Abstract
AIM: To evaluate the relationship between the expression of lipopolysaccharides (LPS) binding protein (LBP) and CD14 mRNA and the severity of liver injury in alcohol-fed rats.
METHODS: Twenty Wistar rats were divided into two groups: ethanol-fed group (group E) and control group (group C). Group E was fed with ethanol (5-12 g·kg¯¹·d¯¹) and group C received dextrose instead of ethanol. Rats of the two groups were sacrificed at 4 wk and 8 wk. Levels of endotoxin and alanine transaminase (ALT) in blood were measured, and liver pathology was observed under light and electronic microscopy. Expressions of LBP and CD14 mRNA in liver tissues were determined by RT-PCR analysis.
RESULTS: Plasma endotoxin levels were increased more significantly in group E (129 ± 21) ng·L¯¹ and (187 ± 35) ng·L¯¹ at 4 and 8 wk than in control rats (48 ± 9) ng·L¯¹ and (53 ± 11) ng·L¯¹, respectively (P < 0.05). Mean values of plasma ALT levels were (1867 ± 250) nkat·L¯¹ and (2450 ± 367) nkat·L¯¹ in Group E. The values were increased more dramatically in ethanol-fed rats than in Group C after 4 and 8 wk. In liver section from ethanol-fed rats, there were marked pathological changes (steatosis, cell infiltration and necrosis). In ethanol-fed rats, ethanol administration led to a significant increase in LBP and CD14 mRNA levels compared with the control group (P < 0.05).
CONCLUSION: Ethanol administration led to a significant increase in endotoxin levels in serum and LBP and CD14 mRNA expressions in liver tissues. The increase of LBP and CD14 mRNA expression might wake the liver more sensitive to endotoxin and liver injury.
Keywords: lipopolysaccharides/analysis; antigens, cd14/analysis; liver diseases; alcoholic/pathology; liver/pathology; liver/ultrastructure; rat; animal
INTRODUCTION
There is an accumulating evidence suggesting a role for endotoxin or lipopoly-saccharide (LPS) in the cause of alcohol-induced liver disease (ALD)[1-5]. Circulating LPS consists of several compounds including its specific carrier, the LPS binding protein (LBP)[6-8]. The LPS-LBP complex has high affinity to the LPS receptor CD14 located on monocytes/macrophages. CD14 is a 55-kD myeloid membrane glycoprotein, expressed mainly by monocytes and macrophages[7,9-11]. Attachment of LPS-LBP complex to the CD14 initiates a process leading to the release of cytokines and liver injury[13-17]. Although indirect evidence cited previously suggested an interaction among LPS, LBP and CD14 during ALD[18-20], a direct link is lacking. To evaluate the role of LBP and CD14 in ALD, the intragastric ethanol po rat model for ALD was used to study the relationship between the expression of LBP and CD14 genes and the severity of liver injury in ALD rats.
MATERIALS AND METHODS
Animals and treatments
Twenty adult female Wistar rats weighing 180 and 220 g were fed ad libitum a liquid diet. They were divided into two groups (ten rats/group): ethanol liquid diet group (group E) and control liquid diet group (group C). Group E were fed ethanol, and group C received the same diet but with isocaloric amounts of dextrose instead of ethanol. In the ethanol-fed rats, an initial dose of 5 g·kg¯¹·d¯¹. The ethanol concentration within the diet was gradually increased up to 12 g·kg¯¹·d¯¹ in 8 wk. All diets were kept fresh daily. They were anesthetized with sodium pentobarbital (30 mg·kg-1 intraperitoneally) and sacrificed at different time points (4 wk and 8 wk). Blood was withdrawn from the tail vein and liver samples were frozen in liquid nitrogen and stored at -70 °C before use.
Blood endotoxin and ALT
For determination of endotoxin, blood was collected into pyrogen-free tubes containing heparin. Plasma was immediately separated at 4 °C by centrifugation at 200 g for 8 minutes and stored in pyrogen-free tubes at -70 °C. Plasma endotoxin levels were measured within a week using the Limulus Amebocyte Lysate assay. Levels of endotoxin in plasma from normal rats were below the limits of detection. Serum alanine transaminase (ALT) was measured by standard enzymatic procedures.
