Abstract
Proopiomelanocortin (POMC) is a multivalent prohormone that can be processed into at least 7 biologically active peptide hormones. Processing can begin in the trans-Golgi network (TGN) and continues in the secretory granules of the regulated secretory pathway (RSP). Sorting of POMC into these granules is a complex process. Previously, a membrane-associated form of carboxypeptidase E (CPE) was shown to bind to POMC and facilitate its trafficking into these granules. More recently, secretogranin III (SgIII) was also found to affect POMC trafficking. Here, we show by RNA silencing that CPE and SgIII play a synergistic role in the trafficking of POMC to granules of the RSP in AtT20 cells. Reduction of either protein resulted in increased constitutive secretion of POMC and chromogranin A, which was increased even further when both proteins were reduced together, indicative of missorting at the TGN. In SgIII-reduced cells, POMC accumulated in a compartment that cofractionated and colocalized with syntaxin 6, a marker of the TGN, on sucrose density gradients and in immunocytochemistry, respectively, indicating an accumulation of this protein in the presumed sorting compartment. Regulated secretion of ACTH, as a measure of sorting and processing of POMC in mature granules, was reduced in the SgIII down-regulated cells but was increased in the CPE down-regulated cells. These results suggest that multiple sorting systems exist, providing redundancy to ensure the important task of continuous and accurate trafficking of prohormones to the granules of the RSP for the production of peptide hormones.
Intrinsic signals exist in proteins that allow trafficking machinery to recognize them as proteins destined to be moved from one cellular location to another. These include amino acid sequences such as the di-leucine motif, the nuclear localization signal and the amino acids, KDEL recognized by the KDEL receptor in the Endoplasmic Reticulum. Three-dimensional structural motifs have also been described in chromogranin A (CgA) (1), CgB (2), brain-derived neurotrophic factor (3), proenkephalin (4), proinsulin (5), and proopiomelanocortin (POMC) (6), which are recognized by a receptor or binding protein to facilitate selective trafficking to the regulated secretory pathway (RSP). POMC is a multivalent prohormone that can be processed to produce ACTH, α-melanocyte-stimulating hormone, and β-endorphin in the mature granules of the RSP depending on the relative levels of the prohormone convertase (PC)1/3 and PC2. The amino terminus of POMC contains a di-sulfide stabilized loop structure (7), which has been shown by classical affinity chromatography, chemical cross-linking, pull-down, and binding assays (6, 8) to be able to interact with carboxypeptidase E (CPE), which in turn is associated with the granule membranes via lipid rafts (9, 10). This interaction facilitates the trafficking of POMC into the granules of the RSP, where it can get processed. In the CPE knock-out mouse, aberrant secretion of POMC/ACTH from the intermediate lobe of the pituitary was observed (6, 11), although some regulated secretion of ACTH was detected from the corticotrophs of the anterior pituitary in a CRH-dependent manner (12). This suggests an additional mechanism for POMC sorting may be active in different cells in the CPE knock-out mouse.
Secretogranin III (SgIII) is a protein that plays a key role in the trafficking of CgA to the granules of the RSP, similar to that of CPE and POMC. SgIII is a member of the granin family of acidic proteins (13) and binds to CgA through one domain (SgIII(187–372)) and to cholesterol in lipid rafts through another (SgIII(23–186)) (14, 15). Similar to CPE, this interaction results in the sorting of CgA to the granules of the RSP, which in turn may sort other cargo protein via interaction with CgA (16). Recent studies have demonstrated SgIII can interact with POMC in pull-down experiments (12), suggesting that SgIII may act as an alternate sorting molecule for POMC in addition to CPE. If this were the case then endocrine cells may have developed a system of redundancy with multiple sorting systems able to accomplish this very important physiological function. Indeed, recent work by Sun et al (17) has suggested a role for SgII, another member of the granin family (13), as a protein with potential for sorting POMC.
Here, we investigated the relative roles that CPE and SgIII play in the trafficking of POMC in AtT20 corticotroph cells, a classic endocrine cell line that normally makes POMC, CPE, and SgIII. We found that stably reducing the levels of CPE or SgIII increased the constitutive secretion of POMC significantly, a result that was augmented when both proteins were reduced together. In addition, regulated secretion of ACTH was reduced when SgIII was chronically down-regulated and reduced further when CPE was acutely reduced by small interfering RNA (siRNA) oligos in the SgIII-knockdown (KD) cells. However, when CPE was chronically reduced in AtT20 cells, the level of regulated secretion of ACTH was increased. These results suggest a capability of AtT20 cells to adjust and compensate when key molecules involved in normal trafficking of POMC are perturbed.
Materials and Methods
Cells
Mouse corticotroph AtT20 cells were cultured in high glucose DMEM supplemented with 10% fetal bovine serum and 1× penicillin/streptomycin in a 37°C incubator under 5% CO2.
RNA silencing
Plasmids encoding small hairpin RNA (shRNA) sequences targeting mouse SgIII were purchased from Origene Technologies, Inc as a set of 4. Preliminary analysis identified sequence 1 (AGC CAG GCA CAT ACT CTG GAA GAT GAA GT) and 4 (ACC AAC AAG CTG ACG CTT ATG TGG AGA AG) as capable of reducing SgIII levels in AtT20 cells and were used together in cotransfection experiments to make stable clones. Selection was with 2-μg/mL puromycin. All 3 forms of SgIII (65, 50, and 35 kDa) were used in the calculations to determine KD effectiveness. A control shRNA plasmid against GFP (green fluorescent protein) was used as a negative control, and stable clones expressing this shRNA were generated in a similar manner.
For mouse CPE, stealth siRNA oligonucleotides were used to acutely KD CPE. These were purchased from Invitrogen, based on the company's website design tools targeting mouse CPE. Sequence 1224 (GGU UUG UCC GUG ACC UUC AGG GUA A) was used to target mouse CPE and a scrambled nucleotide sequence of 1224 was used as the negative control. The oligos were transfected into AtT20 cells using RNAiMax transfection reagent according to the manufacturer's protocol (Invitrogen) and the cells analyzed 24–48 hours after transfection. In addition to the siRNA oligos, Mission lentiviral transduction particles expressing shRNA sequences targeting mouse CPE (NM_013494) were purchased from the Broad Institute RNAi Consortium shRNA library through Sigma-Aldrich. Preliminary analysis identified that sequences 1 (GCT CCT GGA AAC TAT AAA CTT; clone number TRCN0000033084) and 5 (GAC AGG ATC GTG TAT GTT AAT; clone number TRCN0000033088) were capable of reducing CPE in AtT20 cells and was used together in transduction experiments to make stable clones expressing low levels of CPE. Mission Non-Target shRNA control transduction particles (SHC002V) were used as a negative control. Stable clonal cells were batch selected with puromycin (2 μg/mL).
