Abstract
Transmembrane 4 superfamily 3 (TM4SF3) was identified as a novel androgen-regulated gene in prostate cancer (PCa) cells. Our data demonstrate that TM4SF3 exhibits androgen-induced repression of the mRNA but up-regulation of the protein. The androgen positive effect on the TM4SF3 protein is of significant interest in view of the procancer functions of both androgens and tetraspanin proteins. Androgen positively regulates TM4SF3 protein stability by inhibiting its proteasome-dependent degradation. This androgen stabilization of TM4SF3 is involved in promoting PCa cell invasion and migration of both androgen-dependent and androgen-independent PCa cells. Although confirming androgen up-regulation of the TM4SF3 protein, we observed that TM4SF3 is localized not only to the membrane, but also, surprisingly, the nuclei of PCa cells. This novel nuclear localization of TM4SF3 depends on androgen-induced nuclear localization of androgen receptor (AR) in both androgen-dependent and androgen-independent PCa cell lines. TM4SF3 interacts with AR both in PCa cell types and in vitro, strongly suggesting a direct interaction. This direct interaction is required for the stabilization of not only TM4SF3, but also remarkably AR, because down-regulation of TM4SF3 resulted in reduced AR protein levels. As expected of an important AR regulator, TM4SF3 regulates androgen-dependent gene expression in and proliferation of PCa cells. Importantly, a direct correlation between AR and TM4SF3 protein levels and nuclear colocalization were also observed in prostate tumors, strongly suggesting that the mutual stabilization resulting from the AR-TM4SF3 interaction is found in tumors and that this interaction is important in PCa biology.
Prostate cancer (PCa) is the second leading cause of cancer deaths in American men. The standard systemic treatment for PCa is androgen-deprivation therapy, which results in an early positive clinical response but the disease usually relapses to the more aggressive stage called as castration-resistant PCa (CRPC) (1, 2). Androgen receptor (AR) is a master regulator in the development of both normal prostate and PCa. One of the most important mechanisms for CRPC is the restoration of aberrant androgen signaling through the AR (3–5).
AR, a member of nuclear receptor superfamily of transcription factors, regulates the expression of target genes in response to androgenic signals derived from testes that lead to differentiation, proliferation, and transformation of prostate cells (6, 7). Because AR is often overexpressed and AR transcriptional activity is restored in CRPC, AR-regulated genes play significant roles in the progression of this advanced stage of PCa (8–10). Importantly, AR gene amplification and gain-of-function mutations suggests that these advanced prostate tumors are under strong selective pressure to sustain AR transcriptional activity. Based on evidence from the xenograft tumors, AR expression and activity are restored after castration (3–5). Several studies have revealed that the AR transcriptional network is modified during development of CRPC, such as genes involved in androgen synthesis like aldo-keto reductase family 1, member C3 and steroid-5-alpha-reductase 1 (11–13), and M-phase checkpoint inactivator genes like cell division cycle 20, cyclin-dependent kinase 1 promotes tumor growth in mouse models (14). Reactivation of TMPRSS2:ERG fusion gene expression by AR contribute to the tumor progression in CRPC (15), and thus promote invasion of tumors (16, 17).
To shed more light on AR functions in the development of PCa, we performed the gene profiling studies in PCa cells and identified transmembrane 4 superfamily 3 (TM4SF3) as a novel androgen down-regulated gene. TM4SF3 belongs to the tetraspanin family, which consists of 33 mammalian proteins that are conserved from yeast to humans (18). These small proteins are characterized by the presence of 4 hydrophobic transmembrane domains and several conserved amino acids (19, 20). Identified as a tumor-associated antigen due to its high expression in different human carcinomas, TM4SF3 is also known as C0–029 in humans and D6.1 in rats and has proinvasive, prometastatic, and progrowth functions in different tumors (21, 22). For example, TM4SF3 up-regulates the a disintegrin and metalloproteinase expression in esophageal carcinoma and enhances the esophageal cancer cell invasion (22). Up-regulation of TM4SF3 correlates with the progression of hepatocellular (23), colon (24), and pancreatic carcinomas (25). However, the role and regulation of TM4SF3 in PCa is not yet defined.
In the present study, we observed for the first time that TM4SF3 is a target of complex androgen regulation. Our findings showed that androgen represses the TM4SF3 mRNA but up-regulates protein levels by preventing its proteasome-dependent degradation. Androgen-mediated stabilization of TM4SF3 leads to invasion and migration of PCa cells. Most interestingly and perplexingly, in addition to being localized in the plasma membrane, TM4SF3 exhibits androgen-induced nuclear localization in PCa cells and a physical interaction with AR. Moreover, our data show that the TM4SF3 protein up-regulates endogenous AR protein and its biological activities in PCa cells. Importantly, TM4SF3 is overexpressed in prostate tumors and positively correlates with AR and exhibits a nuclear colocalization with AR in advanced prostate tumors.
Materials and Methods
Microarray analysis
The conditions for the microarray are found in our previous publication (see reference 27 below). Briefly, we used the Affymetrix (GeneChip Human Genome U95Av2 Array) with C14 (LNCaP) and A103 (AR-expressing PC-3) PCa cells.
Cell culture, siRNA transfection, and androgen treatment
LNCaP, C81, CWR-22Rv1, A103, and PC-3 cells were grown as previously described (26) (from ATCC, passage 15–30 for all cell lines). For androgen treatment, cells were grown in medium containing 2% fetal bovine serum extracted with dextran-coated charcoal (DCC) and ethanol or 1nM R1881, a synthetic androgen, was added 48 hours later. TM4SF3 On-Target Plus Smart Pool small interfering RNA (siRNA), TM4SF3 (3′UTR, untranslated region) (CAGAUAUCUUCUAGACAUAUU), control siRNA, and AR siRNA (Dharmacon) (27) were transfected at 50nM final concentration into cells using Lipofectamine siMAX (Invitrogen) or Lipofectamine 2000 (Invitrogen). Cell extracts were prepared using 2% sodium dodecyl sulfate (SDS) and then subjected to Western blotting.
Reporter assay and plasmid transfection
LNCaP cells were grown in RPMI 1640 with 2% DCC-stripped serum. After 24 hours, cells were transiently transfected with 0.1-μg ARE-Luc reporter plasmid, control siRNA or TM4SF3 (3′UTR specific) siRNA, and pCH110, which encodes β-galactosidase and was used to control for transfection efficiency (28). Lipofectamine 2000 (Invitrogen) was used for transfection and Luciferase activity was measured using the Luciferase assay system from Promega. LNCaP and C81cells were transiently transfected with TM4SF3 siRNA, control siRNA, 2-μg TM4SF3/myc-DDK (Origene), and 48 hours after transfection, cell extracts were prepared using 2% SDS and subjected to Western blotting.
Cell proliferation
For proliferation assays, LNCaP, C81, and PC-3 cells were transfected with TM4SF3 siRNA and control siRNA using 2% DCC-stripped serum then 72 hours after transfection, 20 000 cells were seeded in 24-well plates. The MTT assay (Sigma) was used as before (29) to determine cell number.