Liver pathology
Liver samples from different liver lobes were fixed with 100 mL·L¯¹ buffered formalin or 25 g·L¯¹ glutaraldehyde immediately. For optical microscopy, the tissue blocks were embedded in paraffin, and stained with hematoxylin and eosin (HE). For electronic microscopy, the tissue blocks were embedded in Epon 618 resin and ultrathin sections were stained with urany acetate and lead citrate. AH-2000 transmission electron microscope was used.
RNA isolation and complementary DNA synthesis
Total RNA was isolated from rat liver tissue using the TRIZOL Reagent (Life Technologies, USA). The quality of RNA was controlled by the intactness of ribosomal RNA bands. A total of 0.5 mg of each intact total RNA samples was reverse-transcribed to complementary DNA (cDNA) using the reverse transcription polymerase chain reaction (RT-PCR) kit (Roche, USA). cDNA was stored at -70 °C until polymerase chain reaction (PCR) analysis.
Determination of LBP and CD14 mRNA by RT-PCR
The PCR primers used were LBP: sense (5’-GAGGCCTGAGTCTCTCCATCT-3’), antisense (5’-TCTGAGATGGCAAAGTAGACC-3’); CD14: sense (5’-CTCAACCTAGAGCCGTTTCT-3’), anti-sense (5’-CAGGATTGTCAGACAGGTCT-3’); β-actin: sense (5’-ACCACAGCTGAGAGGGA-AATCG-3’), antisense (5’-AGAGGTCTTTACGGATGTC-AACG-3’). The sizes of the amplified PCR products were 552 bp for LBP, 267 bp for CD14, and 281 bp for β-actin. The conditions for amplification were as follows: denaturation at 94 °C for 1 min, annealing at 58 °C for 2 min, and extension at 71 °C for 2 min for 28 cycles. The PCR products were electrophoresed in 20 g·L¯¹ agarose gels, and the gels were ethidium bromide stained and video photographed on an ultraviolet transilluminator.
Statistical analysis
All results were expressed as mean ± SD. Statistical difference between means were determined using two-way ANOVA or Student’s t test. A P value of ≤ 0.05 was considered significant.
RESULTS
Blood endotoxin and ALT levels
Plasma endotoxin levels in control rats were (48 ± 9) ng·L¯¹ and (53 ± 11) ng·L¯¹ at 4 wk and 8 wk, respectively. Plasma endotoxin levels in ethanol -fed rats were increased significantly by ethanol to values of (129 ± 21) ng·L¯¹ at 4 wk and (187 ± 35) ng·L¯¹ at 8 wk. The Levels of endotoxin were about 2-and 3-fold higher than the values from control rats (Figure 1). Me an values for ALT in the control animals were (517 ± 200) nkat·L¯¹ and (5 50 ± 150) nkat·L¯¹ at 4 wk and 8 wk, respectively. Plasma ALT levels were increased dramatically to (1867 ± 250) nkat·L¯¹ and (2450 ± 367) nkat·L¯¹ in ethanol-fed rats after 4 wk and 8 wk, respectively (Figure 2).
Figure 1.
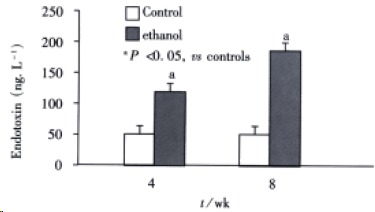
Chenges of endotoxin levels in two groups at different time points.
Figure 2.
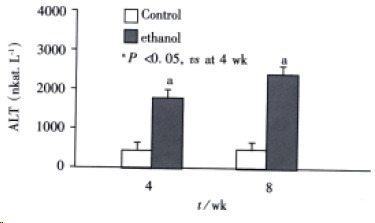
Changes of serum transaminase levels in two groups at different time points.
Pathological changes
Rats in the two groups, increased their body weight. Although the average weight gain was lower in ethanol-fed rats than the control rats, the differences between the two groups were not significant. Histopathological changes of the liver tissues were depicted in rep resentative photomicrographs in Figure 3A and 3B. None of the rats in the control group developed pathological changes in the liver at 4 wk or 8 wk. But, in liver section from rats after 4 wk on ethanol liquid diet, steatosis which was both microvesicular and macrovesicular and few in flammation but accumulation of blood cells in the sinusoidal lining can be seen. In liver section from rats after 8 wk on ethanol diet, there was marked pathological changes (steatosis, cell infiltration and necrosis). Under electron microscopy, focal cytoplasmic degeneration and necrosis could be seen in hepatocytes of ethanol-fed rats (Figure 4A and B).