Western blotting
Standard Western blotting techniques were used to analyze proteins from cell lysates and secretion media (see below) with immunoreactive proteins detected using infrared emitting fluorophore dyes coupled to the secondary antibodies (Odyssey). Primary antibodies used were rabbit anti-CgA (1:5000; generated in our laboratory) (18), rabbit anti-PC1/3 (1:2000; Thermo Fisher Scientific [formerly ABR]), mouse anti-CPE (1:5000; BD Biosciences), rabbit anti-ACTH (1:5000; DP4, generated in our laboratory), rabbit antiaquaporin 1 (AQP1) (1:2000; Alpha Diagnostics), mouse antitubulin (1:10 000; Sigma), rabbit anti-SgIII (1:5000, a generous gift from Dr M. Hosaka, Japan), and mouse antisyntaxin 6 (1:1000; Abcam).
Secretion experiments
Routinely, 6 × 106 cells were plated in 6-cm dishes and allowed to attach and grow overnight. The next day, the cells were rinsed twice with 1-mL serum-free DMEM at 37°C supplemented with 0.01% BSA (basal medium). The cells were then incubated with 1 mL of basal medium for 2 hours at 37°C. The medium was collected, centrifuged at 3000 rpm for 3 minutes, and 720 μL of the supernatant were added to 80 μL of 100% trichloroacetic acid (TCA). The proteins were precipitated overnight at 4°C. After rinsing the cells with fresh warmed basal medium, the cells were incubated for 10 minutes at 37°C with 1-mL stimulation medium (DMEM containing 50mM KCl and 79mM NaCl supplemented with 0.01% BSA). This medium was collected and processed exactly as for the basal medium. The cells were rinsed twice with 2-mL ice-cold PBS and harvested with 150-μL lysis buffer (T-PER; Thermo Fisher Scientific [formerly Pierce]) supplemented with 0.1% Triton X-100 and fresh protease inhibitor cocktail (ProteaseArrest; G-Biosciences). The cell homogenate was centrifuged at 13 000 rpm for 10 minutes to pellet cell debris and the supernatant saved as the soluble cell extract. The TCA-precipitated proteins from the media were centrifuged at 13 000 rpm for 20 minutes and the supernatant discarded. The protein was reconstituted in 72-μL 1× SDS (sodium dodecyl sulphate) sample buffer for gel electrophoresis. For the temperature block secretion experiment, the procedure just described was carried out in parallel at 37°C and 20°C. All reagents for the 20°C experiment were maintained at 20°C.
Subcellular fractionation (on sucrose density gradients)
SgIII-shRNA and control (Ctrl)-shRNA cells were grown in 15-cm culture dishes. The cells were rinsed with PBS, harvested by scraping into homogenization buffer (0.25M sucrose and 10mM Tris-Cl; pH 7.4) and passed through a 27-gauge needle 6 times to shear the cells. The homogenate was centrifuged at 3000 rpm for 10 minutes, and the postnuclear supernatant was applied to a sucrose density gradient (0.6M–1.6M). The gradient was made using a gradient maker (Gradient Master, BioComp), and the samples were centrifuged for 17 hours at 25 000 rpm in a Beckman-Coulter ultracentrifuge using a swing-out rotor, model SW32. The spun gradient was extracted from the top by a densi-flow autofractionator and fractionated into 1-mL fractions. Proteins in these fractions were precipitated by 10% TCA at 4°C for 18 hours. The samples were centrifuged to pellet the protein precipitate (13 000 rpm for 10 min in a benchtop microfuge). The supernatant was discarded and the pellet rinsed with 100% ice-cold acetone. The acetone wash was discarded and the protein allowed to air dry. The protein was then prepared for Western blot analysis of POMC/ACTH and syntaxin 6. This experiment was performed on 2 different Ctrl-shRNA and SgIII-shRNA clonal cell lines each on 2 occasions with similar results.
Immunocytochemistry (ICC)
SgIII-shRNA and Ctrl-shRNA clonal cells were grown on 2-chambered glass slides until 50%–60% confluent. The cells were rinsed once with room temperature (RT) PBS and then fixed with 2% paraformaldehyde for 30 minutes at RT. After, the cells were permeabilized with 0.25% Triton X-100 in 1× PBS for 5 minutes at RT and then blocked with 1% BSA in 1× PBS for 1 hour at RT and then incubated with rabbit anti-ACTH (DP4, generated in our laboratory) and mouse antisyntaxin 6 (Abcam) for 18 hours at 4°C. After washing, the primary antibodies were detected with antirabbit or antimouse secondary antibodies labeled with Alexa Fluor 488 or 546, respectively. Cells were observed on an inverted Zeiss LSM 510 META confocal microscope and the intensity of ACTH-immunoreactivity (IR) that colocalized with syntaxin 6-IR was quantified using Metamorph software.
Metabolic labeling and immunoprecipitation (IP) of POMC
SgIII-shRNA and Ctrl-shRNA cells (3.6 × 106 cells) were cultured overnight in 6-cm dishes. The cells were rinsed with DMEM lacking cysteine and methionine and supplemented with 10% dialyzed fetal bovine serum (starvation medium). The cells were incubated in this medium for 30 minutes, after which they were incubated with fresh starvation medium supplemented with 35[S]-methionine/cysteine (100 μCi/mL; PerkinElmer), for 10 minutes. The cells were then harvested on ice, after rinsing with ice cold PBS, in lysis buffer for IP. A soluble cell lysate was generated as described above and a standard IP procedure was carried out as previously described (19), with rabbit anti-ACTH antibody (DP4) that recognizes all forms of POMC peptides containing ACTH. The eluted immunoreactive POMC-related peptides were analyzed by SDS-PAGE, transferred to nitrocellulose, dried, and exposed to film for 15 hours.
Results
Stable KD of SgIII
Stable KD of SgIII in AtT20 cells expressing shRNA against mouse SgIII, was assessed by Western blot analysis (Figure 1A). The results showed an approximately 75% reduction of SgIII in SgIII-shRNA cells compared with Ctrl-shRNA cells (SgIII-shRNA, 25 ± 1.2% [SEM], when normalized to 100% Ctrl-shRNA, n = 3). Further analysis of other RSP proteins (Figure 1B), including CgA, PC1, CPE, POMC/ACTH, and aquaporin 1 (AQP) 1, showed only minor variations in proteins levels, suggesting that SgIII-KD did not measurably perturb the RSP in these cells.
Figure 1.