Cell invasion and migration assay and adenovirus infection
Cell invasion was measured using the Cell CytoSelect 24-Well Cell Migration and Invasion Assay Combo kit, 8 μm (fluorometric quantitation) (Cell Biolabs) following the manufacturer's protocol. Briefly, cell suspensions containing 100 000 cells/mL (in serum-free medium), which had been treated with or without 1nM R1881, were transfected TM4SF3 siRNA (3′UTR specific) or control siRNA in the absence or presence of TM4SF3 adenovirus (hTSPAN8-His-adenovirus from Abcam). LNCaP, C81, and PC-3 cells were infected with 20 multiplicity of infection of virus. Cells were used to monitor cell invasion into a lower chamber containing RPMI 1640 medium with 10% DCC-stripped serum. After 72 hours of incubation at 37°C, cells were quantified by measuring fluorescence using a microplate reader.
Prostate tissues
Prostate tumors (12 normal and 21 tumors tissues with Gleason scores 3–8 and 2 matched pairs with Gleason scores 6–7) were obtained from the Cooperative Human Tissue Network. The tumor tissue came from 23 different patients. Protein extracts were prepared by boiling tissues in 3× SDS buffer and then subjected to Western blotting to measure TM4SF3 and AR protein levels. A tumor microarray (PR242a from US Biomax) was analyzed by immunocytochemistry for expressions and subcellular localizations of AR and TM4SF3. This tumor microarray contains tumor tissue from 10 patients (Gleason grades 2–5 and Gleason scores 3–9) and 1 normal tissue sample.
Quantitative RT-PCR
Total mRNA was isolated from LNCaP or PC-3 cells using the TRIzol reagent following the manufacturer's protocol (Invitrogen) and then subjected to quantitative real-time PCR (Q-RT-PCR) analysis as described (6). The PCR upstream and downstream primers, respectively, used for each gene were: prostate specific antigen (PSA), 5′-GCAGCATTGAACCAGAGGAG-3′ and 5′-CCCATGACGTGATACCTTGA-3′; AR, 5′-CAATGAGTACCGCATGCAC-3′ and 5′-GCCCATCCACTGGAATAATG-3′; TM4SF3 5′UTR, 5′-TGCCCCAGGAGCTATGACAAGCA-3′ and 5′-TGCTCTGGA GCAACTTGCCTGC-3′; TM4SF3 5′UTR + CDS 5′-CTGGGCTTCCTGGGATGCTGC-3′ and 5′-TACCTGTCGCCACCTGCAGG-3′; TM4SF3 CDS 5′-GCAGGCAAGTTGC TCCAGAGCA-3′ and 5′-ACCTGTCGCCACCTGCAGGA-3′; TM4SF3 3′UTR + CDS 5′-TGGTCCTGTATTGCCAGATCGGGA-3′ and 5′-TCTGTGGTCTAGCTAGCCGA GACA-3′; and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-CGACCACTTTGTCAAGCTCA-3′ and 5′-AGGGGAGATT CAGTGTGGTG-3′. GAPDH was used as a control for mRNA amount. Q-RT-PCR measurements are given relative to GAPDH expression.
Western blotting and cell fractionation
Western blotting was performed as described (27) using antibodies against TM4SF3 (Abcam), AR (Abcam and Santa Cruz Biotechnology, Inc), Lamin A (Sigma-Aldrich), β-tubulin (Abcam), Retinoic Acid Receptor α (Santa Cruz Biotechnology, Inc), Na/K ATPase (Cell Signaling), Flag (Origene), and β-actin (Abcam). LNCaP, C81, A103, and PC-3 cells treated with 1nM R1881 or ethanol using DCC-stripped serum were washed with cold PBS and harvested, and 10% of the cells were saved as Input and the remaining portion was subjected to cell fractionation into cytosolic, membrane, and nuclear fractions using subcellular Fractionation kit (Thermo scientific). The fractions were subjected to Western blotting to measure AR and TM4SF3 protein levels, which were quantified using ImageJ software. Note that for Western blottings containing cell extracts, equal amount (30 μg) of protein was loaded on the gel.
Immunoprecipitation (IP) and cobalt pulldown
IP experiments were performed as described previously (26, 29). Whole-cell extracts from LNCaP and C81cells were subjected to IP using Protein A/G plus Agarose (Santa Cruz Biotechnology, Inc). IP antibodies used were against TM4SF3 (Bioss), AR (Santa Cruz Biotechnology, Inc), and rabbit or mouse IgG (Santa Cruz Biotechnology, Inc) as controls.
For in vitro interaction, we used TNT coupled reticulocyte lysate systems from (Promega). For the IP experiment, the plasmids used were 1-μg AR/CMV and TM4SF3/myc-DDK tag (Origene). The protocol was followed as per the manufacturer's manual. For the Cobalt pulldown experiment, H6-AR/pTL1, TM4SF3-tGFP, and tGFP (pCMV6-Sv-GFP from Origene) were expressed in vitro, mixed together, and subjected first to an IP using an anti-tGFP antibody (Origene) and then to a pulldown using HisPur Cobalt Resin (Origene). Antibodies were against TM4SF3 (Bioss), AR (Santa Cruz Biotechnology, Inc), Flag (Origene), and tGFP (Origene) in Western blotting to detect the AR and TM4SF3 proteins.
Immunocytochemistry/immunohistochemistry
Immunocytochemistry was used to study the subcellular localization of TM4SF3 and AR in LNCaP and C81, PC-3, and A103 cells, as described (28), and in prostate tumors of different stages (US Biomax). Reagents used were anti-TM4SF3 antibody (Abcam) and anti-AR antibody (Santa Cruz Biotechnology, Inc). The anti-TM4SF3 antibody was detected using an antirabbit secondary conjugated to Alexa Fluor 488 and the anti-AR using an antimouse secondary antibody conjugated to Alexa Fluor 546 (Life Technologies). Note that all micrographs were taken at the same microscope settings, using fluorescence microscopy, and all images in a group were processed identically. Note that all images were taken with a magnification of ×60.
Results
TM4SF3 is a target of complex androgen regulation in PCa cells
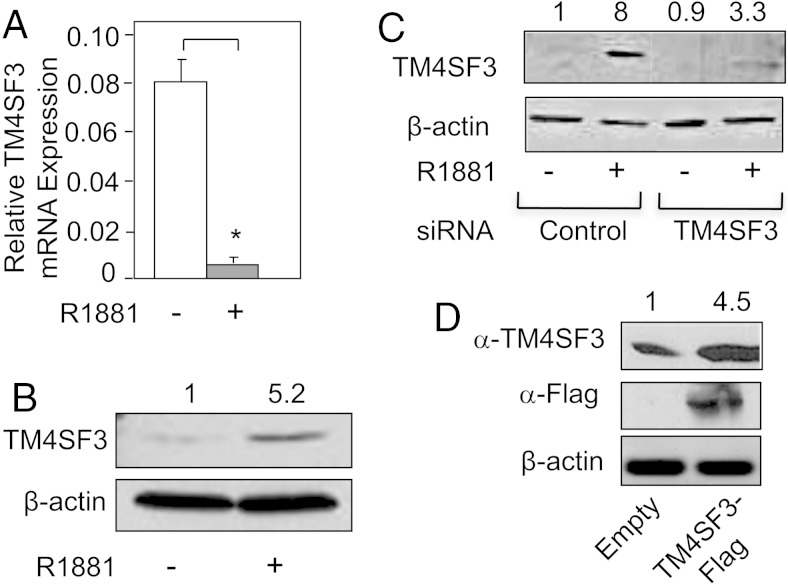
Using gene microarray, our lab has identified several novel androgen-regulated genes. Most of these genes are androgen-induced and several, including Soluble Guanylate Cyclase (sGCα1) (27), Ets Variant (ETV1) (6), and Multi-Drug Resistance Protein (MRP4) (30), have been published to have important biological roles in PCa. Among the fewer androgen-repressed genes, the TM4SF3 gene was unique in its androgen regulation: mRNA expression was reduced, whereas protein expression increased. As shown in Figure 1A, we were able to confirm the gene microarray data by performing Q-RT-PCR, which showed that androgen-repressed TM4SF3 mRNA expression in LNCaP cells. In contrast to the mRNA, the TM4SF3 protein level increased in response to R1881 treatment of LNCaP cells, as measured by Western blotting (Figure 1B). To confirm these surprising results, we employed multiple approaches for the both mRNA and protein.
