Abstract
AIM: To investigate FasL expression in hilar cholangiocarcinoma tissues and cultured cholangiocarcinoma cells, and to assess its ability to induce apoptosis.
METHODS: We studied the expression of FasL by human hilar cholangiocaroinomas tissues by immunohistochemistry, and the QBC939 cholangiocarcinoma cell line by RT-PCR, immunohistochemistry, and Western Blot. TUNEL and flow cytometry were used to detect apoptotic cells.
RESULTS: Prevalent expression of FasL was detected in 39 resected hilar cholangiocarcinoma tissues. TUNEL staining disclosed a high level of cell death among lymphocytes infiltrating FasL positive areas of tumor. FasL mRNA and protein expressions in cholangiocarcinoma cells could induce Jurkat cells.
CONCLUSION: Hilar cholangiocarcinomas may elude immunological surveillance by inducing, via Fas/FasL system, the apoptosis of activated lymphocytes.
Keywords: cholangiocarcinoma/immunology; tumor cells, cultured/immunology; membrane glycoproteins/biosynthesis; lymphocytes/immunology; apoptosis
INTRODUCTION
The evasion, also called ‘tumor escape’, has been suggested to result from the inability of the immune system to react to the tumor, because of either non-recognition of tumor antigens or non-reactivity secondary to insufficient co-stimulation, anergy, tolerance, or immunosuppression. Recent reports showing the expression of FasL in Sertoli cells of the testis and ocular tissues[1,2], have provided new insights into the concepts of tolerance and immune-privilege. FasL triggers apoptotic cell death of sensitive lymphoid cells which express its cell surface receptor (Apo-1/CD95)[3-5]. FasL has been shown to confer immunologic al privilege in tissue transplantation experiments[6]. In rodents, successful allograft survival was obtained from FasL expressing tissues[2,7]. Moreover, FasL has been found to be expressed by non-lymphoid tumors as a mediator of immune evasion, which was initially raised by the finding that colon cancer cell lines express functional FasL[8]. Despite being immunogenic, cholangiocarcinoma overcomes antitumor immune responses by the mechanism that has not yet to be fully elucidated. The fact that many diverse tumors have been found to express FasL, suggests that a ‘Fas counterattack’ against antitumor immune effector cells may contribute to tumor immune escape[9-15]. We studied the expression of FasL on human hilar cholangiocarcinomas and the QBC939 cholangiocarcinoma cell line. We also studied apoptosis of lymphocytes (TILs) in filtrating into tumors. Jurkat cells were cocultured with cholangiocarcinoma cells.
MATERIAL AND METHODS
Human hilar cholangiocarcinoma tissue and cell
Thirty-nine human hilar cholangiocarcinomas of disparate pathological stages were collected from surgical resections performed at Qingdao municipal hospital. The patients were diagnosed as having hilar cholangiocarcinoma by histologic examination, and consisted of 17 males and 22 females. None of the patients have received chemo-, radio- or immuno-therapy before resection. The differentiation of tumors were moderate (n = 12), poor (n = 18), or high (n = 9). The QBC939 cells (a human hilar cholangiocarcinoma cell line) were a generous gift from Professor Wang (Third Military Medical University, China)[16]. The human T cell line Jurkat was purchased from the American Type Culture Collection (Rockville, MD). QBC939 cells were cultured in DMEM supplemented with 100 mL•L¯¹ FBS. Jurkat cells were maintained in RPMI1640 nutrient medium supplemented with 100 mL•L¯¹ FBS, penicillin (100 KLI•L¯¹) and streptomycin (100 mg•L¯¹), and incubated at 37 °C in a 50 mL•L¯¹ CO 2 atmosphere.