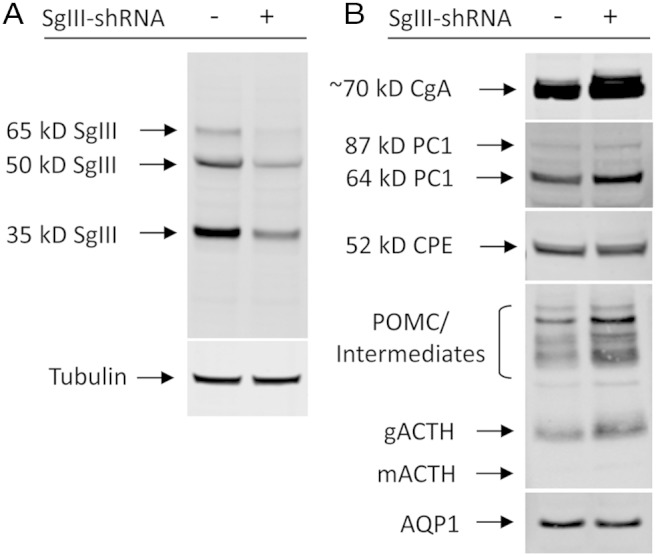
Western blot analysis of proteins from stable clones of AtT20 cells expressing SgIII-shRNA or Ctrl-shRNA. A, Analysis showing an approximately 75% reduction of SgIII in shRNA-SgIII cells (+) compared with control cells (−). Tubulin was used as a protein load control. B, Analysis showing the relative levels of other proteins of the RSP in AtT20 cells. Note the similar levels of PC1, CPE, and AQP1 (∼28 kDa) (38) and a slight increase of CgA and POMC (∼32–34 kDa) and the ACTH biosynthetic intermediates (22–24 kDa) and ACTH (glycosylated ACTH [gACTH], ∼13 kDa; mature ACTH [mACTH], ∼4.5 kDa).
Secretion of POMC/ACTH and CgA
Analysis of the nonstimulated culture medium from Ctrl- and SgIII-shRNA cells showed higher levels of POMC (3.6 ± 0.6-fold) and CgA (1.6 ± 0.1-fold) in the medium from the SgIII-shRNA cells compared with Ctrl-shRNA cells, indicating an increase in constitutive secretion of both of these proteins associated with a reduction of SgIII levels (±SEM, n = 5) (Figure 2A). In stimulated secretion experiments, the fold secretion of ACTH (calculated as the amount of ACTH in the stimulation medium divided by the amount in the basal medium), as a marker of proteins sorted to the RSP, was significantly reduced (Ctrl-shRNA, 11.9 ± 2.5-fold; SgIII-shRNA, 2.5 ± 0.8-fold; ±SEM, n = 4; t test, P < .05) (Figure 2B). This reduction was a combination of increased basal secretion of ACTH as well as a decrease in the quantal release of ACTH upon stimulation by depolarization with 50mM KCl.
Figure 2.
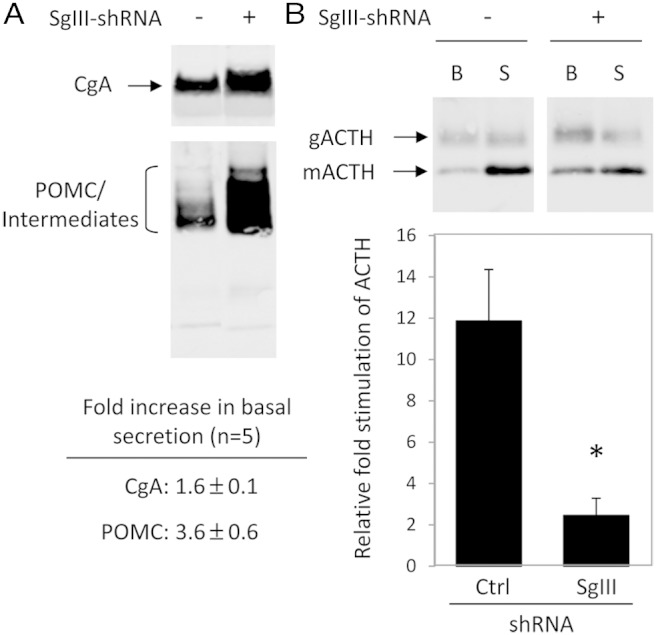
Western blot analysis and quantification of POMC/ACTH and CgA secreted from SgIII-shRNA and Ctrl-shRNA AtT20 cells. A, Analysis of POMC/intermediates and CgA in basal secretion media showing a 3.6- and 1.6-fold increase in basal secretion of POMC and CgA, respectively, in the SgIII-shRNA (+) cells compared with control cells (−) (see panel for ± SEM). B, Analysis and quantification of ACTH (glycosylated [∼13–14 kDa] and mature ACTH [∼4.5 kDa]) in basal and stimulated media showing increased basal and decreased stimulated secretion of ACTH in SgIII-shRNA cells (+) compared with control cells (−). Data are presented as relative fold stimulated secretion of ACTH from these cells (stimulated divided by basal). The results show an approximately 80% reduction in the fold stimulated secretion of ACTH from SgIII-shRNA (SgIII) cells compared with control (Ctrl). B, basal; S, stimulated; gACTH, glycosylated ACTH; mACTH, mature ACTH.
Increased basal secretion of ACTH
To address the source of the basal levels of ACTH, we performed a secretion experiment at 20°C in AtT20 cells and compared the results with those obtained at 37°C. Vesicle budding from the Golgi and subsequent formation of secretory vesicles are prevented when cells are incubated at 20°C, thus reducing the secretion of constitutively secreted proteins originating from the Golgi. Hence, if nonstimulated secretion of ACTH is prevented at 20°C, then its source is interpreted as coming from the Golgi. In wild type AtT20 cells, the amount of ACTH detected in the basal medium (nonstimulated) was reduced by more than 3-fold when the cells were shifted to 20°C (56.3 ± 17.0 at 37°C vs 15.3 ± 7.7 at 20°C, ±SEM, n = 3; t test, P < .05) (Figure 3A). However, upon stimulation at 20°C by depolarization, the amounts of ACTH released were similar to that of cells at 37°C (88.2 ± 38.0 at 37°C vs 93.2 ± 25.5 at 20°C, ±SEM, n = 3; t test, P > .05) (Figure 3B), demonstrating that the mature granules of the RSP were not blocked from being secreted by the 20°C temperature. These results suggest that AtT20 cells secrete ACTH to the culture medium directly from the trans-Golgi network (TGN) via the constitutive or constitutive-like secretory pathway.
Figure 3.
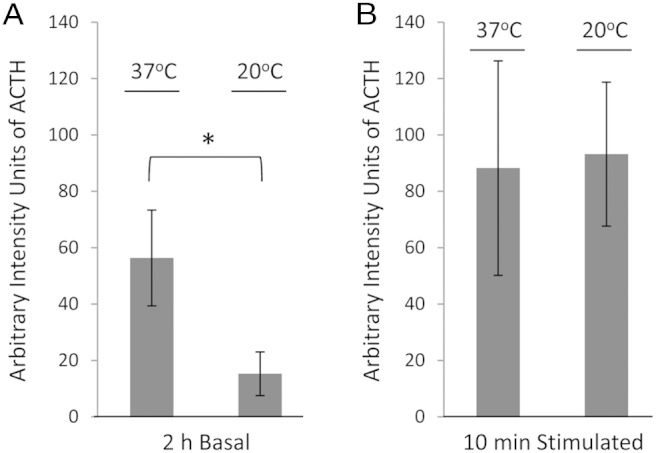
Quantification of ACTH (glycosylated [∼13–14 kDa] and mature ACTH [∼4.5 kDa]) secretion from AtT20 cells at 37°C vs 20°C. A, Quantification by Western blotting of ACTH levels in 2-hour basal media showing a significant approximately 3-fold reduction in the amounts secreted from cells incubated at 20°C compared with cells incubated at 37°C. B, Quantification by Western blotting of ACTH levels in 10-minute stimulation media showing no significant difference in the amounts secreted between cells incubated at 20°C or 37°C.