Figure 1.
Androgen down-regulates TM4SF3 mRNA and up-regulates the protein. LNCaP cells were treated with either ethanol (−) or 1nM R1881 (+) and measured for expression of TM4SF3 (A) mRNA by Q-RT-PCR or (B) protein by Western blotting. CDS primers were used to measure TM4SF3 mRNA expression. LNCaP cells were transfected with (C) control or TM4SF3 siRNA or (D) empty vector or TM4SF3-Flag vector, and protein expression was measured by Western blotting using an anti-TM4SF3 or anti-Flag antibody. The Student's t test was performed to show statistical significance (P < .05), as indicated by asterisks. Note that β-actin (B–D) was used as a loading control. For B–D, the numbers above the gel represent average quantifications of 3 TM4SF3 Western blottings, standardized to β-actin, and relative to the first lane, which was set to 1.
We verified androgen down-regulation of the mRNA using 4 different primer pairs that cover different regions of the TM4SF3 mRNA, including the coding region and untranslated regions (both 5′ and 3′ UTRs) (Supplemental Figure 1A). For the TM4SF3 protein, we first used a siRNA targeting TM4SF3, whose transfection into LNCaP cells resulted in a significant reduction in expression of the TM4SF3 protein detected by our antibody (Figure 1C). Secondly, we expressed exogenous TM4SF3 from an expression plasmid, and this led to a significant increase in the TM4SF3 protein, as once again detected by our antibody (Figure 1D). These data, together with the correct predicted molecular weight based on our Western blotting, confirmed that our antibody was indeed detecting the TM4SF3 protein. Subsequent time-course experiments revealed that androgen up-regulation of TM4SF3 protein (see Supplemental Figure 2B) occurs earlier than androgen down-regulation of TM4SF3 mRNA (Supplemental Figure 1B). It is likely that reduction of mRNA follows the translation process, thereby providing a mechanism of mRNA turnover after it has been used as a template in translation.
Androgen stabilizes the TM4SF3 protein
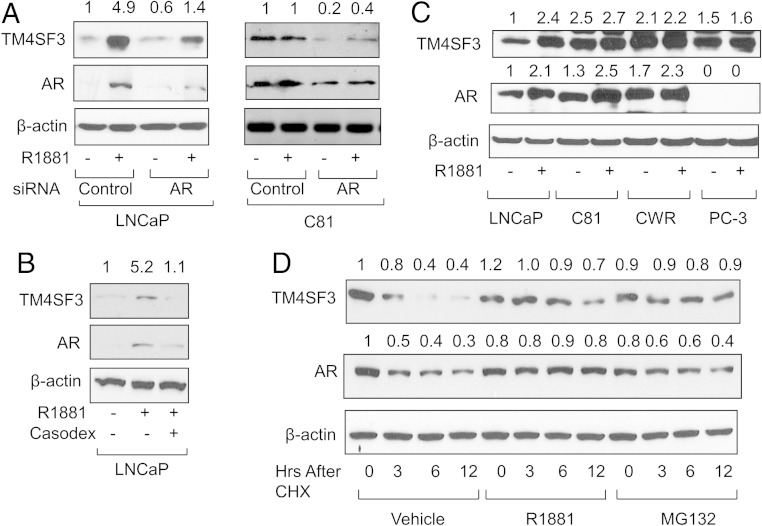
As expected, androgen up-regulation of TM4SF3 protein depends on AR. Knockdown of AR by siRNA resulted in markedly reduced levels of TM4SF3 protein in both the absence and presence of R1881 in both LNCaP and C81 cells (Figure 2A); Casodex treatment had a similar effect (Figure 2B). R1881 treatment increased TM4SF3 protein levels in LNCaP and VCaP (Supplemental Figure 2A) cells but had no effect in the hormone-refractory C81 and CWR-22Rv1 cells (Figure 2C). Indeed, the TM4SF3 protein levels in C81 and CWR-22Rv1 cells without hormone were comparable with those in LNCaP and VCaP cells with hormone (Figure 2C), mimicking what has been seen with AR expression (31). However, this elevated, unresponsive TM4SF3 protein expression was still dependent on AR, because AR knockdown dramatically reduced the TM4SF3 protein in C81 cells (Figure 2A). TM4SF3 protein expression was also high in PC-3 cells and not affected by hormone (Figure 2C). By contrast, TM4SF3 protein was barely detectable in primary prostate epithelial cells (Supplemental Figure 2A) (32).
Figure 2.
AR is required for androgen up-regulation of TM4SF3 protein. Western blotting was used to measure TM4SF3 and AR expression in LNCaP or C81 cells transfected with (A) control or AR siRNA or (B) LNCaP cells treated with 10μM Casodex, (C) or in various other PCa cells. CWR represents CWR-22Rv1 cells. Cells were treated with either ethanol (−) or 1nM R1881 (+). D, LNCaP cells were grown for 0–12 hours in 50-μg/mL CHX and treated with ethanol (vehicle), 1nM R1881, or 10μM MG132 and measured for expression of TM4SF3 or AR by Western blotting. Note that β-actin was used as a loading control. For all the gels, the numbers above the gel represent average quantifications of 3 TM4SF3 and AR Western blottings, standardized to β-actin, and relative to the first lane, which was set to 1.
One possible mechanism for protein up-regulation is decreased degradation. Therefore, we studied the stability of the endogenous TM4SF3 protein in LNCaP cells using cycloheximide (CHX), which showed that steady state levels of TM4SF3 protein decreased over 12 hours, but only in the absence of R1881 (Figure 2D). R1881 treatment maintained these steady state levels during the first 6 hours and a decrease was observed after 12 hours (Figure 2D), consistent with the hypothesis that androgen disrupts TM4SF3 protein degradation.
These data suggest that the mechanism of androgen up-regulation of the TM4SF3 protein is by enhancing its stability. This is further supported by our time-course experiment, which showed that androgen begins to have a positive effect on the TM4SF3 after 8 hours, during which time, the TM4SF3 protein levels drop strongly without androgen (Supplemental Figure 2B). Additionally, the proteasome inhibitor MG132 was able to disrupt the time-dependent reduction in TM4SF3 protein (Figure 2D), having a similar effect on TM4SF3 protein as R1881, strongly suggesting that the protein reduction is due to proteasome-dependent degradation. Collectively, these data suggest that androgen up-regulates TM4SF3 protein levels by decreasing its proteasomal-dependent degradation.