Immunohistochemistry for FasL and CD45
Detection of FasL expression and CD45 positive cells was performed using a rabbit polyclonal anti-human FasL specific IgG and a mouse anti-human monoclonal antibody on paraffin sections of human cholangiocarcinoma respectively (Boster Biological Technology Company, Wuhan, China). Five μm thick sections on the slides were deparaffinized, rehydrated and blocked for removing endogenous peroxides activity with 3 mL•L¯¹ H2O2 in methanol. Then the sections were washed in PBS and pre-incubated with 50 mL •L¯¹ normal goat serum for 30 min. The slides were incubated with antibodies against FasL and CD45 for 3 h at room temperature respectively. After washing, antibody binding was localized using a biotinylated secondary antibody with the ABC detection kit. The slides were counterstained with haematoxylin.
RT-PCR for FasL mRNA
Total RNA was prepared from the QBC 939 cell line with Trizol reagent (Gibco) according to the manufacturer’s instructions. RT-PCR was performed using RT-PCR kit (Promega) according to the manufacturer’s protocol. cDNA synthesis was carried out with 2 μg of total RNA. The primers for PCR were 5’-TCCAACTCAAGGTCCATGCC-3’ (forward) and 5’-CAGAGAGAGCTCAGATACGTTT-3’ (reverse). PCR reaction smith total volume of 50 μL were processed in a MJ. PTC 100 Thermocycler under the following conditions: 94 °C for 2 min; 94 °C for 30 s, 57 °C for 45 s, and 72 °C for 1 min for 35 cycles; and 72 °C at 5 min. The RT-PCR products (342 bp fragments) were analyzed on 20 g•L¯¹ agarose gels. Amplication of human β-actin served as control for sample loading and integrity.
Western blotting
Immunoblotting was performed for detection of FasL. Cells (1 × 106) were scraped, centrifuged briefly, and lysed for 30 min on ice in 50 mmol •L¯¹ Tris-HCl buffer (pH8), containing 120 mmol•L¯¹ NaCl and 10 g•L¯¹ lyepal supplemented with the complete-TM mixture of proteinase inhibitors. The total protein was collected by centrifugation (14, 000 r•min-1, 30 min, 4 °C) and assessed for protein concentration. SDS-PAGE (120 g•L¯¹) was performed, and the proteins were electroblotted onto nitrocellulose membranes. After 3 h incubation in blocking solution (200 mL•L¯¹ IgG-free normal horse serum in PBS), the membrane was exposed to the primary antibody overnight at 4 °C. After washing in PBS, the secondary peroxidase-labeled antibody was added at a 1:10000 dilution for 40 min at room temperature. The proteins were visualized with the enhanced chemiluminescence technique. The primary anti-FasL antibody was the clone 33 (Jingmei Biotech Co. Ltd. China) mAb (1:1000 dilution). The secondary antibody was peroxidase-labeled anti-mouse IgG antibody.
Cell death detection in situ by TUNEL
Cell death was detected in situ in resected tissues by enzymic labelling of DNA strand breaks using a TUNEL assay (Boehringer Mannheim GmbH, Germany) according to the manufacturer’s instructions. Only those cells with positive TUNEL staining and of apoptotic morphology were considered apoptotic.
T-cell apoptosis analysis
Cholangiocarcinoma cells were seeded in 6-well tissue culture plates and allowed to grow to 90% confluence. The cells were then washed twice with PBS and fixed with 20 g•L¯¹ paraformaldeyhde at 4 °C for 1 h. After the cell s were washed 3 times with PBS, they were layered with 2 mL of Jurkat cell suspension (5 × 108 T cells•L¯¹) in serum-containing media. After 48 h of coculture, Jurkat cells were collected from the 6-well plates, centrifuged, fixed in 700 mL•L¯¹ ethanol, and stored at -20 °C prior to analysis. Apoptotic cells were detected as a sub-G1 fraction after propidiumiodide staining and analysis using a FACScan.