Sucrose gradient analysis of POMC in SgIII-shRNA cells
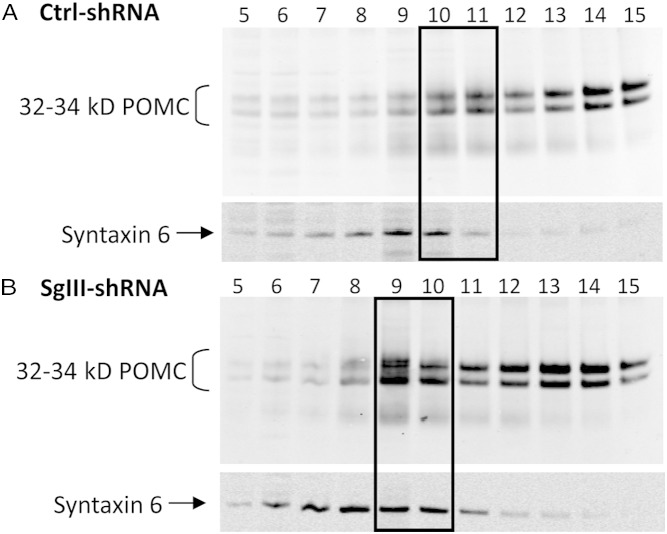
To identify whether cellular organelles, derived from cell lines expressing SgIII-shRNA or Ctrl-shRNA, were enriched in POMC, postnuclear supernatants were analyzed by sucrose density gradients. POMC-IR was generally localized to fraction numbers 10–15 on the sucrose gradients from both Ctrl-shRNA (Figure 4A) and SgIII-shRNA (Figure 4B) cells. In the SgIII-shRNA cells, however, there was an increase in the amount of POMC in fraction number 9 that cofractionated with syntaxin 6, a marker of the TGN, indicating an accumulation of POMC in the TGN.
Figure 4.
Sucrose density fractionation and immunoblot analyses of SgIII-shRNA and Ctrl-shRNA cells. Western blot analysis of Ctrl-shRNA (A) and SgIII-shRNA (B) cells showing POMC predominantly present in fraction numbers 10–15 from sucrose density fractionation of postnuclear supernatants. Note the shift and increase in levels of POMC to fraction number 9 in the SgIII-shRNA cells that cofractionated with syntaxin 6, a TGN marker (compare boxed areas). This experiment was performed on 2 different Ctrl-shRNA and 2 different SgIII-shRNA clonal cell lines on 2 occasions with similar results.
Quantification of ICC
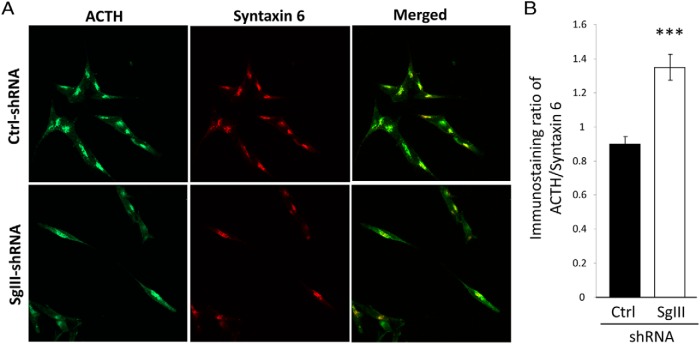
To validate the sucrose density gradient findings, clonal cell lines expressing SgIII-shRNA or Ctrl-shRNA were analyzed by ICC for the cellular localization of POMC/ACTH-IR. Immunostaining was found in the TGN as evidenced by colocalization with syntaxin 6 (Figure 5A). Quantification of the ICC showed the intensity of the POMC/ACTH staining was significantly increased in the syntaxin 6 compartment of the SgIII-shRNA cells compared with the Ctrl-shRNA cells (SgIII-shRNA 1.34 ± 0.07 vs Ctrl-shRNA 0.89 ± 0.5, ±SEM, n = 45 cells per group; t test, P < .001) (Figure 5B), consistent with an accumulation of POMC-IR in this compartment.
Figure 5.
ICC analysis of SgIII-shRNA and Ctrl-shRNA AtT20 cells. A, Confocal images of clonal AtT20 cells stained for POMC/ACTH and syntaxin 6. B, Quantification of the images for POMC-IR colocalized with syntaxin 6 using Metamorph software, showing the increased relative level of POMC in the TGN in SgIII-shRNA cells compared with Ctrl-shRNA cells.
Transient KD of CPE
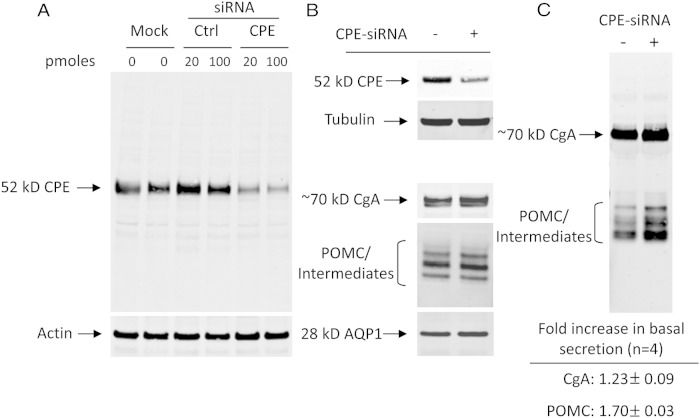
Stealth siRNA oligos directed against mouse CPE (CPE-siRNA) were used to transiently KD CPE in WT AtT20 cells. Western blot analysis showed reduced levels of CPE protein by 70%–80% compared with the Ctrl-siRNA (0.67 integrated units for Ctrl-siRNA vs 0.19 and 0.14 U for 20 and 100 pmol CPE-siRNA-treated cells, respectively) (Figure 6A). Other RSP marker proteins showed no significant differences (Figure 6B). To study the secretion behavior of POMC and CgA in response to acute KD of CPE, the basal levels of POMC and CgA were measured by Western blotting. The results showed an increase in the amount of constitutively secreted POMC (1.70 ± 0.03-fold) and CgA (1.23 ± 0.09-fold) when compared with Ctrl-siRNA-treated cells (±SEM, n = 4) (Figure 6C).