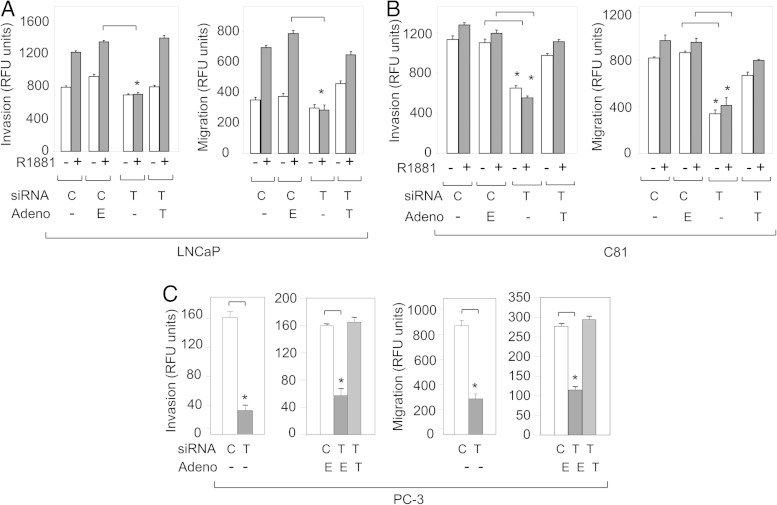
TM4SF3 promotes the migration and invasiveness of prostate cancer cells
Because tetraspanins are well known to function in metastasis (33, 34), we studied the possibility that TM4SF3 serves this function in PCa cells. Androgen treatment of LNCaP cells enhanced both invasion and migration, as expected (6), and interestingly, knockdown of TM4SF3 completely eliminated both of these cell processes (Figure 3A). Importantly, these TM4SF3 siRNA-treated cells were completely rescued for invasion and migration by an adenovirus expressing a siRNA-resistant TM4SF3-His (Figure 3A). The same findings were made in androgen-independent C81 cells (Figure 3B). A strong TM4SF3 effect on invasion and migration was also observed in PC-3 cells (Figure 3C), which express significant levels of TM4SF3 protein (see Figure 2C); these cells only express membrane-bound TM4SF3 (see figure 5 below). Just as with LNCaP and C-81 cells, PC-3 cells were completely rescued for invasion and migration by the siRNA-resistant TM4SF3-His (Figure 3C), clearly demonstrating that these metastatic processes are under TM4SF3 regulation.
Figure 3.
TM4SF3 induces invasion and migration of PCa cells. (A) LNCaP or (B) C81 cells were treated with either ethanol (−) or 1nM R1881 (+), or (C) PC-3 cells were transfected with control (C) or TM4SF3 (T) siRNA and infected with empty (E) or TM4SF3-His adenovirus (T), and monitored (A–C) for invasion and migration. Bar graphs represent averages of 3 independent experiments ± SDs. The Student's t test was performed to show statistical significance (P < .005), as indicated by asterisks.
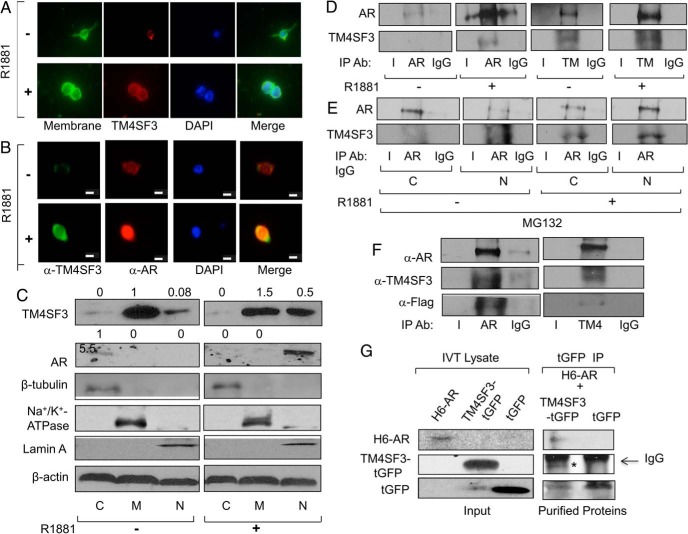
A nuclear TM4SF3 is found in PCa cells that associates with AR
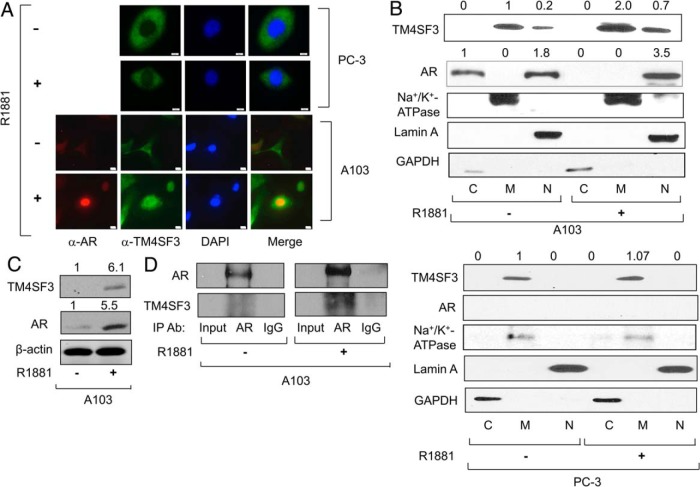
Immunocytochemistry was used to confirm the androgen-mediated up-regulation of TM4SF3 protein that was detected by Western blotting (see Figures 1 and 2). As shown in Figure 4A, androgen strongly enhances endogenous TM4SF3 levels in LNCaP cells, which colocalizes with the plasma membrane marker, as would be expected for a transmembrane protein. To study AR as well as TM4SF3, LNCaP cells were stained for both proteins, and, as expected, both proteins exhibited markedly increased levels under androgen treatment (Figure 4B). Remarkably, a merge of the 2 signals showed that a subset of endogenous TM4SF3 was nuclear in the presence of androgen, and this nuclear TM4SF3 was colocalized with nuclear AR in both LNCaP (Figure 4B) and C81 cells (Supplemental Figure 2C); note that, as expected, no signal was detected when primary antibodies or secondary antibodies were used alone (Supplemental Figure 2D). Interestingly, although androgen had little effect on AR and TM4SF3 protein levels in C81 cells, it strongly induced nuclear colocalization of AR and TM4SF3 (Supplemental Figure 2C), mimicking what was observed in LNCaP cells (see Figure 4B). Indeed, quantification of immunocytochemistry showed that nuclear localization of TM4SF3 was found in 0% cells without R1881 and 66.7% with R1881 for LNCaP cells, and 17% and 45% for C81 cells. To confirm this surprising nuclear localization of TM4SF3, we performed cell fractionation followed by Western blotting. Without hormone, TM4SF3 was mainly localized in the membrane fraction with a small amount of nuclear protein in LNCaP cells, whereas with hormone, the nuclear levels of TM4SF3 increased strongly (Figure 4C), consistent with the immunocytochemistry data (see Figure 4B). A similar androgen-dependent nuclear localization of AR and TM4SF3 finding was made in hormone-refractory C81 cells (Supplemental Figure 2E). Collectively, these data show that a fraction of endogenous TM4SF3 in PCa cells is localized in the nucleus, and this nuclear fraction increases substantially when cells are treated with androgens and the AR is translocated into the nucleus. These findings represent the first demonstration of the TM4SF3 protein being localized in the nucleus and open the possibility that this nuclear localization has biological functions in PCa.
Figure 4.