RESULTS
Immunohistochemical localization of FasL
Paraffin sections from hilar cholangicarcinomas (n = 39) were stained for FasL. Positive staining for FasL was seen in the tissue of all 39 patients with hilar cholangiocarcinomas assessed (Figure 1). Positive staining of neoplastic tissue varied in both intensity and extent from individual tumor cell to cell, region to region in tumor and among tumors. Intensity of staining varied from weakly positive neoplastic areas to intense regions and was stronger than that observed in local FasL positive TILs staining areas where staining was locally uniform with nests of tum or cells. All tumors examined were predominantly FasL positive (> 70% of tumor area). To generate further evidence that FasL is expressed by human hilar cholangiocarcinoma cells, we also identified the protein expression of FasL in QBC939 cholangiocarcinoma cell line (Figure 2).
Figure 1.
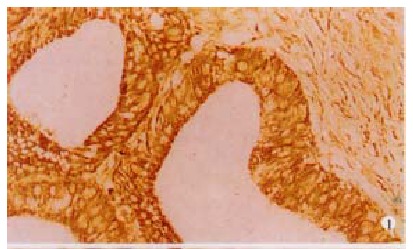
FasL positive in human hilar cholangiocarcinom as (brown). SABC × 200
Figure 2.
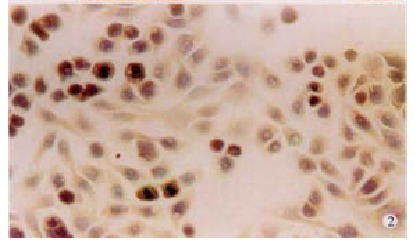
Expression of FasL in cholangiocarcinoma cell line. QBC939 × 200
Expression of FasL mRNA
To confirm the results obtained from the immunohistochemical studies, we evaluated the expression of FasL mRNA in the QBC939 human cholangiocarcinoma cell line. Total RNA was extracted and tested using RT-PCR. FasL mRNA was identified in QBC939 cells (Figure 3).
Figure 3.
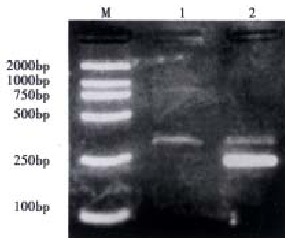
Expression of FasL mRNA in human cholangiocarcinoma cells QBC939. M: DL 2000 Marker; 1: FasL; 2: FasL+β-actin
Apoptosis of TILs
CD45 immunohistochemistry showed immunocyte infiltration in all 39 carcinomas (Figure 4). Most of the CD45 positive cells were of lymphoid morphology. Apoptosis was detected by TUNEL among TILs adjacent to FasL positive areas of the hilar cholangiocarcinomas. These TUNEL positive TILs exhibited morphological features of apoptosis, including nuclear condensation and fragmentation (Figure 5). This was a consistent finding in all the tumors examined (n = 15).
Figure 4.
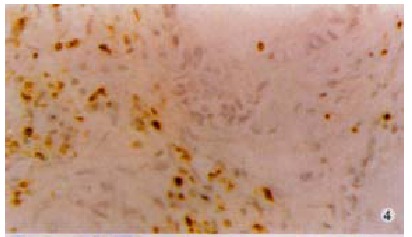
CD45 positive cells (brown) of lymphoid morphology adjacent to carcinoma. × 200
Figure 5.
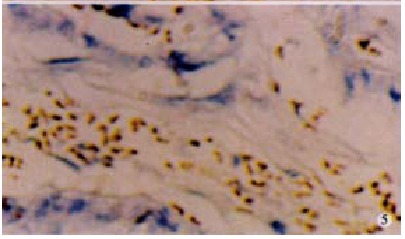
Positive apoptotic TUNEL stainig in situ (brown) with apoptotic morphology.
FasL by Western blot
Whole cell lysates from QBC939 cell cultures were electrophoresed in a polyacry lamide gel. We had previously obtained mRNA from the same samples, Mr 37000 protein was recognized on line 06 clone 33 (Figure 6).
Figure 6.
