Abstract
Exposure to excess glucocorticoids during fetal development has long-lasting physiological and behavioral consequences, although the mechanisms are poorly understood. The impact of prenatal glucocorticoids exposure on stress responses in juvenile and adult offspring implicates the developing hypothalamus as a target of adverse prenatal glucocorticoid action. Therefore, primary cultures of hypothalamic neural-progenitor/stem cells (NPSCs) derived from mouse embryos (embryonic day 14.5) were used to identify the glucocorticoid transcriptome in both males and females. NPSCs were treated with vehicle or the synthetic glucocorticoid dexamethasone (dex; 100nM) for 4 hours and total RNA analyzed using RNA-Sequencing. Bioinformatic analysis demonstrated that primary hypothalamic NPSC cultures expressed relatively high levels of a number of genes regulating stem cell proliferation and hypothalamic progenitor function. Interesting, although these cells express glucocorticoid receptors (GRs), only low levels of sex-steroid receptors are expressed, which suggested that sex-specific differentially regulated genes identified are mediated by genetic and not hormonal influences. We also identified known or novel GR-target coding and noncoding genes that are either regulated equivalently in male and female NPSCs or differential responsiveness in one sex. Using gene ontology analysis, the top functional network identified was cell proliferation and using bromodeoxyuridine (BrdU) incorporation observed a reduction in proliferation of hypothalamic NPSCs after dexamethasone treatment. Our studies provide the first characterization and description of glucocorticoid-regulated pathways in male and female embryonically derived hypothalamic NPSCs and identified GR-target genes during hypothalamic development. These findings may provide insight into potential mechanisms responsible for the long-term consequences of fetal glucocorticoid exposure in adulthood.
During late gestation, there is a surge of endogenous glucocorticoids necessary for the development of many organ systems such as the lungs, thyroid, kidney, brain, gut, and pituitary (1, 2). Because glucocorticoids are required for fetal organ maturation, synthetic glucocorticoids (eg, dexamethasone [dex] or betamethasone) are administered to pregnant women in premature labor or at risk for preterm delivery and lead to dramatic reductions in premature infant morbidity and mortality due in large part to reduced respiratory distress, iv hemorrhage and necrotizing enterocholitis (3, 4). However, these treatments are not without risk as both preclinical and clinical studies reveal potential adverse effects of antenatal glucocorticoids on brain development. For example, antenatal exposure to excess glucocorticoid alters hypothalamic development and subsequently its ability to function in adolescence (5) and adulthood (6). Although direct effects of glucocorticoids on primary cultures of fetal neural stem cells derived from the telencephalon have been demonstrated (7, 8), the impact of these hormones on stem cells derived from the hypothalamus have not been investigated.
The hypothalamus is a sexually dimorphic brain region that integrates inputs from various central and peripheral sources to maintain homeostasis of various organ systems and whole body metabolism. Hypothalamic nuclei originate from neurogenesis in the proliferative zone of the third ventricle (9) that later will exit the proliferative zone and yield terminally mitotic cells. In animal models, excess glucocorticoids during fetal development results in increased anxiety-like (10) and depression-like (11) behavior, hypertension (12), and in humans alter hypothalamic-pituitary-adrenal axis function (5, 6). Furthermore, the impact of antenatal glucocorticoids on hypothalamic regulation of adult metabolism has been found in some cases to be sexually dimorphic. Specifically, hepatic steatosis and other growth deficits in female rats exposed to dex as fetuses derive in part from hypothalamic defects in the GH axis (ie, via GH-releasing hormone) (13). Additionally, a decrease in body core temperature in adult female rats caused by antenatal dex exposure is associated with decreased TRH expression in the hypothalamus (14). Sex-specific effects of antenatal glucocorticoids on hypothalamic programming may occur in humans as evidenced by the altered stress response in young girls but not boys associated with their fetal exposure to synthetic glucocorticoids (15).
Identifying glucocorticoid actions on cells that populate the hypothalamus could provide a better understanding of the long-term consequences on physiology and behavior resulting from fetal exposure to synthetic glucocorticoids and the basis for potential sex-specific effects of these hormones on hypothalamic programming. We therefore performed RNA-Seq analysis to identify the dex transcriptome in primary hypothalamic neural-progenitor/stem cell (NPSC) cultures derived from individual male and female embryos. Our results identify a number of genes and pathways that could play important roles in sexually dimorphic patterns of hypothalamic development and responses to glucocorticoids during fetal development.