TM4SF3 colocalizes and interacts with AR in PCa cells. LNCaP cells were treated with either ethanol (−) or 1nM R1881 (+) for 24 hours and measured for expression of TM4SF3 and AR by (A and B) immunocytochemistry or (C) Western blotting of cell fractions cytosol (C), membrane (M), and nuclear (N) using antibodies against TM4SF3, AR, and β-actin, which was used as loading control. Note that that the membrane marker (CellMask from Life Technologies) in A stains the plasma membrane, and DAPI was used to stain the nuclei in both A and B. Scale bar in B, is 10 μm. Cell fractions markers are β-tubulin (cytosol), Na+/K+ ATPase (membrane), and Lamin A (nuclear). LNCaP cells were treated with ethanol (−) or 1nM R1881 (+) for 24 hours and (D) whole-cell extracts or (E) cytosolic (C) and nuclear (N) extracts were subjected to IP using antibodies against AR or TM4SF3 or IgG. Note that cells in E were also treated with 10μM MG132. F, TM4SF3-Flag and AR or (G) TM4SF3-tGFP, tGFP, and H6-AR were expressed in vitro using TNT system and (F) mixed and subjected to IP using anti-AR or anti-TM4SF3 antibody or (G) Cobalt pull-down purified H6-AR was added to IP-purified TM4SF3-tGFP or tGFP. Western blotting was used to measure levels of AR, H6-AR, TM4SF3, TM4SF3-Flag, and TM4SF3-tGFP, and tGFP in C–G, including an anti-Flag antibody in F. In tGFP IP of G; **, TM4SF3-tGFP and *, IgG heavy chain. For C, the numbers above the gel represent average quantifications of 3 TM4SF3 and AR Western blottings, standardized to β-actin, and relative to the first lane, which was set to 1.
The colocalization data of Figure 4, B and C, and Supplemental Figure 2, C and E, suggest that that TM4SF3 interacts with AR in PCa cells. To study this possibility, we performed IP experiments with endogenous proteins. IP purification of AR resulted in copurification of TM4SF3 in the presence of R1881 (Figure 4D). The complementary experiment of TM4SF3 IP purification yielded some copurified AR without hormone and significantly more AR with hormone (Figure 4D). We observed a similar hormone-dependent AR-TM4SF3 interaction in C81 cells (Supplemental Figure 2F). To determine whether the AR-TM4SF3 interaction occurs in the cytoplasm, nucleus, or both, LNCaP cells were treated with MG132, in the absence or presence of R1881, to block proteasomal degradation, and cell fractions were subjected to IP using an anti-AR antibody. As shown in Figure 4E, significant levels TM4SF3 were copurified with AR in both cytosolic and nuclear fractions with hormone, suggesting that the AR-TM4SF3 interaction may begin in the cytoplasm before AR has undergone an androgen-induced nuclear translocation.
The above results (see Figure 4 and Supplemental Figure 2) show an R1881-dependent interaction and nuclear colocalization of AR and TM4SF3. To determine whether exogenous proteins can exhibit the same interaction, transfected LNCaP and PC-3 cells were used. To study exogenous TM4SF3-Flag, we expressed a FLAG-tagged TM4SF3 protein in LNCaP cells, which exhibited a small, but reproducible increase in response to androgen treatment (Supplemental Figure 3A). A similar, but stronger androgen positive effect was observed on TM4SF3-tGFP, whereas there was no effect on tGFP (Supplemental Figure 3B). Equally important, cell fractionation in LNCaP cells treated with R1881 indicated that the exogenous TM4SF3 is distributed mainly in the membrane fraction and significant amount in the nuclear fraction (Supplemental Figure 3C), clearly demonstrating that the exogenous TM4SF3 protein behaves similarly to its endogenous counterpart. To study exogenous AR, we used PC-3 cells stably expressing AR (A103 cells) (26, 35). As expected, A103 cells exhibited both a cytosolic and nuclear AR localization without hormone (26, 35) and exclusively nuclear and elevated levels with hormone (Figure 5, A and B), mimicking the androgen effects observed in AR-positive cells (36). With respect to TM4SF3 without hormone, most of the protein in A103 cells was found in the membrane fraction with a small level of nuclear protein (Figure 5, A and B), whereas in PC-3 cells, TM4SF3 was found exclusively in the membrane fraction (Figure 5, A and B). Interestingly, R1881 increased both the total TM4SF3 cellular protein levels (Figure 5, A and C) and the amount of TM4SF3 protein colocalizing with AR in the nucleus (Figure 5, A and B) in A103 cells; R1881 had no effect in PC-3 cells (Figure 5B). Theses colocalization data suggest that AR and TM4SF3 interact in A103 cells, and this was confirmed by IP experiments. As shown in Figure 5D, IP of AR resulted in copurification of TM4SF3, with more AR and TM4SF3 copurified in the presence of R1881. Collectively, these results show that we have successfully reconstituted the AR-TM4SF3 interaction in PC-3 cells with exogenous AR that mimics what we observed with endogenous proteins in AR-positive PCa cells.
Figure 5.
TM4SF3 colocalizes and interacts with AR in AR-expressing PC-3 cells. PC-3 or A103 cells were treated with either ethanol (−) or 1nM R1881 (+) and measured for expression of TM4SF3 and AR by (A) immunocytochemistry or (B and C) Western blotting of (B) A103 or PC-3 cell fractions (cytosol [C], membrane [M], and nuclear [N]) or of (C) A103 whole-cell extracts. Scale bar in A, 10μM. Cell fractions markers are GAPDH (cytosol) marker, Na+/K+ ATPase (membrane), and Lamin A (nuclear marker); β-actin controls for protein loading. D, IP experiments were performed with whole-cells extracts from A103 cells treated with ethanol (−) or 1nM R1881 (+) using antibodies against AR or IgG. Western blotting was used to measure levels of copurified AR and TM4SF3. DAPI was used to stain the nuclei in A. For B and C, the numbers above the gel represent average quantification of 3 TM4SF3 and AR Western blottings, standardized to β-actin, and relative to the first lane, which was set to 1.
The above all results show an androgen induced AR-TM4SF3 interaction in both hormone-dependent and hormone-independent PCa cells and the immunocytochemistry and cell fractionation data suggest that this interaction takes place in the cell nucleus. To determine whether AR and TM4SF3 directly associate, we expressed both proteins in vitro using reticulocyte lysate. In vitro expressed AR and TM4SF3-Flag were incubated together and then subjected to an AR IP, resulting in purification of AR and, importantly, copurification of TM4SF3-Flag, as detected by anti-TM4SF3 and anti-Flag antibodies (Figure 4F). Stronger data for a direct interaction came from a second in vitro translation experiment, in which Cobalt-purified H6-AR interacted with IP-purified TM4SF3-tGFP, but not tGFP (Turbo-GFP) (Figure 4G). These data strongly suggest that AR and TM4SF3 interact directly and demonstrate that either the Flag or GFP tag at the C terminus of TM4SF3 does not interfere with its association with AR and R1881 is not required for this in vitro interaction.