Figure 6.
Acute reduction of CPE in AtT20 cells by siRNA oligos. A, Western blot analysis of CPE levels in AtT20 cells transiently transfected with CPE-siRNA or Ctrl-siRNA oligos showing dose-dependent reduction of CPE protein in CPE-siRNA-transfected cells. Actin was used as a protein load control. B, Western blot analysis showing the relative levels of several other proteins of the RSP in AtT20 cells. Note the similar levels of AQP1, and POMC (32–34 kDa) and its intermediates (22–24 kDa), and a slight increase of CgA. Tubulin was used as a protein load control. C, Analysis of secreted POMC and CgA in basal secretion media showing a 1.7- and 1.2-fold increase in basal secretion of POMC and CgA, respectively, in the CPE-siRNA (+) cells compared with control cells Ctrl-siRNA (−).
Secretion of ACTH in double KD of SgIII and CPE
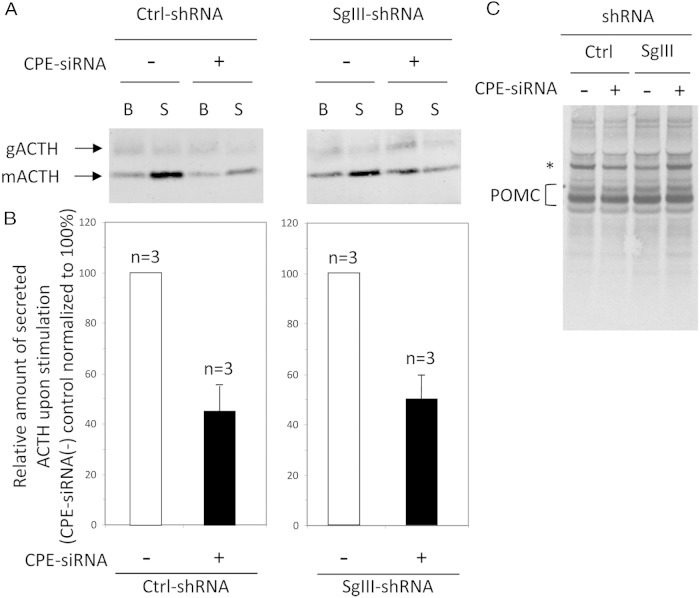
To investigate the secretion behavior of ACTH in response to the double KD of SgIII and CPE, stealth siRNA oligos against CPE were transfected into SgIII-shRNA or Ctrl-shRNA cells to generate a double KD of SgIII and CPE. In both cases (Ctrl-shRNA and SgIII-shRNA cells treated with CPE-siRNA), a more than or equal to 50% decrease in the quantal release of ACTH upon stimulation was observed, indicating that an acute reduction of CPE in AtT20 cells reduces the levels of ACTH in the granules of the RSP (Ctrl-shRNA treated with CPE-siRNA, 45.8 ± 10.2% fold reduction; SgIII-shRNA treated with CPE-siRNA, 50.2 ± 9.9% fold reduction when normalized to 100% Ctrl-shRNA, ±SEM, n = 3) (Figure 7, A and B). Because the SgIII-shRNA cells have been shown to have a perturbed secretory pathway for POMC and ACTH compared with Ctrl-shRNA cells (Figure 2), the additional reduction in ACTH stimulated secretion by CPE-siRNA oligos, demonstrated that when both SgIII and CPE are reduced in AtT20 cells, an additive effect in the missorting of POMC/ACTH occurred.
Figure 7.
Western blot analysis and quantification of ACTH secreted from CPE-siRNA and Ctrl-siRNA-transfected AtT20 cells. Representative Western blottings of secreted ACTH (glycosylated [gACTH], ∼13 kDa; and mature [mACTH], ∼4.5 kDa) (A) and quantification of secreted ACTH in basal and stimulated media from Ctrl-shRNA and SgIII-shRNA cells transfected with either CPE-siRNA or Ctrl-siRNA oligos (B). The results show a more than or equal to 50% reduction overall in the stimulated secretion of ACTH in CPE-siRNA-treated cells (+) compared with control cells (−), whether in Ctrl-shRNA or SgIII-shRNA cells. Note the increased basal secretion and decreased stimulated secretion of ACTH (mACTH) in the cells knocked down in SgIII and CPE together. B, basal; S, stimulated. C, Metabolic labeling and analysis of intracellular POMC (32–34 kDa) in SgIII-shRNA or Ctrl-shRNA cells transfected with CPE-siRNA or Ctrl-siRNA oligos. Note the newly synthesized POMC protein is similar in all 4 cell conditions, indicating that its rate of synthesis is similar. An asterisk indicates a nonspecific band.
Metabolic labeling of POMC
SgIII-shRNA and Ctrl-shRNA cells, transfected with Ctrl-siRNA or CPE-siRNA oligos, were radio-labeled with 35[S]-Met/Cys for 10 minutes. IP of POMC/ACTH showed equivalent levels of radio-labeled full-length POMC in both cell types, whether transfected with CPE-siRNA or Ctrl-siRNA, indicating the rate of POMC synthesis was similar in AtT20 cells reduced in SgIII or CPE or both (Figure 7C).
Stable KD of CPE and secretion of POMC/ACTH and CgA
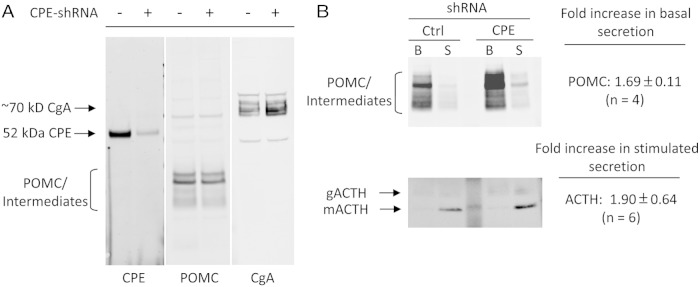
To assess the chronic effect of CPE-KD, AtT20 cells were stably transduced with CPE-shRNA lentiviral particles. These cells had reduced levels of CPE protein by approximately 90% compared with Ctrl-shRNA (CPE-shRNA, 9.1 ± 1.5%, when normalized to 100% Ctrl-shRNA, n = 2) (Figure 8A). Similar to the results with the CPE-siRNA oligos, this reduction in CPE resulted in a significant increase in the constitutive secretion of POMC (1.69 ± 0.11-fold; ±SEM, n = 4) (Figure 8B) relative to the Ctrl-shRNA cells. However, in contrast to the CPE-siRNA oligo-treated AtT20 cells, the fold secretion of ACTH was increased 1.90 ± 0.64-fold (±SEM, n = 6) primarily as a result of an increase in the quantal release of ACTH upon stimulation. Further analysis also showed a 2.52 ± 0.17-fold increase (±SD, n = 2) in basal secretion of CgA and a 4.14 ± 0.65-fold increase (±SD, n = 2) in the stimulated secretion of CgA compared with Ctrl-shRNA transduced cells (data not shown).