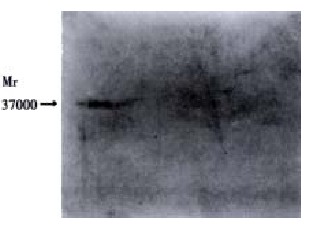
Western blotting of FasL protein with mAb from QBC939 cell cultures clone 33 from QBC939 cell cultures
Is functional FasL expression
To determine if FasL expressed by cholangiocytes in culture was capable of inducing cell death, we cocultured fixed cholangiocacinoma cells with FasL-sensitive (Jurkat) thymocytes. The QBC939 cholangiocarcinoma cell line induced cell death in FasL-sensitive T cells. At 48 h of coincubation, the cell line induced cell death in 50% of FasL-sensitive cells. These data show that the FasL expressed by the cholangiocarcinoma cells is capable of inducing T-cell apoptosis and therefore is functional.
DISCUSSION
It has been shown that hilar cholangiocarcinomas could express FasL, an inducer of immunocyte apoptosis in our study. The expression of FasL potentially enables hilar cholangiocarcinomas to counterattack and kill antitumor immune effector cells that seem Fas sensitive. As an established mediator of immune privilege and immunological tolerance in the eye and testis, functional FasL expressed in hilar cholangiocarcinomas was recognized as a contributor to the immune evasion of hilar cholangiocarcinoma. Expression of FasL in human hilar cholangiocarcinomas was prevalent; all 39 samples resected from hilar cholangiocarcinomas were found to express FasL protein. FasL staining was variable in both intensity and extent within tumors. The fact that extensive expression (> 70% of the tumor area) occurred in all tumors irrespective of tumor stage or degree of differentiation suggest that FasL may be expressed throughout hilar cholangiocarcinoma progression. In contrast with the extensive expression detected in the hilar cholangiocarcinomas, FasL expression was consistently restricted to only those epithelial cells at the luminal surface in control normal biliary epithelial sections (n = 6).It was estimated that a down-regulation and upregulation of FasL expression occurs during the transformation process. Using well-characterized, stable primary culture system of human cholangiocarcinoma cells, we found that cholangiocarcinomas in culture could express mRNA and protein of FasL and induce cell death in T cells. Apoptosis of TILs is an evidence in vivo which indicated that FasL expressed by the tumor is functional and can kill Fas sensitive antitumor immune effector cells.
Several cancers have been reported to express FasL. The tumor derived cell lines in all of the cases could induce apoptosis in Fas sensitive, but not Fas resistant lymphoid target cells in vitro[17-30]. Tumors and cell lines themselves usually exhibit resistance to FasL mediated apoptosis because of various defects acquired in Fas signal transduction[31-37,44-48]. Immunogenic tumor cells are probably subjected to a barrage of cell mediated cytotoxic antitumor immune assaults[38-43,49-52]. The fact that most tumor cells are efficiently killed by LAK cells in vitro suggests that cancer cells probably exhibit some degree of susceptibility to cell mediated cytotoxic mechanisms. Expression of a molecule to defuse antitumor immune challenge clearly offers a protective advantage to tumor growth and development. As an established mediator of immunological tolerance and privilege, FasL is such a molecule. Our findings have conclusively show that human hilar cholangiocarcinomas express functional FasL. Hilar cholangiocarcinoma may therefore be added to the growing list of malignancies that appear to be immunologically privileged through FasL expression. The high prevalence of FasL expression in the tumors suggests that this molecule may be critical to tumor immune privilege. In conclusion, the Fas counterattack appears to prevail as a potentially critical mechanism of immune privilege in human hilar cholangiocarcinoma.