Materials and Methods
Mouse NPSC cultures
The entire hypothalamic region was dissected from individual C57BL/6 mice embryonic day 14.5 embryos, dissociated into single cells, and grown as 3-dimensional neurosphere cultures as previously described (7, 8, 16, 17). These cultures were typically passaged every 7 days and transcriptional analysis performed at the third passage (P3). Fetal sex was determined by digesting tail tissue overnight in 200 μL of nonionic detergent buffer with 1.2 μL of proteinase K overnight at 56°C. The next day, the samples were heat inactivated at 95°C for 10 minutes and isolated DNA subjected to PCR analysis to detect the Y chromosome Sry gene.
RNA-Seq library preparation
Hypothalamic NPSCs were treated with 100nM dex (Sigma Chemicals) or vehicle (ethanol) for 4 hours. Cells were harvested in TRIzol (15596-026; Invitrogen) and RNAs isolated using the Machery-Nagel Nucelospin RNA II kit. The RNA-Seq library was generated according to manufacturer's instructions using the TruSeq Stranded Total RNA kit (RS-122-2201; Illumina) and then submitted to Tufts University Genomics Core for next generation sequencing.
RNA-Seq data analysis
After sequencing, the reads were assessed for quality using FastQC tool. Because each of the 12 samples was run on 2 lanes, the technical replicates were merged and then aligned to Ensembl GRCm38 Reference Mouse genome using Tophat2 v2.0.9. Transcript assembly was performed using Cufflinks v2.2.1 and the resulting Cufflinks assemblies from each sample were merged with the reference GTF file using Cuffmerge. Cuffdiff was run with the merged GTF file and default parameters on these samples to find the genes differentially expressed between different conditions and further refined to only contain genes induced by dex by greater than 1.5-fold. Differentially expressed genes from each of the comparisons were uploaded to Ingenuity Pathway Analysis for functional analysis (version 1.0; QIAGEN). The raw and processed data were submitted to Gene Expression Omnibus (accession number GSE55113).
Comparison with published datasets
The in-house dataset was compared with 3 previously published studies. The reads per kilobase of transcript per million mapped reads (RPKM) values for the samples belonging to the different embryonic zones in the mouse cortex by high-throughput sequencing were downloaded from the author's website (http://rakiclab.med.yale.edu/transcriptome/index.aspx) (18). The dataset was filtered to remove the rows where multiple transcripts were represented by the same gene symbols in the Cufflinks output. Similar filtering was performed with the in-house dataset. It is important to note that the data (18) were analyzed using the University of California Santa Cruz mm9 reference genome, whereas the in-house data were analyzed using the Ensemble mm10 reference genome. For the next comparison, the RMA normalized data for the C57BL/6 strain (male and female) were downloaded from Gene Expression Omnibus (accession number GSE21278) and reduced to single probe set per gene by selecting the probe set with the highest IQR to represent the genes (19). Lastly, for the last comparison, the fragments per kilobase of transcript per million mapped reads (FPKM) values for the 8 cell types were downloaded from the author's website (http://web.stanford.edu/group/barres_lab/brain_rnaseq.html) and used for comparison with the in-house dataset (20). Each of the preprocessed dataset was matched to the filtered in-house data by gene symbols to contain only the common genes present in both datasets being compared. The low expressing genes were filtered out and Pearson correlation was then performed to compare the gene expression profiles of samples from the 2 datasets.
Quantitative real-time-PCR (qRT-PCR)
For qRT-PCR, P3 hypothalamic NPSCs from multiple litters were exposed to either 100nM dex or vehicle for 4, 8, or 24 hours. Cells were harvested in TRIzol and processed for RNA isolation using chloroform extraction. cDNA synthesis was performed using iScript Select cDNA Synthesis kit (170-8897; Bio-Rad). For microRNAs, total RNAs were size fractionated and gel purified (21). The NCode VILO cDNA synthesis and qRT-PCR kit was used to polyadenylate the resulting RNA and oligo(DT) primers were used to synthesize the first strand cDNA (Invitrogen) and one universal qPCR reverse primer was used to amplify the poly-dT region of the cDNA T1 along with small RNA-specific forward primers (3′ or 5′). qRT-PCRs were performed on a Bio-Rad CFX qRT-PCR machine using iTaq Universal SYBR Green Supermix (172-5121; Bio-Rad) and primers with efficiencies calculated to be greater than 80% (Supplemental Table 1). There was high-level expression (>330 FPKMs) and no differences observed due to sex or treatment for glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in our RNA-Seq dataset, and therefore, relative expression was calculated with Gapdh as the reference gene, whereas for microRNAs, U6 served as the reference gene.