Figure 8.
Western blot analysis of POMC/intermediates, ACTH and CgA levels and their secretion behavior from AtT20 cells stably down-regulated in CPE by CPE-shRNA lentiviral vectors. A, Western blot analysis of cell lysates for CPE, POMC/intermediates, and CgA showing a 90% reduction in CPE in the CPE-shRNA cells. Levels of intracellular POMC (∼32 kDa) and its intermediates (23–25-kDa ACTH biosynthetic intermediate) are similar, and CgA is slightly elevated. B, Western blot analysis of culture medium for POMC/intermediates and ACTH secreted under basal and stimulated conditions. POMC/intermediates are elevated by 1.7-fold (top blot) in the CPE-KD cells compared with the Ctrl-shRNA cells (compare B lanes). The stimulated secretion of ACTH (combined glycosylated and mature ACTH) was increased by 1.9-fold compared with the control cells (lower blot, compare S lanes). B, basal; S, stimulated; gACTH, glycosylated ACTH (∼13 kDa); mACTH, mature ACTH (∼4.5 kDa).
Discussion
Endocrine cells are professional secretory cells that synthesize, store, and ultimately secrete bioactive peptides in a secretagogue-dependent manner. Defects in any aspect of this process results in endocrine disorders such as GH deficiency (20), hyperproinsulinemia (5, 21), and Cushing's syndrome (22). How endocrine cells determine which proteins go into the RSP and which do not, is a complex issue (23, 24), and one where endocrine cells may have redundant or interconnected mechanisms. Most researchers in this field are in agreement that aggregation plays a significant role because many RSP cargo proteins have the ability to aggregate under conditions of mild acidity and increased Ca2+ concentrations; conditions reported to be present in the TGN (25, 26) where sorting can occur (25, 27). Additionally, there has to be a membrane component to which the aggregated cargo can attach, to allow localization in the nascent secretory granule. Several reports have identified membrane bound proteins capable of binding prohormone cargo; 2 of which are CPE (6, 8) and SgIII (12, 14, 15), studied here.
Both CPE and SgIII have been shown to be tightly associated with secretory granule membranes through their interaction with cholesterol in lipid microdomains (9, 15) and both proteins have been shown to bind to POMC (6, 12). In order to study the relative roles that each protein plays in POMC sorting we used a model endocrine cell line, AtT20 cells that normally make POMC, SgIII, and CPE. AtT20 cells are corticotroph cells from a mouse anterior pituitary adenoma and although they secrete higher levels of POMC and ACTH than primary corticotrophs they are an ideal model cell line to study the mechanisms of POMC and ACTH secretion. The major function of corticotrophs in vivo is to generate and store ACTH, a peptide hormone generated from POMC in the secretory granules of these cells. Hence, studying the secretion patterns of POMC and ACTH from these cells will shed light on the importance of CPE and SgIII in the secretory pathway. Stable KD of SgIII (Figure 1), increased the constitutive secretion of both POMC and CgA (Figure 2A), a finding similar to that reported by Hosaka and coworkers for CgA (17). This is not surprising for CgA, because the role of SgIII in CgA sorting to the RSP has been well documented previously (12, 14, 15). The observation that a 3.6-fold increase in POMC secretion via the constitutive secretory pathway (CSP), without an increase in POMC synthesis (Figure 7C), suggests an inability to be sorted into the granules of the RSP but diversion to the CSP in the SgIII-KD cells. Notably, stimulated secretion of ACTH from the RSP was markedly reduced (Figure 2B), and in combination with elevated basal secretion of ACTH, the fold stimulation of ACTH was reduced by more than or equal to 80% compared with the control cells. Current dogma based in the literature (28, 29) accepts that ACTH, an end product of POMC processing in AtT20 cells, is predominantly produced in the mature secretory granule by PC1. When we noted that more ACTH was being secreted under basal conditions we questioned its source; because to account for this, the results indicated that basal secretion of mature granules via spontaneous fusion with the plasma membrane was elevated. However, using a temperature block (Figure 3), to prevent secretory vesicles budding from the Golgi apparatus (30), we found evidence that the source of ACTH was not from mature granules but from the TGN, because the basal secretion of ACTH was significantly reduced at 20°C (Figure 3A). In addition, subsequent stimulation of these cells at 20°C demonstrated that the mature granules were not affected by the temperature block, indicating that the machinery for secretion of mature secretory granules was intact under both temperatures conditions.
The elevated POMC and ACTH secretion via the CSP indicated a possible bottleneck is occurring in the Golgi. Subcellular fractionation on sucrose density gradients identified higher amounts of POMC accumulated in lighter fractions in the SgIII-KD cells that cofractionated with syntaxin 6, a TGN marker (Figure 4B). In addition, ICC and confocal microscopy demonstrated a relative increase of POMC/ACTH staining in the syntaxin 6 compartment of SgIII-KD cells (Figure 5). Both sets of results suggest that POMC accumulates in the TGN in the absence of SgIII, indicating that SgIII plays an important role in POMC trafficking in AtT20 cells. It is conceivable that such an accumulation of POMC in this compartment, which is shared by PC1, could be partially processed to generate ACTH at low levels. The predominant form of PC1 in this compartment is the 87-kDa form and is known to be active (31–33), albeit at a low level due to the nonoptimum pH and Ca2+ levels. Thus, we speculate that the increased constitutive secretion of ACTH from these cells is generated by PC1 in the TGN, due to the accumulation of the substrate, POMC, in this compartment, and secretion via constitutive secretory vesicles with POMC (for schematic diagram, see Figure 9).
Figure 9.