Figure 3.
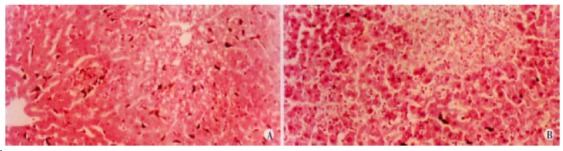
Liver section from rats (HE × 200) A: 4wk after ethanol liquid diet, steatosis and accumulation of blood cells in the sinusoidal lining; B: 8wk after ethanol diet, steatosis, cell infiltration and necrosis.
Figure 4.
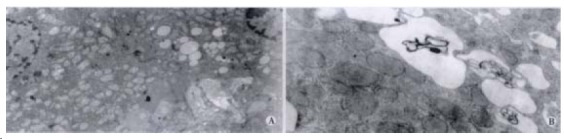
Ethanol-fed rats. A: Steatosis degeneration and necrosis in hepatocytes TEM × 5000 B: Focal cytoplasmic degeneration and many myelin figures (TEM × 20000).
Expression of LBP and CD14 mRNA in the liver
The livers of rats in the individual group were examined for LBP and CD14 mRNA expression by RT-PCR (Figure 5). In the control rats, there was no significant difference in the levels of LBP and C D14 at 4 wk and 8 wk. Ethanol administration led to a significant increase in LBP and CD14 mRNA levels compared with the control group (P < 0.05).The levels of LBP and CD14 mRNA in ethanol-fed rats were significantly higher in 8 wk than in 4 wk (P < 0.05).The highest levels of CD14 mRNA were seen in ethanol-fed rats after 8 wk (Figure 6, Figure 7).
Figure 5.
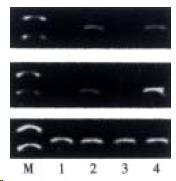
Expression of LBP and CD14 mRNA by RT-PCR analysis. M: Marker; Lane 1, 3: Group C in 4, 8 wk respectively. Lane 2, 4: Group E in 4, 8 wk.
Figure 6.
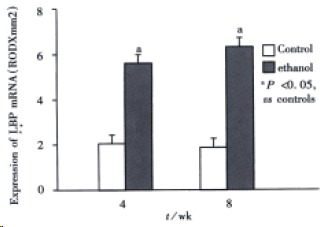
Expression of LBP mRNA.
Figure 7.
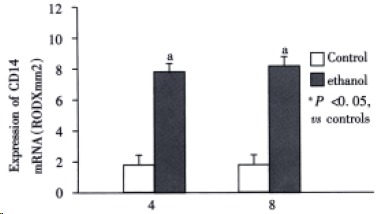
Expression of CD14 mRNA.
DISCUSSION
It is well documented that liver disease can result from the dose- and time-dependent consumption of alcohol[23-25], female rats exhibit greater susceptibity to early alcohol-induced injury than males[26-28], Glycine prevents alcohol-induced liver injury by decreasing alcohol in the stomach[29]. However, mechanisms remain unclear. There appears to be increasing evidences that ethanol toxicity is associated with increased level of endotoxin in plasma[1,2,31]. Endotoxin or LPS is believed to exert many of its effects on the liver injury via interaction with LBP and CD14[11-18]. LBP and CD14 are clearly implicated in the molecular and cellular basis of the interaction between endotoxin and monocytes/macrophages. LBP in serum can recognize and bind LPS to form LPS-LBP complexes and activate cells through the CD14 receptor on membrane of these cells, initiate a process leading to the release of cytokines (e.g. tumor necrosis factor a and interleukines), prostanoids, and other soluble mediators[24,31-34].The release of these mediators is considered to be an early key step in the pathogenesis of liver disease because they trigger inflammatory events in the liver and alter the parenchymal homeostasis, ultimately initiating liver injury[35-38].
