottaro DP, Breckenridge D, Larochelle WJ, Merlino G. Disassociation of met-mediated biological responses in vivo: the natural hepatocyte growth factor/scatter factor splice variant NK2 antagonizes growth but facilitates metastasis. Mol Cell Biol. 2000;20:2055–2065. doi: 10.1128/mcb.20.6.2055-2065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang WG, Hiscox SE, Parr C, Martin TA, Matsumoto K, Nakamura T, Mansel RE. Antagonistic effect of NK4, a novel hepatocyte growth factor variant, on in vitro angiogenesis of human vascular endothelial cells. Clin Cancer Res. 1999;5:3695–3703. [PubMed] [Google Scholar]
- 43.Maehara N, Matsumoto K, Kuba K, Mizumoto K, Tanaka M, Nakamura T. NK4, a four-kringle antagonist of HGF, inhibits spreading and invasion of human pancreatic cancer cells. Br J Cancer. 2001;84:864–873. doi: 10.1054/bjoc.2000.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuba K, Matsumoto K, Ohnishi K, Shiratsuchi T, Tanaka M, Nakamura T. Kringle 1-4 of hepatocyte growth factor inhibits proliferation and migration of human microvascular endothelial cells. Biochem Biophys Res Commun. 2000;279:846–852. doi: 10.1006/bbrc.2000.4034. [DOI] [PubMed] [Google Scholar]
- 45.Bell A, Chen Q, DeFrances MC, Michalopoulos GK, Zarnegar R. The five amino acid-deleted isoform of hepatocyte growth factor promotes carcinogenesis in transgenic mice. Oncogene. 1999;18:887–895. doi: 10.1038/sj.onc.1202379. [DOI] [PubMed] [Google Scholar]
- 46.Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–6743. [PubMed] [Google Scholar]
- 47.Matsumoto K, Kataoka H, Date K, Nakamura T. Cooperative interaction between alpha- and beta-chains of hepatocyte growth factor on c-Met receptor confers ligand-induced receptor tyrosine phosphorylation and multiple biological responses. J Biol Chem. 1998;273:22913–22920. doi: 10.1074/jbc.273.36.22913. [DOI] [PubMed] [Google Scholar]
- 48.Tulasne D, Paumelle R, Weidner KM, Vandenbunder B, Fafeur V. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering. Mol Biol Cell. 1999;10:551–565. doi: 10.1091/mbc.10.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abounader R, Ranganathan S, Lal B, Fielding K, Book A, Dietz H, Burger P, Laterra J. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J Natl Cancer Inst. 1999;91:1548–1556. doi: 10.1093/jnci/91.18.1548. [DOI] [PubMed] [Google Scholar]
- 50.Webb CP, Hose CD, Koochekpour S, Jeffers M, Oskarsson M, Sausville E, Monks A, Vande Woude GF. The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network. Cancer Res. 2000;60:342–349. [PubMed] [Google Scholar]
- 51.Michieli P, Basilico C, Pennacchietti S, Maffè A, Tamagnone L, Giordano S, Bardelli A, Comoglio PM. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene. 1999;18:5221–5231. doi: 10.1038/sj.onc.1202899. [DOI] [PubMed] [Google Scholar]