Proliferation assay using bromodeoxyuridine (BrdU) incorporation quantified using flow cytometry
P3 male and female hypothalamic NPSCs were plated and allowed to form neurospheres before 24-hour treatment with either vehicle or dex (100nM). Four hours before collection BrdU (10μM, B-9285; Sigma Chemicals) was added (ie, after a 20-h dex treatment). Cells were then harvested, manually dissociated into single cells, washed with ice-cold PBS, and fixed with 4% paraformaldehyde. Fixed cells were processed for immunocytochemistry according to standard methods and immunolabeled using rat anti-BrdU antibody (1:500, ab6326; Abcam) followed by antirat antibody conjugated to Alexa Flour 488 (1:500, A21208; Invitrogen) (Peffer et al [8]). Cells were counted using an LSRII flow cytometer (BD Biosciences) and data analyzed with FACSDiva (BD Biosciences).
In vivo analysis
Time-mated pregnant C57BL/6 mice received a single injection at embryonic day 14.5 of either dex (0.4 mg/kg) or vehicle. Embryos were collected by cesarean section 24 hours later. The entire hypothalamic region was dissected from individual embryos and homogenized in TRIzol for RNA analysis.
Statistical analysis
For RNA-Seq analysis, statistical analysis and visualization were performed using R (Bioconductor). Unique and common dex-regulated genes were obtained by taking the intersection of the significant (false detection rate of 5%) dex-regulated genes significant in both male and female dex vs veh gene lists. For in vivo gene expression, relative expression values were compared using a t test (vehicle vs dex). All other statistical significance was determined by two-way ANOVA for sex (male vs females) X treatment (vehicle vs dex) using Prism (GraphPad), values are reported as mean ± SEM, and P < .05 was considered significant. For treatment (dex, vehicle) and sex (males, females), n = 3 was used for RNA-Seq analysis, n = 4–6 for qRT-PCR (in vivo and in vitro), and n = 4 for flow cytometry.
Results
Characteristics of the mouse fetal hypothalamic NPSC transcriptome
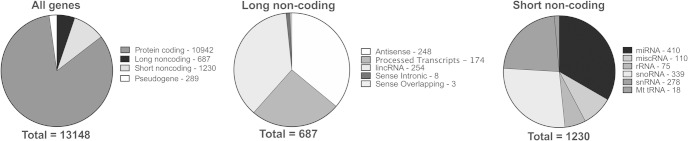
Previous studies have shown that hypothalamic NPSCs can be maintained in culture, express hypothalamic-specific transcripts and peptides, and can differentiate into hypothalamus neurons that are responsive to hormones such as grehlin and leptin (16, 17). We have adopted established protocols from our laboratory (7, 8) and generated primary cultures of proliferating NPSCs obtained from dissected hypothalamus of individual embryonic day (E) 14.5 C57Bl6 mice embryos. Total RNA was then prepared from NPSC cultures derived from 3 independent male or female embryos (E14.5) and subjected to RNA-Seq analysis to identify the dex-regulated transcriptome. The RNA and libraries prepared for this analysis did not enrich for small RNAs (eg, miRNAs), although microRNA precursors were identified as highlighted below. The number of sequence reads ranged from 20 to 35 million, and the average reads mapped were about 85%. In our samples, there were 13 148 genes identified with significant reads of FPKMs more than 1 with the vast majority of transcripts derived from protein coding genes (10 942). Pie charts display total genes identified (13 148) (Figure 1A), long noncoding RNAs (687) (Figure 1B), and short noncoding RNAs (1230) (Figure 1C) in our samples that display significant reads. Examination of protein coding genes with significant expression in both male and female hypothalamic NPSCs revealed the presence of multiple stem/progenitor cell cycle genes, including Nes, Olig2, and Sox2 (FPKMs >100) (Supplemental Table 2). In fact, 10 of the top 20 genes with known functions in hypothalamic development are involved in the regulation of stem and/or progenitor cell function (19, 22–24). For cell cycle markers, there was high expression for Ccnd1 (>500 FPKM), Ccna2 (>100FPKM), Fstl1 (>15 FPKM), and Fzd2 (>2 FPKM) consistent with previous results from E10–E14 mouse hypothalamus gene expression analysis (19). There was minimal detectable expression (<1 FPKM) of cell differentiation genes Sim1, Sim2, and Arx or for the terminal differentiation genes Gad1 and Gad2 (19). The expression of genes known to delineate-specific hypothalamic nuclei during embryonic development (Shh, Lef1, Pomc, Nr5a1, Lhx8, or Sim1) (19) was also at the limits of detection (<1 FPKM). This comparison demonstrates that these NPSCs originating from the embryonic hypothalamus in vitro are most similar to stem cells and have not begun to express genes involved in hypothalamic patterning and development.