A major goal of this study was to observe the expression of LBP and CD14 mRNA in ethanol-fed rats and evaluate the in role in ALD. It was found that endotoxin levels in the plasma of rats treated with ethanol were increased significantly when compared with control animals and fatty liver, necrosis, and inflammation were developed in the ethanol treated rat. Control rats showed no liver pathology. In the present study, we found the severity of pathological changes in ethanol-fed rats were accompanied by an increase in intrahepatic LBP and CD14 mRNA levels and serum ALT levels. The increase in LBP and CD14 mRNA levels in the ethanol-fed rats is correlated with the degree of inflammation and necrosis in the livers of these animals. A similar pattern of changes was observed by Yin, et al[39]. They found that blood endotoxin and hepatic levels of CD14 messenger RNA and protein were increased by ethanol. Therefore, the sensitivity of rat liver to alcohol-induced injury is directly related to CD14 expression in the liver that lead to increasing the production of TNF-α, free radicals, interleukins and other cytokines[40-44]. The marked increase in CD14 expression suggests a new mechanism by which alcohol increases the LPS-mediated cytokine signaling by the liver macrophages, thus promoting the interaction between alcohol and endotoxins in the development of liver damage[45-50].
It has been well established that the role of LBP is to augment the response of monocytes/macrophages to low levels of endotoxin via interaction with CD14 protein and play an important role in alcoholic liver injury[36]. The increase in intrahepatic CD14 m RNA expression may represent either an increase in the expression of CD14 within cells that reside in the liver or may represent recruitment of inflammatory cells (e.g., infiltrating mononuclear cells or macrophages) that have high expression of CD14 gene and CD14 protein [39,51-53]. In either case, an increase of CD14 may result in greater sensitivity to endotoxin and NF-κB activation and production of pro-inflammatory cytokines which mediate liver injury[54-57].
In summary, our results show that ethanol administ ration led to a significant increase in LBP and CD14 mRNA levels in ethanol-fed rats when compared with the control rats. Increase of LBP and CD14 mRNA expression may result in greater sensitivity to endotoxin and liver injury. However, the mechanism of LBP increase and CD14 mRNA express ion is thus as yet unclear and needs further studies.
Footnotes
Edited by Wang YQ and Ma JY
Supported by the National Natural Science Foundation of China (No.39970719).
References
- 1.Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- 2.Zhang SC, Dai Q, Wang JY, He BM, Zhou K. Gut derived endotoxemia: one of the factors leading to production of cytokines in liver diseases. World J Gastroenterol. 2000;6(Suppl 3):16. [Google Scholar]
- 3.Gordon H. Detection of alcoholic liver disease. World J Gastroenterol. 2001;7:297–302. doi: 10.3748/wjg.v7.i3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331–334. doi: 10.3748/wjg.v7.i3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680–1689. doi: 10.1002/hep.510290633. [DOI] [PubMed] [Google Scholar]
- 6.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- 7.Heumann D, Adachi Y, Le Roy D, Ohno N, Yadomae T, Glauser MP, Calandra T. Role of plasma, lipopolysaccharide-binding protein, and CD14 in response of mouse peritoneal exudate macrophages to endotoxin. Infect Immun. 2001;69:378–385. doi: 10.1128/IAI.69.1.378-385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amura CR, Kamei T, Ito N, Soares MJ, Morrison DC. Differential regulation of lipopolysaccharide (LPS) activation pathways in mouse macrophages by LPS-binding proteins. J Immunol. 1998;161:2552–2560. [PubMed] [Google Scholar]
- 9.Fearns C, Loskutoff DJ. Role of tumor necrosis factor alpha in induction of murine CD14 gene expression by lipopolysaccharide. Infect Immun. 1997;65:4822–4831. doi: 10.1128/iai.65.11.4822-4831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler-Rodriguez AM, Zhang H, Lichenstein HS, Qureshi N, Niesel DW, Crowe SE, Peterson JW, Klimpel GR. Neutrophil activation by bacterial lipoprotein versus lipopolysaccharide: differential requirements for serum and CD14. J Immunol. 2000;164:2674–2683. doi: 10.4049/jimmunol.164.5.2674. [DOI] [PubMed] [Google Scholar]