TM4SF3 regulates AR protein stability and activity in PCa cells
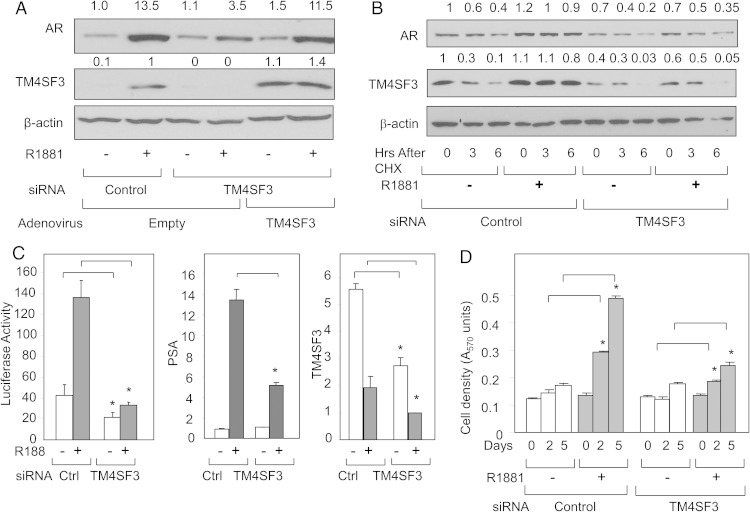
To begin understanding what functions a nuclear TM4SF3 has, we focused on its new interaction partner, AR. First, we monitored AR protein levels when TM4SF3 expression was depleted by siRNA, and, to our surprise, this elicited a marked decrease in AR protein levels (Figure 6A). To ensure that this effect was not due to some off-target effect of the TM4SF3 siRNA (a Blast search showed that the siRNA sequence is specific for TM4SF3), we used an adenovirus to express exogenous TM4SF3. Adenovirus expression of TM4SF3 fully rescued AR protein levels (Figure 6A), clearly demonstrating that the TM4SF3 protein is directly involved in regulating AR protein concentration. The same TM4SF3 positive effect on the AR protein was observed in the hormone-refractory C81 cells (Supplemental Figure 5A). Using TM4SF3 siRNA in the presence of CHX, we have determined that TM4SF3 affects the stability of the AR protein (Figure 6B), mimicking the effect AR has on TM4SF3 protein (see Figure 2D). Collectively, our data strongly suggest that the interaction between TM4SF3 and AR results in the mutual stabilization of both proteins in PCa cells.
Figure 6.
TM4SF3 up-regulates the AR protein and androgen signaling. LNCaP cells were transfected with control or TM4SF3 siRNA and (A) infected with empty or TM4SF3 adenovirus in the presence of ethanol (−) or 1nM R1881 (+) or (B) treated with 50-mg/mL CHX for the indicated times. Expression of TM4SF3 and AR was measured by Western blotting. Note that β-actin was used as loading control. LNCaP cells were transfected with control or TM4SF3 siRNA and treated with ethanol (−) or 1nM R1881 (+) and then measured for (C) activity of transfected luciferase or expression of endogenous PSA or TM4SF3, or (D) cell proliferation using the MTT assay. Bar graphs represent averages of 3 independent experiments ± SDs. CDS primers were used to measure TM4SF3 mRNA expression. The Student's t test was performed to show statistical significance (P < .05), when comparing cells transfected with TM4SF3 siRNA with control siRNA as indicated by asterisks. For A and B, the numbers above the gel represent average quantifications of 3 AR and TM4SF3 Western blottings, standardized to β-actin, and relative to the first lane, which was set to 1.
To monitor a possible TM4SF3 function on AR activity, we measured transcriptional activity and cell proliferation in LNCaP cells. TM4SF3 knockdown markedly compromised AR transcriptional activity, as measured by both a reporter gene assay and expression of the endogenous target gene PSA (Figure 6C). The same negative effect was observed on androgen-induced cell growth, as TM4SF3 depletion severely attenuated LNCaP cell proliferation (Figure 6D). Similar results were obtained in C81 cells, as TM4SF3 knockdown resulted in significantly compromised androgen-induced AR transcriptional activity (Supplemental Figure 5B) and cell proliferation (Supplemental Figure 5C). Importantly, there is no effect of TM4SF3 knockdown on the growth of PC-3 cells (Supplemental Figure 5D), which express TM4SF3 but not AR (see Figure 2C). Together, these data clearly demonstrate that the reduced AR protein levels resulting from TM4SF3 knockdown have significant consequences on AR-induced gene expression and cell proliferation only in AR-positive PCa cells.
TM4SF3 is overexpressed in prostate tumors and correlates with AR overexpression
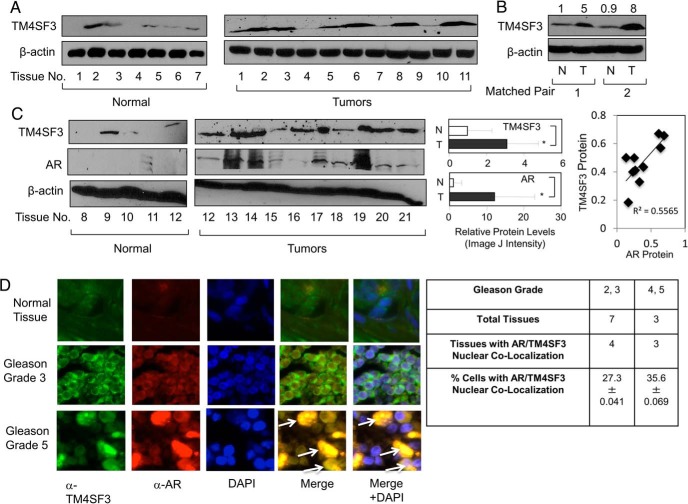
Validation of TM4SF3 importance in PCa depends on demonstrating its expression in human tumor tissues. As shown in Figure 7A, most prostate tumors expressed markedly higher levels of TM4SF3 protein than normal prostate tissues. To control for variability among different patients, we examined matched pairs of tissues, and in both cases studied TM4SF3 was significantly higher in PCa than normal tissues (Figure 7B). Because the tissues of Figure 7, A and B, were no longer available to examine AR protein expression, we studied additional tissues and these too showed elevated levels of TM4SF3 and AR in tumors as compared with normal (Figure 7C, right panel). Importantly, the second set of tissue also strong a correlation between AR and TM4SF3 protein levels (Figure 7C, far-right panel). When protein levels were quantified in all tissues, we observed significantly higher levels of both AR and TM4SF3 proteins in tumors as compared with normal prostate (Figure 7C, right panel) and a direct correlation between TM4SF3 and AR (Figure 7C, far-right panel). These expression data in tumors mimic what was observed in PCa cells (see Figure 2C) and suggest that costabilization of AR and TM4SF3 also occurs in prostate tumors. To directly test this possibility, we used tumor arrays and immunofluorescence to study the subcellular localizations of AR and TM4SF3. As shown in Figure 7D, AR and TM4SF3 fluorescent signals were higher in Gleason grade 5 tumors than Gleason grade 3 tumors, whereas normal tissue expressed the little of either protein. More importantly, a Gleason grade 5 tumor had multiple cells exhibiting a nuclear colocalization of AR and TM4SF3 (Figure 7D). Quantification showed that AR/TM4SF3 nuclear colocalization was found in 57% (4/7) of Gleason grade 2, 3 tissues (27.3% of total cells were positive) and 100% (3/3) of Gleason grade 4, 5 tissues (35.6% cells) (Figure 7D, right panel). These data provide strong evidence for a nuclear-localized TM4SF3 in prostate tumor cells.
Figure 7.
Nuclear TM4SF3 and AR are overexpressed in PCa. Western blotting was used to measure TM4SF3 and AR expression in (A and C) normal and tumor tissues and (B) matched pairs of normal (N) and tumor (T) from same patients. Note that β-actin was used as a loading control. TM4SF3 and AR protein levels were significantly higher (P < .05) in tumors as compared with normal, analyzed by Student's t test. Linear regression analysis was done to compare protein levels of TM4SF3 and AR, which were significant as represented by R2 = 0.55655 and P = .03. It is important to mention that both normal prostate and cancer tissues have been previously shown (see Ref. 6) to have mainly epithelial cells based, on expression of cytokeratin 18 (K18), a maker of epithelial cells, and keratinocyte growth factor (KGF), a marker gene for prostate stromal cells. For B, the numbers above the gel represent quantification of the TM4SF3 Western blotting signals, standardized to β-actin, and relative to the first lane, which was set to 1. D, Normal prostate tissue and different grades of prostate tumors (from US Biomax) were subjected to immunocytochemistry to monitor the relative expression levels and subcellular localization TM4SF3 and AR. DAPI stains nuclei. Arrows in merge images shows colocalization of TM4SF3 and AR in both nucleus and cytoplasm. Table shows quantification of number of tumors and % of cells having positive AR/TM4SF3 nuclear colocalization (±SD). Student's t test showed that a statistically significant higher (P < .05) number of cells of grade 4/5 than grade 2/3.