Figure 1.
Gene expression profiling of hypothalamic NPSCs using RNA-Seq analysis. Pie charts detailing the distribution of total genes identified (A), long noncoding RNAs (B), and short noncoding RNAs (C) in male and female hypothalamic NPSCs that display significant reads.
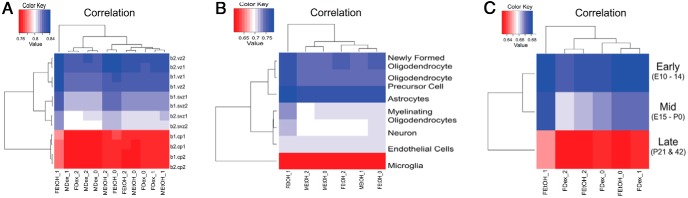
To provide a more comprehensive assessment of the fate of cells in hypothalamic NPSC cultures, the transcript profile we obtained was compared with 3 published datasets (Figure 2): cortical regions at E14.5 (Figure 2A) (18), different cell types present in the cortex (Figure 2B) (20), and hypothalamic development (E10 to postnatal d [P]42) (Figure 2C) (19). For genes expressed in different regions of the cortex determined using RNA-Seq analysis, the ventricular zone (VZ) has the highest correlation with RNAs expressed in both male and female hypothalamic NPSCs (Figure 2A). For the subventricular zone (SVZ) (Figure 2A), there was a lower correlation compared with the VZ. The lowest correlation in this analysis using hypothalamic NPSCs was with transcripts derived from E14.5 cortical plate (CP) (Figure 2A). There were no changes in correlation observed due to sex or dex treatment for hypothalamic NPSCs and different cells populations at E14.5 in the cortex. In the developing cortex, neural stems cells are maintained in the VZ and migrate through the SVZ to the CP (shown not to contain stems cells at E14.5) (18). Therefore, because hypothalamic NPSCs derived at E14.5 had the highest correlation with the VZ and the lowest correlation with the CP (18), they maintain their stem cell fate in vitro, and transcriptome analyses of our hypothalamic NPSC cultures can provide insights into glucocorticoid-regulated genes in stem cells of the embryonic brain.
Figure 2.
Comparison of gene expression in male and female hypothalamic NPSCs with published datasets. Compared with different embryonic zones (SVZ and VZ) in 2 technical replicates (represented as CP1, CP2, etc) using biological replicates (B1 and B2) in the embryonic (E)14.5 cortex (A), multiple cell types in the adult cortex (B), and multiple time points (early, E10–E14; mid, E15–P0; and late, P21 and P42) during hypothalamic development (C).
We next examined the relationship between the RNA expression profiles generated using RNA-Seq analyses in hypothalamic NPSCs to cerebral cortical astrocytes, neurons, oligodendrocytes (precursor cells, newly formed and myelinating), microglia, and endothelial cells isolated by a variety of cell sorting techniques (Figure 2B) (20) These analyses showed that the gene expression profile in both male and female hypothalamic NPSCs was highly correlated with that from astrocytes, followed by oligodendrocytes precursor cells, and newly formed oligodendrocytes. There was less correlation for neurons, myelinating oligodendrocytes, and endothelial cells followed by the lowest correlation for transcripts derived from microglial cells. Overall, this comparison demonstrates there are gene sets that are in common with specific cell types (eg, oligodendrocyte precursor cells) and provides a resource of the transcriptome of these neural stem cells.
We then correlated our hypothalamic NPSC gene expression profile with a microarray dataset derived from male and female hypothalamic tissue dissected from different (early, mid, and late) developmental periods (19). As shown in Figure 2C, the highest correlation of gene expression for primary embryonic hypothalamic NPSCs culture was with the mRNA expression profile from hypothalamic tissue obtained early in development (E10–E14). There was less correlation between the hypothalamic NPSC gene expression profile and that from midhypothalamic development (E15–P0) and the lowest correlation with genes expressed in the postnatal male and female hypothalamus (P21–P42). This is not surprising because our cultures highly express transcripts found in neural stem cells (eg, Sox2 [FPKMs >121]) and Pax6 [FPKMs >24]) (Figure 2A) and in oligodendrocyte precursor cells (Figure 2B). Thus, the culture conditions used to propagate and enrich for NPSCs in general does not disrupt the overall genetic program established in stem or progenitor cells of hypothalamus at the time of isolation.