- 12.Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saitoh O, Sugi K, Lojima K, Matsumoto H, Nakagawa K, Kayazawa M, Tanaka S, Teranishi T, Hirata I. Increased prevalence of intestinal inflammation in patients with liver cirrhosis. World J Gastroenterol. 1999;5:391–396. doi: 10.3748/wjg.v5.i5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LT, Zhang B, Chen JJ. Effect of anti-fibrosis compound on collagen expression of hepatic cells in experimental liver fibrosis of rats. World J Gastroenterol. 2000;6:877–880. doi: 10.3748/wjg.v6.i6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai WJ, Jiang HC. Advances in gene therapy of liver cirrhosis: a review. World J Gastroenterol. 2001;7:1–8. doi: 10.3748/wjg.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu BH, Chen HS, Zhou JH, Xiao N. Effects of endotoxin on endothelin receptor in hepatic and intestinal tissues after endotoxemia in rats. World J Gastroenterol. 2000;6:298–300. doi: 10.3748/wjg.v6.i2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtman SN, Wang J, Lemasters JJ. LPS receptor CD14 participates in release of TNF-alpha in RAW 264.7 and peritoneal cells but not in kupffer cells. Am J Physiol. 1998;275:G39–G46. doi: 10.1152/ajpgi.1998.275.1.G39. [DOI] [PubMed] [Google Scholar]
- 18.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- 19.Järveläinen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148–1153. doi: 10.1053/jhep.2001.24236. [DOI] [PubMed] [Google Scholar]
- 20.Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, Seitz HK. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- 21.Järveläinen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. J Hepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 22.Järveläinen HA, Oinonen T, Lindros KO. Alcohol-induced expression of the CD14 endotoxin receptor protein in rat Kupffer cells. Alcohol Clin Exp Res. 1997;21:1547–1551. [PubMed] [Google Scholar]
- 23.Reeves HL, Burt AD, Wood S, Day CP. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25:677–683. doi: 10.1016/s0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- 24.Bautista AP. Chronic alcohol intoxication induces hepatic injury through enhanced macrophage inflammatory protein-2 production and intercellular adhesion molecule-1 expression in the liver. Hepatology. 1997;25:335–342. doi: 10.1002/hep.510250214. [DOI] [PubMed] [Google Scholar]
- 25.Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin Exp Res. 2001;25:579–589. [PubMed] [Google Scholar]
- 26.Iimuro Y, Frankenberg MV, Arteel GE, Bradford BU, Wall CA, Thurman RG. Female rats exhibit greater susceptibility to early alcohol-induced liver injury than males. Am J Physiol. 1997;272:G1186–G1194. doi: 10.1152/ajpgi.1997.272.5.G1186. [DOI] [PubMed] [Google Scholar]
- 27.Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- 28.Thurman RG. Sex-related liver injury due to alcohol involves activation of Kupffer cells by endotoxin. Can J Gastroenterol. 2000;14 Suppl D:129D–135D. doi: 10.1155/2000/735262. [DOI] [PubMed] [Google Scholar]
- 29.Iimuro Y, Bradford BU, Forman DT, Thurman RG. Glycine prevents alcohol-induced liver injury by decreasing alcohol in the rat stomach. Gastroenterology. 1996;110:1536–1542. doi: 10.1053/gast.1996.v110.pm8613061. [DOI] [PubMed] [Google Scholar]
- 30.Yin M, Ikejima K, Arteel GE, Seabra V, Bradford BU, Kono H, Rusyn I, Thurman RG. Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther. 1998;286:1014–1019. [PubMed] [Google Scholar]
- 31.Järveläinen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology. 1999;29:1503–1510. doi: 10.1002/hep.510290508. [DOI] [PubMed] [Google Scholar]
- 32.von Baehr V, Döcke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, Baumgarten R, Volk HD. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281–287. doi: 10.1136/gut.47.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128–130. doi: 10.3748/wjg.v7.i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiki N, Berger D, Mimura Y, Frick J, Dentener MA, Buurman WA, Seidelmann M, Kaminishi M, Beger HG. Release of endotoxin-binding proteins during major elective surgery: role of soluble CD14 in phagocytic activation. World J Surg. 2000;24:499–506. doi: 10.1007/s002689910080. [DOI] [PubMed] [Google Scholar]
- 35.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304–1309. [PubMed] [Google Scholar]