Footnotes
Edited by Wang YQ and Ma JY
References
- 1.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 2.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 4.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu XH, Li QF, Wang YM. Expression of hepatocyte apoptosis and Fas/Fas L, in liver tissues of patients with hepatitis D. Shijie Huaren Xiaohua Zazhi. 2000;8:35–38. [Google Scholar]
- 6.Zhang H, Yang Y, Horton JL, Samoilova EB, Judge TA, Turka LA, Wilson JM, Chen Y. Amelioration of collagen-induced arthritis by CD95 (Apo-1/Fas)-ligand gene transfer. J Clin Invest. 1997;100:1951–1957. doi: 10.1172/JCI119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart PM, Griffith TS, Usui N, Pepose J, Yu X, Ferguson TA. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Que FG, Phan VA, Phan VH, Celli A, Batts K, LaRusso NF, Gores GJ. Cholangiocarcinomas express Fas ligand and disable the Fas receptor. Hepatology. 1999;30:1398–1404. doi: 10.1002/hep.510300618. [DOI] [PubMed] [Google Scholar]
- 10.Qian QJ, Xue HB, Qu ZQ, Fang SG, Cao HF, Wu MC. In situ detection of tumor infiltrating lymphocytes expressing perforin and fas ligand genes in human HCC. World J Gastroenterol. 1999;5:12–14. doi: 10.3748/wjg.v5.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu HF, Liu WW, Fang DC. Induction of apoptosis in human gastric carcinoma cell line SGC-901 by anti Fas monoclonal antibody. Shijie Huaren Xiaohua Zazhi. 1999;7:476–478. [Google Scholar]
- 12.Gutierrez LS, Eliza M, Niven-Fairchild T, Naftolin F, Mor G. The Fas/Fas-ligand system: a mechanism for immune evasion in human breast carcinomas. Breast Cancer Res Treat. 1999;54:245–253. doi: 10.1023/a:1006102601215. [DOI] [PubMed] [Google Scholar]
- 13.Mitsiades N, Poulaki V, Mastorakos G, Tseleni-Balafouta ST, Kotoula V, Koutras DA, Tsokos M. Fas ligand expression in thyroid carcinomas: a potential mechanism of immune evasion. J Clin Endocrinol Metab. 1999;84:2924–2932. doi: 10.1210/jcem.84.8.5917. [DOI] [PubMed] [Google Scholar]
- 14.Bennett MW, O'connell J, O'sullivan GC, Roche D, Brady C, Kelly J, Collins JK, Shanahan F. Expression of Fas ligand by human gastric adenocarcinomas: a potential mechanism of immune escape in stomach cancer. Gut. 1999;44:156–162. doi: 10.1136/gut.44.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimonishi T, Isse K, Shibata F, Aburatani I, Tsuneyama K, Sabit H, Harada K, Miyazaki K, Nakanuma Y. Up-regulation of fas ligand at early stages and down-regulation of Fas at progressed stages of intrahepatic cholangiocarcinoma reflect evasion from immune surveillance. Hepatology. 2000;32:761–769. doi: 10.1053/jhep.2000.18192. [DOI] [PubMed] [Google Scholar]
- 16.Wang SG, Han BL, Duan HC, Chen YS, Peng ZM. Establishment of the extrahepatic cholangiocarcinoma cell line. Zhonghua Shiyan Waike Zaizhi. 1997;14:67–68. [Google Scholar]
- 17.Que FG, Phan VA, Phan VH, Celli A, Batts K, LaRusso NF, Gores GJ. Cholangiocarcinomas express Fas ligand and disable the Fas receptor. Hepatology. 1999;30:1398–1404. doi: 10.1002/hep.510300618. [DOI] [PubMed] [Google Scholar]
- 18.Liu QY, Rubin MA, Omene C, Lederman S, Stein CA. Fas ligand is constitutively secreted by prostate cancer cells in vitro. Clin Cancer Res. 1998;4:1803–1811. [PubMed] [Google Scholar]