To further characterize our hypothalamic NPSCs, we examined the transcriptome data for nuclear receptor (NR) gene expression (Table 1). The most highly expressed (ie, >10 FPKM) NRs in male and female hypothalamic NPSC cultures were COUP-TF1, EAR2, ERRα, GR, LXRβ, RORβ, RXRβ, SF1, TR4, and TRα, whereas COUP-TF2, LRH1, MR, NUR77, PPARβ/δ, RARα, RARβ, REVERBα, RORα, RXRα, TLX, TR2, and TRβ were expressed at moderate levels (ie, >1 FPKM and <10 FPKM). There were no significant differences observed between male and female hypothalamic NPSCs for these NRs. Sex steroid receptors AR, ERα, ERβ, and PR were among the other NRs expressed at very low to undetectable levels (<1 FPKM), suggesting that any sex differences in gene expression in the hypothalamic NPSCs was unlikely to be driven by sex steroids.
Table 1.
Nuclear Receptors Gene Expression (FPKM)
| >10 | 10 < 1 | 1 < 0.1 | <0.1 |
|---|---|---|---|
| ERRα | COUP-TFII | ERRβ | CAR |
| TRα | Rev-ErbAβ | LXRα | ERβ |
| LXRβ | NUR77 | RORγ | PR |
| SF1 | RXRα | NURR1 | PXR |
| EAR-2 | RARα | NOR1 | ERα |
| RORβ | TLX | RARγ | VDR |
| GR | TR2 | GCNF | DAX1 |
| COUP-TFI | Rev-ErbAα | RXRγ | FXR |
| RXRβ | RORα | PPARα | FXRβ |
| TR4 | PPARβ/δ | AR | HNF4α |
| LRH-1 | PPARγ | HNF4β | |
| RARβ | ERRγ | PNR | |
| TRβ | SHP | ||
| MR |
Nuclear receptors expressed identified using RNA-Seq analysis in both male and female hypothalamic NPSCs.
We next used gene ontology analysis to determine pathways and biological significance of differential gene expression observed between male and female hypothalamic NPSCs (Supplemental Figures 1 and 2). Using pathway analysis the top 2 diseases and function networks that are significantly differentially expressed genes in males vs females were cell death and survival, cellular assembly and organization, cellular compromise, and lipid metabolism, molecular transport, and small molecule biochemistry (Supplemental Figure 1, A and B). Gene ontology identified significant changes in a number of genes established to have a functional role in these 2 networks. The top significant disease associated pathway identified was morphology of the nervous system (Supplemental Figure 2) and contained 20 genes that were different in expression between male and female hypothalamic NPSCs.
The glucocorticoid receptor (GR) transcriptome in fetal mouse hypothalamic NPSC cultures