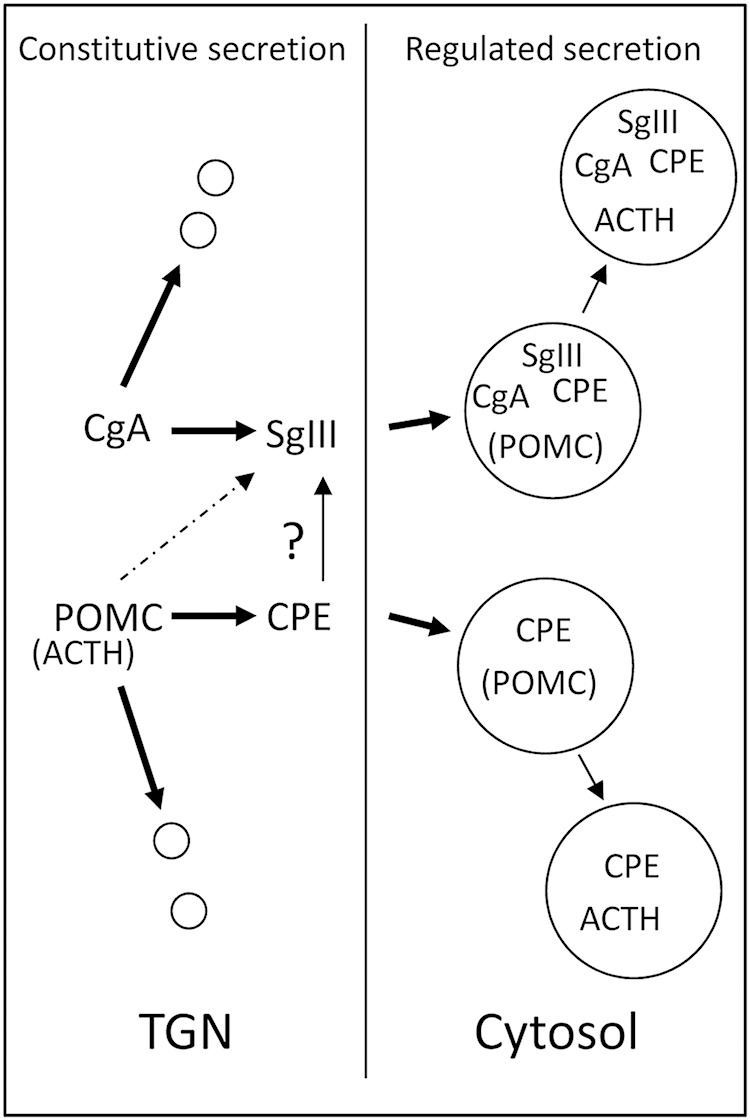
Schematic diagram depicting a predicted steady state mechanism for POMC and CgA trafficking in AtT20 cells. POMC and CgA are synthesized and trafficked to the TGN where sorting occurs. SgIII and CPE can facilitate CgA and POMC sorting into the granules of the RSP, because in all cases, reduction of these proteins result in increased constitutive secretion of POMC/ACTH and CgA and accumulation in the TGN. Chronic reduction of SgIII alone prevented a component of POMC/ACTH sorting to the RSP, whereas chronic reduction of CPE enhanced the sorting to the RSP. In contrast, acute KD of CPE prevented a component of POMC/ACTH sorting to the RSP. This suggests that AtT20 cells with long-term reduction of CPE levels have adapted to maintain and even increase the sorting efficiency of POMC/ACTH in AtT20 cells, presumably through the SgIII pathway.
Transiently reducing CPE in AtT20 cells with siRNA oligos (Figure 6) also demonstrated a similar, but not as pronounced, phenotype with respect to constitutive secretion of POMC and CgA, giving a 1.7- and 1.2-fold increase, respectively (Figure 6C). When used in Ctrl-shRNA or SgIII-shRNA cells, the fold stimulation of ACTH was also reduced by approximately 50%, due to increased basal secretion and reduced stimulated secretion of ACTH. This result is in contrast to that reported previously where acute reduction of CPE in AtT20 cells had no effect on the stimulated secretion of ACTH (34). We cannot explain the differences between these 2 sets of results. However, in that same report, a significant increase in constitutive secretion of POMC was observed which is consistent with our current data, even though POMC synthesis was similar between control and CPE-siRNA cells. Not surprisingly, when SgIII-KD cells were further treated with CPE-siRNA, to generate a double KD, the already perturbed secretory behavior of ACTH due to SgIII-KD, was further perturbed in that the stimulation of ACTH was reduced more (Figure 7B), supporting the idea that both proteins contribute to the efficient trafficking of POMC and hence its processing to ACTH in AtT20 cells (Figure 9).
Unexpectedly, when CPE was stably reduced in AtT20 cells (Figure 8A), stimulated secretion of ACTH was almost doubled, due primarily to increased quantal release of ACTH indicating increased levels of ACTH in the mature granules. This suggests more efficient sorting of POMC into the RSP when CPE was chronically down-regulated in these cells. We also noted a similar result for CgA, although in both cases, POMC and CgA were secreted constitutively at an elevated rate consistent with the acute reduction of CPE by the siRNA oligos. How an increase in stimulated secretion of these 2 RSP proteins happens is unknown, especially when cellular levels of SgIII and SgII were not increased in these cells to support a simple compensatory mechanism (data not shown). Although it is possible that yet a different “granin” may contribute, we speculate that the capacity of SgIII may have increased to accommodate the POMC that cannot get sorted due to the reduction of CPE. Such an idea of differing responses is readily found in biological systems. An example is seen with hexokinase and glucokinase; both perform the exact same function on glucose, but one will predominate over the other depending on the concentration of glucose due to their differing KM values. For POMC sorting, a similar mechanism may be in play between CPE and SgIII. It is possible that CPE interacts with SgIII in vivo as indicated by in vitro binding assays (12); thus long term reduction of CPE may free up additional binding sites on SgIII for cargo proteins such as POMC and CgA.
The secretion of ACTH from corticotrophs in the anterior pituitary in vivo is a highly regulated procedure and is typically tied into the control of POMC expression. POMC expression is modulated by many secretagogues (35), including glucocoticoids, melatonin and bone morphogenic protein-4 (36, 37). How these molecules might affect expression levels of SgIII and CPE, and hence trafficking of POMC is unknown, however, due to the compensatory ability of these 2 proteins, and possibly SgII also (17), modulating the levels of these may not affect this specific aspect of POMC/ACTH trafficking and secretion significantly, because others may compensate. In support of this, in looking at the CPEfat/fat mouse, where CPE is degraded in the ER, SgIII is up-regulated in the anterior pituitary and the corticotrophs respond normally to CRH to secrete ACTH (12). This suggests that even if expression levels of CPE are reduced, SgIII may compensate for its trafficking function in vivo.
Hence, in the regulation of sorting prohormones to the granules of the RSP, we propose that endocrine cells have evolved mechanisms that can partially or completely compensate for each other with the goal of maintaining accurate and specific delivery of bioactive peptides to the circulation and that at least 2 proteins, CPE and SgIII, play a role as demonstrated in this study (Figure 9).
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AQP1
- aquaporin 1
- CgA
- chromogranin A
- CPE
- carboxypeptidase E
- ICC
- immunocytochemistry
- IP
- immunoprecipitation
- IR
- immunoreactivity
- KD
- knockdown
- PC
- prohormone convertase
- POMC
- proopiomelanocortin
- RSP
- regulated secretory pathway
- RT
- room temperature
- SgIII
- secretogranin III
- shRNA
- small hairpin RNA
- siRNA
- small interfering RNA
- TCA
- trichloroacetic acid
- TGN
- trans-Golgi network.