- 19.Peduto Eberl L, Guillou L, Saraga E, Schröter M, French LE, Tschopp J, Juillerat-Jeanneret L. Fas and Fas ligand expression in tumor cells and in vascular smooth-muscle cells of colonic and renal carcinomas. Int J Cancer. 1999;81:772–778. doi: 10.1002/(sici)1097-0215(19990531)81:5<772::aid-ijc18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Koomägi R, Volm M. Expression of Fas (CD95/APO-1) and Fas ligand in lung cancer, its prognostic and predictive relevance. Int J Cancer. 1999;84:239–243. doi: 10.1002/(sici)1097-0215(19990621)84:3<239::aid-ijc7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Shibakita M, Tachibana M, Dhar DK, Kotoh T, Kinugasa S, Kubota H, Masunaga R, Nagasue N. Prognostic significance of Fas and Fas ligand expressions in human esophageal cancer. Clin Cancer Res. 1999;5:2464–2469. [PubMed] [Google Scholar]
- 22.Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN, Owen-Schaub LB. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- 23.Kornmann M, Ishiwata T, Kleeff J, Beger HG, Korc M. Fas and Fas-ligand expression in human pancreatic cancer. Ann Surg. 2000;231:368–379. doi: 10.1097/00000658-200003000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellone G, Smirne C, Carbone A, Mareschi K, Dughera L, Farina EC, Alabiso O, Valente G, Emanuelli G, Rodeck U. Production and pro-apoptotic activity of soluble CD95 ligand in pancreatic carcinoma. Clin Cancer Res. 2000;6:2448–2455. [PubMed] [Google Scholar]
- 25.Liu ZG, Yin PZ, Wang BM, Kong LF, Zhao YW. Expression of Fas, FasL and HLA-DR in colon carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:428. [Google Scholar]
- 26.Tan LJ, Jiang N, Zhang N, Zhang XR, Qiu DH. Fas/FasL expression of esophageal squamous cell carcinoma, dysplasia tissues and normal mucosa. Shijie Huaren Xiaohua Zazhi. 2001;9:15–19. [Google Scholar]
- 27.Gao Y, Yang JZ. Pathological and clinical studies on pancreatoplastoma. Huaren Xiaohua Zazhi. 1998;6:64–65. [Google Scholar]
- 28.Li J, Wang WL, Liu B. Angiogenesis and apoptosis in human hepatocellular carcinoma. Huaren Xiaohua Zazhi. 1998;6:1057–1060. [Google Scholar]
- 29.Wang XW, Xie H. Presence of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells. World J Gastroenterol. 1998;4:540–543. doi: 10.3748/wjg.v4.i6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu HF, Liu WW, Fang DC, Liu FX, He GY. Clinical significance of Fas antigen expression in gastric carcinoma. World J Gastroenterol. 1999;5:90–91. doi: 10.3748/wjg.v5.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Bernstorff W, Spanjaard RA, Chan AK, Lockhart DC, Sadanaga N, Wood I, Peiper M, Goedegebuure PS, Eberlein TJ. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery. 1999;125:73–84. doi: 10.1067/msy.2099.93570. [DOI] [PubMed] [Google Scholar]
- 32.Nagao M, Nakajima Y, Hisanaga M, Kayagaki N, Kanehiro H, Aomatsu Y, Ko S, Yagita H, Yamada T, Okumura K, et al. The alteration of Fas receptor and ligand system in hepatocellular carcinomas: how do hepatoma cells escape from the host immune surveillance. in vivo Hepatology. 1999;30:413–421. doi: 10.1002/hep.510300237. [DOI] [PubMed] [Google Scholar]
- 33.Tang YC, Li Y, Qian GX. Reduction of tumorigenicity of SMMC-7721 hepatoma cells by vascular endothelial growth factor antisense gene therapy. World J Gastroenterol. 2001;7:22–27. doi: 10.3748/wjg.v7.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu HP, Wu KC, Li L, Yao LP, Lan M, Wang X, Fan DM, Wang X, Fan DM. Cloning of human cyclooxygenase 2 (COX) encoding gene and study of gastric cancer cell transfected with its antisense vector. Shijie Huaren Xiaohua Zazhi. 2000;8:1211–1217. [Google Scholar]