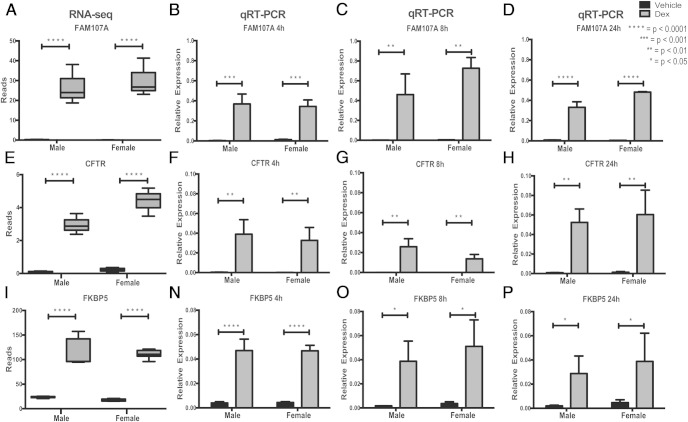
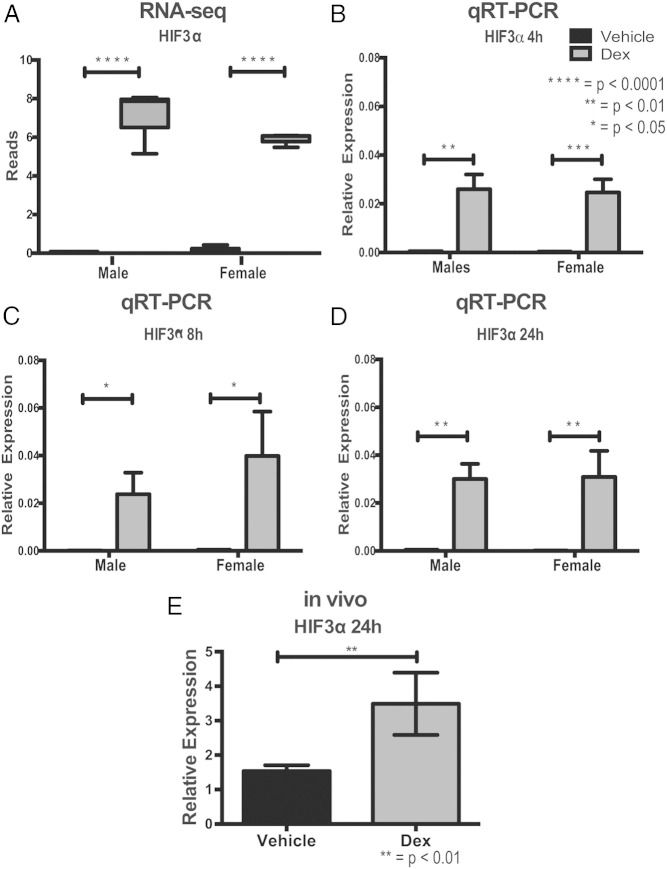
Overall, there were 175 genes that were dex regulated in both male and female hypothalamic NPSCs (Table 2). The top protein-coding genes with more than 4-fold change include Fam107a, Hif3α, Cftr, Zbtb16, Ptk2B, Rasgef1c, Sphkap, Kent1, Ada, Thrsp, Tsc22d3, Kcnn2, Crip1, Mfsd2a, Fkbp5, Wnt3, Gbx2, Nedd9, Cio2, Fam101b, Pdk4, Tprn, Klf9, Per1, Amigo2, Gfpt2, and Map7d2; for repressed protein-coding genes Ercc2, Man1c1, and Syt4 showed a fold change less than 0.2. We then selectively validated a number of GR-induced genes after a 4-hour dex treatment using qRT-PCR and observed a significant induction of previously identified GR-target genes Fam107a, Cftr, Fkbp5, and a novel GR-target gene Hif3α induced by dex in both male and female hypothalamic-NPSCs across multiple litters. Top GR-targets Fam107a, Cftr, Fkbp5, and Hif3α were also examined at 8 and 24 hours to verify that their induction is not transient and may therefore exert a functional biological outcome in hypothalamic NPSCs. Figure 3 shows box plots of RNA-Seq reads for Fam107a (Figure 3A), Cftr (Figure 3E), and Fkbp5 (Figure 3J) followed by qRT-PCR showing significant induction after a 4-, 8-, and 24-hour dex treatment (Figure 3, B–D, F–H, and N–P). Figure 4A shows similar box plots of RNA-Seq reads for the novel GR-target gene Hif3α that was validated by qRT-PCR, which showed induction of gene expression in NPSC cultures after 4 (Figure 4B), 8 (Figure 4C), and 24 (Figure 4D) hours of dex treatment. Hif3α is also induced in the fetal hypothalamus in vivo at embryonic day 15.5 after a 24-hour antenatal dex treatment initiated in pregnant mice carrying fetuses at 14.5 gestational age (Figure 4E).
Table 2.
GR-Target Genes Identified Using RNA-Seq Analysis in Both Male and Female Hypothalamic NPSCs
| Gene | Fold Change | Gene | Fold Change | Gene | Fold Change | Gene | Fold Change |
|---|---|---|---|---|---|---|---|
| Fam107a | 353.69 | Nkd2 | 3.38 | Klf13 | 2.20 | Mbnl2 | 1.59 |
| Hif3a | 75.57 | Rhou | 3.35 | Mgll | 2.18 | Ncl | 0.65 |
| Cftr | 26.43 | Bcat1 | 3.32 | Tlcd2 | 2.16 | Mtss1 | 0.64 |
| Zbtb16 | 22.79 | Kcnj12 | 3.27 | Lgalsl | 2.11 | Sema6d | 0.64 |
| Rasgef1c | 11.85 | Chst2 | 3.27 | Zfp189 | 2.10 | Pcdha2 | 0.63 |
| Ptk2b | 11.80 | Cables1 | 3.18 | Per2 | 2.09 | Jun | 0.63 |