References
- 1. Thiele C, Huttner WB. The disulfide-bonded loop of chromogranins, which is essential for sorting to secretory granules, mediates homodimerization. J Biol Chem. 1998;273:1223–1231. [DOI] [PubMed] [Google Scholar]
- 2. Glombik MM, Krömer A, Salm T, Huttner WB, Gerdes HH. The disulfide-bonded loop of chromogranin B mediates membrane binding and directs sorting from the trans-Golgi network to secretory granules. EMBO J. 1999;18:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45:245–255. [DOI] [PubMed] [Google Scholar]
- 4. Loh YP, Maldonado A, Zhang C, Tam WH, Cawley N. Mechanism of sorting proopiomelanocortin and proenkephalin to the regulated secretory pathway of neuroendocrine cells. Ann NY Acad Sci. 2002;971:416–425. [DOI] [PubMed] [Google Scholar]
- 5. Dhanvantari S, Shen FS, Adams T, et al. Disruption of a receptor-mediated mechanism for intracellular sorting of proinsulin in familial hyperproinsulinemia. Mol Endocrinol. 2003;17:1856–1867. [DOI] [PubMed] [Google Scholar]
- 6. Cool DR, Normant E, Shen F, et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. [DOI] [PubMed] [Google Scholar]
- 7. Tam WW, Andreasson KI, Loh YP. The amino-terminal sequence of pro-opiomelanocortin directs intracellular targeting to the regulated secretory pathway. Eur J Cell Biol. 1993;62:294–306. [PubMed] [Google Scholar]
- 8. Cool DR, Loh YP. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol Cell Endocrinol. 1998;139:7–13. [DOI] [PubMed] [Google Scholar]
- 9. Dhanvantari S, Loh YP. Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J Biol Chem. 2000;275:29887–29893. [DOI] [PubMed] [Google Scholar]
- 10. Dhanvantari S, Arnaoutova I, Snell CR, et al. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry. 2002;41:52–60. [DOI] [PubMed] [Google Scholar]
- 11. Shen FS, Loh YP. Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in cpefat mice associated with a carboxypeptidase E mutation. Proc Natl Acad Sci USA. 1997;94:5314–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction between secretogranin III and carboxypeptidase E facilitates prohormone sorting within secretory granules. J Cell Sci. 2005;118:4785–4795. [DOI] [PubMed] [Google Scholar]
- 13. Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011;32:755–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosaka M, Watanabe T, Sakai Y, Uchiyama Y, Takeuchi T. Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic β-cells. Mol Biol Cell. 2002;13:3388–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosaka M, Suda M, Sakai Y, Izumi T, Watanabe T, Takeuchi T. Secretogranin III binds to cholesterol in the secretory granule membrane as an adapter for chromogranin A. J Biol Chem. 2004;279:3627–3634. [DOI] [PubMed] [Google Scholar]
- 16. Montero-Hadjadje M, Elias S, Chevalier L, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun M, Watanabe T, Bochimoto H, et al. Multiple sorting systems for secretory granules ensure the regulated secretion of peptide hormones. Traffic. 2013;14:205–218. [DOI] [PubMed] [Google Scholar]
- 18. Koshimizu H, Cawley NX, Kim T, Yergey AL, Loh YP. Serpinin: a novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol Endocrinol. 2011;25:732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cawley NX, Rodriguez YM, Maldonado A, Loh YP. Trafficking of mutant carboxypeptidase E to secretory granules in a β-cell line derived from Cpe(fat)/Cpe(fat) mice. Endocrinology. 2003;144:292–298. [DOI] [PubMed] [Google Scholar]
- 20. Zhu YL, Conway-Campbell B, Waters MJ, Dannies PS. Prolonged retention after aggregation into secretory granules of human R183H-growth hormone (GH), a mutant that causes autosomal dominant GH deficiency type II. Endocrinology. 2002;143:4243–4248. [DOI] [PubMed] [Google Scholar]
- 21. Gabbay KH, DeLuca K, Fisher JN, Jr, Mako ME, Rubenstein AH. Familial hyperproinsulinemia. An autosomal dominant defect. N Engl J Med. 1976;294:911–915. [DOI] [PubMed] [Google Scholar]
- 22. Pivonello R, De Martino MC, De Leo M, Lombardi G, Colao A. Cushing's syndrome. Endocrinol Metab Clin North Am. 2008;37:135–149, ix. [DOI] [PubMed] [Google Scholar]
- 23. Canaff L, Bennett HP, Hendy GN. Peptide hormone precursor processing: getting sorted? Mol Cell Endocrinol. 1999;156:1–6. [DOI] [PubMed] [Google Scholar]
- 24. Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol. 2007;177:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seksek O, Biwersi J, Verkman AS. Direct measurement of trans-Golgi pH in living cells and regulation by second messengers. J Biol Chem. 1995;270:4967–4970. [DOI] [PubMed] [Google Scholar]
- 27. Rindler MJ, Colomer V, Jin Y. Immature granules are not major sites for segregation of constitutively secreted granule content proteins in NIT-1 insulinoma cells. Biochem Biophys Res Commun. 2001;288:1071–1077. [DOI] [PubMed] [Google Scholar]
- 28. Bloomquist BT, Eipper BA, Mains RE. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991;5:2014–2024. [DOI] [PubMed] [Google Scholar]
- 29. Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993;268:1763–1769. [PubMed] [Google Scholar]
- 30. Matlin KS, Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983;34:233–243. [DOI] [PubMed] [Google Scholar]
- 31. Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]
- 32. Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem. 1994;269:18408–18413. [PubMed] [Google Scholar]
- 33. Friedman TC, Loh YP, Birch NP. In vitro processing of proopiomelanocortin by recombinant PC1 (SPC3). Endocrinology. 1994;135:854–862. [DOI] [PubMed] [Google Scholar]
- 34. Kemppainen RJ, Behrend EN. Acute inhibition of carboxypeptidase E expression in AtT-20 cells does not affect regulated secretion of ACTH. Regul Pept. 2010;165:174–179. [DOI] [PubMed] [Google Scholar]
- 35. Aoki Y, Iwasaki Y, Katahira M, Oiso Y, Saito H. Regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. I: effects of the common secretagogues. Endocrinology. 1997;138:1923–1929. [DOI] [PubMed] [Google Scholar]
- 36. Tsukamoto N, Otsuka F, Miyoshi T, et al. Effects of bone morphogenetic protein (BMP) on adrenocorticotropin production by pituitary corticotrope cells: involvement of up-regulation of BMP receptor signaling by somatostatin analogs. Endocrinology. 2010;151:1129–1141. [DOI] [PubMed] [Google Scholar]
- 37. Tsukamoto N, Otsuka F, Ogura-Ochi K, et al. Melatonin receptor activation suppresses adrenocorticotropin production via BMP-4 action by pituitary AtT20 cells. Mol Cell Endocrinol. 2013;375:1–9. [DOI] [PubMed] [Google Scholar]
- 38. Arnaoutova I, Cawley NX, Patel N, Kim T, Rathod T, Loh YP. Aquaporin 1 is important for maintaining secretory granule biogenesis in endocrine cells. Mol Endocrinol. 2008;22:1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]