Discussion
AR is key to the development and progression of PCa (27). Much of the research on AR in PCa has focused on its regulation of gene expression, which has led to identification of several genes shown important roles in PCa (37). In this study, we have identified a novel mechanism of AR action, regulating the stability of the TM4SF3 protein. TM4SF3 is member of the tetraspanin family of transmembrane proteins, whose functions in cancer are well established (21, 38). AR regulation of target protein stability is poorly understood, with the only published example being Cdc25C, which exhibits androgen-mediated protein stabilization; androgen also up-regulates the Cdc25C mRNA (39). On the other hand, we show here that androgen positively affects the TM4SF3 protein and negatively the mRNA.
Among the tetraspanins, TM4SF3 is the most poorly understood with regard to its role in PCa. TM4SF3 is highly expressed in various human cancers, including hepatocarcinoma (23), esophageal carcinoma (22), and pancreatic adenocarcinoma (21). The limited studies in these tumors have determined that TM4SF3 has metastasis-promoting activities (40). Importantly, our data here clearly demonstrate similar functions in PCa, as knockdown of endogenous TM4SF3 significantly diminished cell migration and invasiveness of both LNCaP and PC-3 cells, strongly demonstrating that prometastatic functions of TM4SF3 do not depend on interaction with AR. Thus, in LNCaP cells, TM4SF3 functions in metastasis are likely happening in concert with AR and independent of AR, and the significance of each will be a focus of future research. These metastatic functions TM4SF3 support what has already been published for the related protein CD151 in hepatocellular carcinoma cells (41). In contrast to this role of TM4SF3 in cell migration/invasion, its role in cell proliferation depends on interaction with AR, because LNCaP exhibit reduced proliferation when TM4SF3 is depleted while PC-3 proliferation is unaffected.
Although the prometastatic functions of TM4SF3 described above were expected, its nuclear interaction with AR and effect on androgen-induced cell proliferation were completely surprising. All tetraspanin proteins thus far have shown to be localized to the cell surface, with the only exception being CD9, which exhibits a nuclear localization in melanoma cells (42). TM4SF3 is the first example of a tetraspanin exhibiting a nuclear localization in PCa cells and, importantly, prostate tumor cells. Interestingly, our data suggest that the TM4SF3 nuclear localization depends on androgen binding to AR, which is known to trigger nuclear localization of this receptor (43). More evidence for the necessity of AR in TM4SF3 nuclear localization came from our studies here with PC-3 cells, which show that TM4SF3 is localized to the cell surface in parental cells but becomes also localized to the nucleus in A103 cells, PC-3 cells expressing exogenous AR (26, 35), and just like LNCaP cells, this TM4F3 nuclear localization depends on androgen treatment. Thus, in this report, we demonstrate this nuclear interaction in both LNCaP cells, with endogenous AR, and PC-3 cells, with exogenously expressed AR, strongly suggesting that the TM4SF3-AR interaction does not depend on endogenous AR or cell-specific factors. Indeed, our data strongly suggest a direct interaction between TM4SF3 and AR, because in vitro-translated proteins can interact. Thus, ligand binding induces not only dimerization and nuclear localization of AR but also its interaction with TM4SF3. How might this happen? It is possible that ligand binding induces a conformation on AR that allows it to interact with TM4SF3 in the cytoplasm and the complex then translocates into the cell nucleus. In support of this model, we were able to detect an AR-TM4SF3 interaction, not only in the nucleus but also in the cytoplasm of PCa cells, and this interaction occurred in the absence of hormone. Thus, it is possible that AR and TM4SF3 began their interaction in the cytosol and androgen binding to AR will enhance this binding and cause AR nuclear localization. Although we cannot rule out that TM4SF3 has a cryptic nuclear localization signal, our failure to identify a putative nuclear localization signal using bioinformatics analysis and our finding that ligand-activated AR is required for TM4SF3 nuclear localization strongly argue against this possibility. Our working hypothesis is that TM4SF3 moves into the nucleus via its interaction with AR. Such a “piggy-back” mechanism has been demonstrated for several proteins, including subunits of the heterotrimeric CCAAT-binding complex (44) and several viral proteins (45).
Posttranslational modifications of AR affect its stability and enhance AR transactivation in response to androgen-depleted conditions in CRPC (46). It was recently published that CDK5-mediated serine phosphorylation of AR prevents its proteasome-dependent degradation and subsequent activation of AR (47). With regard to TM4SF3 protein turnover, there are no published data; in fact, our data here provide the first evidence for proteasomal degradation of TM4SF3. Although an ubiquitination consensus site does not exist, it is a well-established fact that ubiquitin molecules are predominantly added to lysine residues on target proteins (48). Using this knowledge and the conservation found among human tetraspanins, Lineberry et al (49) identified several lysines at the cytosolic amino terminus of CD151 and CD81 as ubiquitination sites. Interestingly, TM4SF3 has a lysine as residue 9, at a similar position to the ubiquitinated lysines on CD151 and CD81 (49); other lysines are found away from the amino terminus.
The mutual stabilization exhibited by AR and TM4SF3 is uncommon for interacting proteins. There are only a few examples published, including the interaction between LZAP (LXXLL/Leucine Zipper-containing alternative reading frame [ARF]-binding protein) and novel LZAP-binding protein in liver cancer cells (50) and between stimulated by retinoic acid gene 13 protein and 58-kda microspherule protein in mammalian cells (51). Although the AR-TM4SF3 interaction may be rare for interacting proteins, the mutual stabilization resulting from it provides PCa with an enhanced ability to survive, grow, and undergo metastasis, 3 essential steps for the deadly form of this disease. Although it is possible that that AR and TM4SF3 are regulated by a common upstream factor, interaction between these 2 proteins is still necessary for their mutual enhanced stabilization. Because this interaction is likely direct, as our data suggest, further studying the AR-TM4SF3 interaction in the future will shed light on a novel regulation of these 2 proteins and provide a means by which to disrupt the complex and thus destabilize both proteins. Thus, this would provide a new therapeutic mechanism for targeting AR, distinct from both the recently published small molecules that inhibit AR interaction with Bromodomain-containing proteins (52) and the long-used antiandrogen therapy that has limited efficacy in CRPC (53).
Additional material
Supplementary data supplied by authors.
Acknowledgments
Present address for M.B.: Johns Hopkins University School of Medicine, 1550 Orleans Street, CRB2, Baltimore, MD 21231.
This work was supported by grants from the National Institutes of Health and internal money from the University of Toledo.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- CHX
- cycloheximide
- CRPC
- castration-resistant PCa
- DCC
- dextran-coated charcoal
- IP
- immunoprecipitation
- PCa
- prostate cancer
- PSA
- prostate specific antigen
- Q-RT-PCR
- quantitative real-time PCR
- SDS
- sodium dodecyl sulfate
- siRNA
- small interfering RNA
- TM4SF3
- transmembrane 4 superfamily 3.