| Sphkap | 8.84 | Rcan2 | 3.15 | Adm | 2.07 | Rassf3 | 0.62 |
| Ada | 7.30 | Cpne7 | 3.14 | F3 | 2.06 | Fosl2 | 0.62 |
| Kcnt1 | 7.15 | Errfi1 | 3.13 | Prss23 | 2.05 | Anp32b | 0.61 |
| Kcnn2 | 6.19 | Fam43a | 3.08 | Endod1 | 2.03 | Uaca | 0.60 |
| Thrsp | 6.12 | Rhob | 3.08 | Klf15 | 2.03 | Rprm | 0.59 |
| Tsc22d3 | 6.10 | Rhoj | 3.07 | Ndnl2 | 2.03 | Cxxc5 | 0.59 |
| Crip1 | 5.68 | Bcl2l1 | 3.03 | BC029214 | 2.02 | S1pr1 | 0.57 |
| Fkbp5 | 5.65 | Sesn1 | 2.94 | Sepp1 | 1.99 | Arl4c | 0.57 |
| Mfsd2a | 5.64 | Plekhf1 | 2.94 | Galnt16 | 1.97 | Fn1 | 0.57 |
| Wnt3 | 5.44 | Adamts9 | 2.94 | Lpin1 | 1.95 | Hdac9 | 0.57 |
| Gbx2 | 5.15 | Pla2g3 | 2.87 | Gpd1 | 1.95 | Arl4a | 0.56 |
| Pdk4 | 4.87 | Bcl6 | 2.84 | Sdc4 | 1.94 | Mfap3l | 0.56 |
| Nedd9 | 4.74 | Agt | 2.79 | Arid5b | 1.92 | Sox9 | 0.56 |
| Dio2 | 4.65 | Parp1 | 2.66 | Lfng | 1.89 | Lonrf1 | 0.54 |
| Fam101b | 4.60 | Hspa12a | 2.66 | Slc37a2 | 1.89 | Irf2bpl | 0.53 |
| Tprn | 4.53 | Smox | 2.56 | Ralgps1 | 1.88 | Hbegf | 0.51 |
| Klf9 | 4.41 | Ociad2 | 2.55 | Pim3 | 1.88 | Mtss1l | 0.51 |
| Per1 | 4.28 | Fkbp14 | 2.55 | Pik3r1 | 1.87 | Sytl2 | 0.50 |
| Amigo2 | 4.20 | Mt1 | 2.52 | Ddit4 | 1.86 | Adamts3 | 0.50 |
| Gfpt2 | 4.16 | Arrdc2 | 2.50 | Elmo1 | 1.85 | Nhs | 0.49 |
| Map7d2 | 4.13 | Dyrk3 | 2.49 | Mapk4 | 1.84 | Pde1b | 0.48 |
| Pgbd5 | 3.97 | Fam13a | 2.45 | Ptprj | 1.82 | Fbxo32 | 0.45 |
| Mertk | 3.96 | Tmem229b | 2.42 | Tob2 | 1.80 | Pde3a | 0.45 |
| Ctgf | 3.92 | Htr3a | 2.41 | Dock9 | 1.80 | Kcnk3 | 0.43 |
| Arl4d | 3.79 | 1810011O10Rik | 2.29 | Nmnat2 | 1.78 | Fam135b | 0.33 |
| Pgf | 3.66 | Col5a3 | 2.29 | Megf9 | 1.78 | Brd3 | 0.31 |
| Ccl2 | 3.63 | Cdh19 | 2.27 | Cspg4 | 1.76 | Diras2 | 0.30 |
| Frmpd1 | 3.54 | Adrb2 | 2.26 | Mxd4 | 1.69 | Scg2 | 0.27 |
| Mt2 | 3.54 | Cebpa | 2.25 | Sema4b | 1.67 | Rerg | 0.27 |
| Kank1 | 3.51 | Tbc1d10a | 2.24 | Usp53 | 1.66 | Syt4 | 0.24 |
| Phf15 | 3.47 | Paqr8 | 2.24 | Tigd2 | 1.64 | Man1c1 | 0.19 |
| Camkk1 | 3.47 | Nfkbia | 2.23 | Nek7 | 1.63 | Ercc2 | 0.12 |
| Rhod | 3.44 | Sap30 | 2.22 | Akap6 | 1.62 | ||
| Crispld2 | 3.43 | Pmp22 | 2.21 | Abhd17b | 1.60 |
Figure 3.
Boxplots of top GR-target genes identified by RNA-Seq analysis (A, E, and I) and qRT-validations of GR-target genes FAM107a (B–D), CFTR (F–H), and FKBP5 (N–P) in both male and female hypothalamic NPSCs at 4, 8, and 24 hours.
Figure 4.