- 35.Liu HF, Liu WW, Fang DC. Effect of combined anti Fas mAb and IFN-γ on the induction of apoptosis in human gastric carcinoma cell line SGC-7901. Shijie Huaren Xiaohua Zazhi. 2000;8:1361–1364. [Google Scholar]
- 36.Kong XD, Zhang SZ, Hu JK, Xiao CY, Sun Y, Xia QJ. Abnormalities of p15 gene and protein expression in gastric cancers. Shijie Huaren Xiaohua Zazhi. 2001;9:513–516. [Google Scholar]
- 37.Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664–670. doi: 10.3748/wjg.v6.i5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang S, Song MJ, Shin EC, Lee MO, Kim SJ, Park JH. Apoptosis in human hepatoma cell lines by chemotherapeutic drugs via Fas-dependent and Fas-independent pathways. Hepatology. 1999;29:101–110. doi: 10.1002/hep.510290102. [DOI] [PubMed] [Google Scholar]
- 39.Petak I, Douglas L, Tillman DM, Vernes R, Houghton JA. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6:4119–4127. [PubMed] [Google Scholar]
- 40.Petak I, Tillman DM, Houghton JA. p53 dependence of Fas induction and acute apoptosis in response to 5-fluorouracil-leucovorin in human colon carcinoma cell lines. Clin Cancer Res. 2000;6:4432–4441. [PubMed] [Google Scholar]
- 41.Tillman DM, Petak I, Houghton JA. A Fas-dependent component in 5-fluorouracil/leucovorin-induced cytotoxicity in colon carcinoma cells. Clin Cancer Res. 1999;5:425–430. [PubMed] [Google Scholar]
- 42.Suzuki A, Hayashida M, Kawano H, Sugimoto K, Nakano T, Shiraki K. Hepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology. 2000;32:796–802. doi: 10.1053/jhep.2000.17738. [DOI] [PubMed] [Google Scholar]
- 43.Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421–425. doi: 10.3748/wjg.v4.i5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Cuadrado S, López-Armada MJ, Gómez-Guerrero C, Subirá D, Garcia-Sahuquillo A, Ortiz-Gonzalez A, Neilson EG, Egido J, Ortiz A. Anti-Fas antibodies induce cytolysis and apoptosis in cultured human mesangial cells. Kidney Int. 1996;49:1064–1070. doi: 10.1038/ki.1996.155. [DOI] [PubMed] [Google Scholar]
- 45.Schlosser SF, Azzaroli F, Dao T, Hingorani R, Crispe IN, Boyer JL. Induction of murine hepatocyte death by membrane-bound CD95 (Fas/APO-1)-ligand: characterization of an in vitro system. Hepatology. 2000;32:779–785. doi: 10.1053/jhep.2000.18422. [DOI] [PubMed] [Google Scholar]
- 46.Sung KJ, Paik EM, Jang KA, Suh HS, Choi JH. Apoptosis is induced by anti-Fas antibody alone in cultured human keratinocytes. J Dermatol. 1997;24:427–434. doi: 10.1111/j.1346-8138.1997.tb02816.x. [DOI] [PubMed] [Google Scholar]
- 47.Toyozaki T, Hiroe M, Tanaka M, Nagata S, Ohwada H, Marumo F. Levels of soluble Fas ligand in myocarditis. Am J Cardiol. 1998;82:246–248. doi: 10.1016/s0002-9149(98)00300-2. [DOI] [PubMed] [Google Scholar]
- 48.Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 1995;85:3183–3190. [PubMed] [Google Scholar]
- 49.Tanaka S, Sugimachi K, Shirabe K, Shimada M, Wands JR. Expression and antitumor effects of TRAIL in human cholangiocarcinoma. Hepatology. 2000;32:523–527. doi: 10.1053/jhep.2000.9716. [DOI] [PubMed] [Google Scholar]
- 50.Patel T, Steer CJ, Gores GJ. Apoptosis and the liver: A mechanism of disease, growth regulation, and carcinogenesis. Hepatology. 1999;30:811–815. doi: 10.1002/hep.510300334. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Fu XY, Plate J, Chong AS. IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res. 1998;58:2832–2837. [PubMed] [Google Scholar]
- 52.Böhm W, Thoma S, Leithäuser F, Möller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161:897–908. [PubMed] [Google Scholar]