References
- 1. Huggins C, Stevens RE, Jr, Hodges CV. Prostatic cancer. II: the effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 2. Ross RW, Xie W, Regan MM, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112:1247–1253. [DOI] [PubMed] [Google Scholar]
- 3. Gregory CW, Hamil KG, Kim D, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58:5718–5724. [PubMed] [Google Scholar]
- 4. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. [DOI] [PubMed] [Google Scholar]
- 5. Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 6. Cai C, Hsieh CL, Omwancha J, et al. ETV1 is a novel androgen receptor-regulated gene that mediates prostate cancer cell invasion. Mol Endocrinol. 2007;21:1835–1846. [DOI] [PubMed] [Google Scholar]
- 7. Gaughan L, Logan IR, Neal DE, Robson CN. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005;33:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144:735–746. [PMC free article] [PubMed] [Google Scholar]
- 9. Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. [DOI] [PubMed] [Google Scholar]
- 10. Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofland J, van Weerden WM, Dits NF, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–1264. [DOI] [PubMed] [Google Scholar]
- 13. Cai C, Wang H, Xu Y, Chen S, Balk SP. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res. 2009;69:6027–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. [DOI] [PubMed] [Google Scholar]
- 16. Tomlins SA, Laxman B, Varambally S, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu J, Yu J, Mani RS, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang S, Yuan S, Dong M, et al. The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics. 2005;86:674–684. [DOI] [PubMed] [Google Scholar]
- 19. Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci. 2003;28:106–112. [DOI] [PubMed] [Google Scholar]
- 20. Seigneuret M, Delaguillaumie A, Lagaudrière-Gesbert C, Conjeaud H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J Biol Chem. 2001;276:40055–40064. [DOI] [PubMed] [Google Scholar]
- 21. Gesierich S, Berezovskiy I, Ryschich E, Zöller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. [DOI] [PubMed] [Google Scholar]
- 22. Zhou Z, Ran YL, Hu H, et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin Exp Metastasis. 2008;25:537–548. [DOI] [PubMed] [Google Scholar]
- 23. Kanetaka K, Sakamoto M, Yamamoto Y, et al. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J Hepatol. 2001;35:637–642. [DOI] [PubMed] [Google Scholar]
- 24. Greco C, Bralet MP, Ailane N, et al. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 2010;70:7674–7683. [DOI] [PubMed] [Google Scholar]
- 25. Gesierich S, Paret C, Hildebrand D, et al. Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: impact on cell motility. Clin Cancer Res. 2005;11:2840–2852. [DOI] [PubMed] [Google Scholar]
- 26. Cai C, Hsieh CL, Gao S, et al. Soluble guanylyl cyclase α1 and p53 cytoplasmic sequestration and down-regulation in prostate cancer. Mol Endocrinol. 2012;26:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai C, Chen SY, Zheng Z, et al. Androgen regulation of soluble guanylyl cyclasealpha1 mediates prostate cancer cell proliferation. Oncogene. 2007;26:1606–1615. [DOI] [PubMed] [Google Scholar]
- 28. Cai C, Hsieh CL, Shemshedini L. c-Jun has multiple enhancing activities in the novel cross talk between the androgen receptor and Ets variant gene 1 in prostate cancer. Mol Cancer Res. 2007;5:725–735. [DOI] [PubMed] [Google Scholar]
- 29. Gao S, Hsieh CL, Bhansali M, Kannan A, Shemshedini L. A peptide against soluble guanylyl cyclase α1: a new approach to treating prostate cancer. PLoS One. 2013;8:e64189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai C, Omwancha J, Hsieh CL, Shemshedini L. Androgen induces expression of the multidrug resistance protein gene MRP4 in prostate cancer cells. Prostate Cancer Prostatic Dis. 2007;10:39–45. [DOI] [PubMed] [Google Scholar]
- 31. Karan D, Kelly DL, Rizzino A, Lin MF, Batra SK. Expression profile of differentially-regulated genes during progression of androgen-independent growth in human prostate cancer cells. Carcinogenesis. 2002;23:967–975. [DOI] [PubMed] [Google Scholar]
- 32. Sobel RE, Wang Y, Sadar MD. Molecular analysis and characterization of PrEC, commercially available prostate epithelial cells. In Vitro Cell Dev Biol Anim. 2006;42:33–39. [DOI] [PubMed] [Google Scholar]
- 33. Hemler ME. Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer. 2014;14:49–60. [DOI] [PubMed] [Google Scholar]
- 34. Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- 36. Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266(1):510–518. [PubMed] [Google Scholar]
- 37. Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2014;33:2815–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berthier-Vergnes O, Kharbili ME, de la Fouchardière A, et al. Gene expression profiles of human melanoma cells with different invasive potential reveal TSPAN8 as a novel mediator of invasion. Br J Cancer. 2011;104:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chou YW, Zhang L, Muniyan S, et al. Androgens upregulate Cdc25C protein by inhibiting its proteasomal and lysosomal degradation pathways. PLoS One. 2013;8:e61934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. [DOI] [PubMed] [Google Scholar]
- 41. Ke AW, Shi GM, Zhou J, et al. CD151 amplifies signaling by integrin α6β1 to PI3K and induces the epithelial-mesenchymal transition in HCC cells. Gastroenterology. 2011;140:1629–1641 e1615. [DOI] [PubMed] [Google Scholar]
- 42. Fan J, Zhu GZ, Niles RM. Expression and function of CD9 in melanoma cells. Mol Carcinog. 2010;49:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–968. [DOI] [PubMed] [Google Scholar]
- 44. Steidl S, Tuncher A, Goda H, et al. A single subunit of a heterotrimeric CCAAT-binding complex carries a nuclear localization signal: piggy back transport of the pre-assembled complex to the nucleus. J Mole Biol. 2004;342:515–524. [DOI] [PubMed] [Google Scholar]
- 45. Arnoys EJ, Wang JL. Dual localization: proteins in extracellular and intracellular compartments. Acta Histochem. 2007;109:89–110. [DOI] [PubMed] [Google Scholar]
- 46. Gioeli D, Paschal BM. Post-translational modification of the androgen receptor. Mol Cell Endocrinol. 2012;352:70–78. [DOI] [PubMed] [Google Scholar]
- 47. Hsu FN, Chen MC, Chiang MC, et al. Regulation of androgen receptor and prostate cancer growth by cyclin-dependent kinase 5. J Biol Chem. 2011;286:33141–33149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mattiroli F, Sixma TK. Lysine-targeting specificity in ubiquitin and ubiquitin-like modification pathways. Nat Struct Mol Biol. 2014;21:308–316. [DOI] [PubMed] [Google Scholar]
- 49. Lineberry N, Su L, Soares L, Fathman CG. The single subunit transmembrane E3 ligase gene related to anergy in lymphocytes (GRAIL) captures and then ubiquitinates transmembrane proteins across the cell membrane. J Biol Chem. 2008;283:28497–28505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kwon J, Cho HJ, Han SH, No JG, Kwon JY, Kim H. A novel LZAP-binding protein, NLBP, inhibits cell invasion. J Biol Chem. 2010;285:12232–12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ivanova AV, Ivanov SV, Lerman ML. Association, mutual stabilization, and transcriptional activity of the STRA13 and MSP58 proteins. Cell Mol Life Sci. 2005;62:471–484. [DOI] [PubMed] [Google Scholar]
- 52. Asangani IA, Dommeti VL, Wang X, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.