- 36.Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol. 1998;152:841–849. [PMC free article] [PubMed] [Google Scholar]
- 37.Arai M, Mochida S, Ohno A, Arai S, Fujiwara K. Selective bowel decontamination of recipients for prevention against liver injury following orthotopic liver transplantation: evaluation with rat models. Hepatology. 1998;27:123–127. doi: 10.1002/hep.510270120. [DOI] [PubMed] [Google Scholar]
- 38.Kelly JL, O'Sullivan C, O'Riordain M, O'Riordain D, Lyons A, Doherty J, Mannick JA, Rodrick ML. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury. Ann Surg. 1997;225:530–41; discussion 541-3. doi: 10.1097/00000658-199705000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin M, Ikejima K, Wheeler MD, Bradford BU, Seabra V, Forman DT, Sato N, Thurman RG. Estrogen is involved in early alcohol-induced liver injury in a rat enteral feeding model. Hepatology. 2000;31:117–123. doi: 10.1002/hep.510310119. [DOI] [PubMed] [Google Scholar]
- 40.Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kono H, Bradford BU, Rusyn I, Fujii H, Matsumoto Y, Yin M, Thurman RG. Development of an intragastric enteral model in the mouse: studies of alcohol-induced liver disease using knockout technology. J Hepatobiliary Pancreat Surg. 2000;7:395–400. doi: 10.1007/s005340070034. [DOI] [PubMed] [Google Scholar]
- 42.Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA, et al. Kupffer cell-derived prostaglandin E (2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G100–G106. doi: 10.1152/ajpgi.2000.279.1.G100. [DOI] [PubMed] [Google Scholar]
- 43.Bautista AP. Impact of alcohol on the ability of Kupffer cells to produce chemokines and its role in alcoholic liver disease. J Gastroenterol Hepatol. 2000;15:349–356. doi: 10.1046/j.1440-1746.2000.02174.x. [DOI] [PubMed] [Google Scholar]
- 44.Batey R, Cao Q, Madsen G, Pang G, Russell A, Clancy R. Decreased tumor necrosis factor-alpha and interleukin-1alpha production from intrahepatic mononuclear cells in chronic ethanol consumption and upregulation by endotoxin. Alcohol Clin Exp Res. 1998;22:150–156. [PubMed] [Google Scholar]
- 45.Järveläinen HA, Oinonen T, Lindros KO. Alcohol-induced expression of the CD14 endotoxin receptor protein in rat Kupffer cells. Alcohol Clin Exp Res. 1997;21:1547–1551. [PubMed] [Google Scholar]
- 46.French SW. Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J Biomed Sci. 2001;8:20–27. doi: 10.1007/BF02255967. [DOI] [PubMed] [Google Scholar]
- 47.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 48.French SW. Mechanisms of alcoholic liver injury. Can J Gastroenterol. 2000;14:327–332. doi: 10.1155/2000/801735. [DOI] [PubMed] [Google Scholar]
- 49.Crabb DW. Pathogenesis of alcoholic liver disease: newer mechanisms of injury. Keio J Med. 1999;48:184–188. doi: 10.2302/kjm.48.184. [DOI] [PubMed] [Google Scholar]
- 50.Oneta CM, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine selectively modulates lipopolysaccharide-induced Kupffer cell activation. J Lab Clin Med. 1999;134:466–470. doi: 10.1016/s0022-2143(99)90167-1. [DOI] [PubMed] [Google Scholar]
- 51.McClain CJ, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19:205–219. doi: 10.1055/s-2007-1007110. [DOI] [PubMed] [Google Scholar]
- 52.Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Wall C, Raleigh JA, Frankenberg MV, et al. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13 Suppl:S39–S50. [PubMed] [Google Scholar]
- 53.Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 54.Tsukamoto H, Lin M, Ohata M, Giulivi C, French SW, Brittenham G. Iron primes hepatic macrophages for NF-kappaB activation in alcoholic liver injury. Am J Physiol. 1999;277:G1240–G1250. doi: 10.1152/ajpgi.1999.277.6.G1240. [DOI] [PubMed] [Google Scholar]
- 55.Nanji AA, Jokelainen K, Rahemtulla A, Miao L, Fogt F, Matsumoto H, Tahan SR, Su GL. Activation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology. 1999;30:934–943. doi: 10.1002/hep.510300402. [DOI] [PubMed] [Google Scholar]
- 56.Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999;14:618–633. doi: 10.1046/j.1440-1746.1999.01928.x. [DOI] [PubMed] [Google Scholar]
- 57.Bautista AP. Impact of alcohol on the ability of Kupffer cells to produce chemokines and its role in alcoholic liver disease. J Gastroenterol Hepatol. 2000;15:349–356. doi: 10.1046/j.1440-1746.2000.02174.x. [DOI] [PubMed] [Google Scholar]