Boxplots of novel GR-target gene HIF3α identified by RNA-Seq analysis (A) and qRT-PCR validations in both male and female hypothalamic NPSCs in vitro at 4 (B), 8 (C), and 24 (D) hours and within the hypothalamus at E15.5 after 24 hours of dex exposure in vivo (E).
We next determined whether dex altered gene expression in a sex-dependent manner in hypothalamic NPSCs (Figure 5A). In addition to dex-regulated genes identified in both male and female hypothalamic NPSCs, 137 female-unique and 53 male-unique dex-regulated genes were identified (Figure 5B). Previously in the liver, dex treatment was shown to significantly increase Nfkbib and Hnf1α in male livers, whereas Il-10 and Adora2 were only suppressed in the female liver (25). For male hypothalamic NPSCs, Klf4 was identified and validated as significantly repressed in response to dex (Figure 5C). In female hypothalamic NPSCs, Map7 was identified through RNA-Seq and validated using qRT-PCR to be significantly increased due to dex treatment only in female cells (Figure 5D). In addition, the long noncoding RNA H19 (FPKMs >1000), and its embedded microRNA miR-675 (FPKMs >10) was induced in dex-treated compared with vehicle-treated females but not males (H19 FPKMs >1437 vs 783 and miR-675 FPKMs 13 vs 8). qRT-PCR validations found H19 and miR-675 were significantly increased only in dex-treated female HT-NPSCs (Figure 5, E and F). These results demonstrate sex-specific GR-target genes are likely to vary between organ systems.
Figure 5.
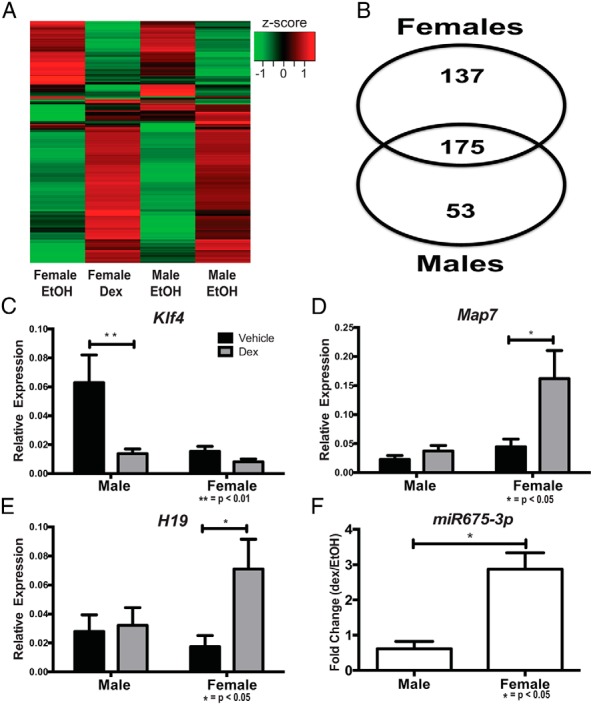
qRT-PCR validations of sex-specific GR-target genes in hypothalamic NPSCs. Heat map depicting significant GR-target genes (A), quantification of GR-target genes similarly and differentially in male and female hypothalamic NPSCs (B), qRT-PCR validation of male-specific GR-target gene Klf4 (C) (P < .01), and female-specific GR-target genes Map7 (D) (P < .05), H19 (E) (P < .05), and miR675 (F) (P < .05).
Using gene ontology analysis in male and female hypothalamic NPSCs in response to dex treatment, the top functional network identified in vehicle vs dex was proliferation of cells (Figure 6), which included our top GR-target genes (FAM107a, CFTR, and FKBP5) (Table 2). To assess whether dex indeed regulated proliferation of hypothalamic NPSCs, we used a BrdU incorporation assay to accurately measure dex effects on proliferation. We observed an overall main effect for a reduction in Brdu+ positive cells after dex treatment demonstrating that GC reduced proliferation in hypothalamic NPSCs (P < .001) (Figure 6B). P value and an activation z-score (Figure 6A) suggest that females were more impacted than males after dex treatment and post hoc analysis showed a significant reduction in BrdU+ cells in dex-treated vs vehicle-treated females (P < .01) (Figure 6B). During embryonic hypothalamic development, cell proliferation occurs along the third ventricle, and after a final division, neurons and glia cells migrate towards their final location in the hypothalamus where they are either maintained or undergo cell death (26). The reduction in NPSC proliferation observed after dex treatment, with a greater reduction occurring in females, suggests that antenatal glucocorticoid exposure could differentially limit (ie, with respect to sex) the ultimate composition of differentiated neurons that populate the adult hypothalamus.
Figure 6.
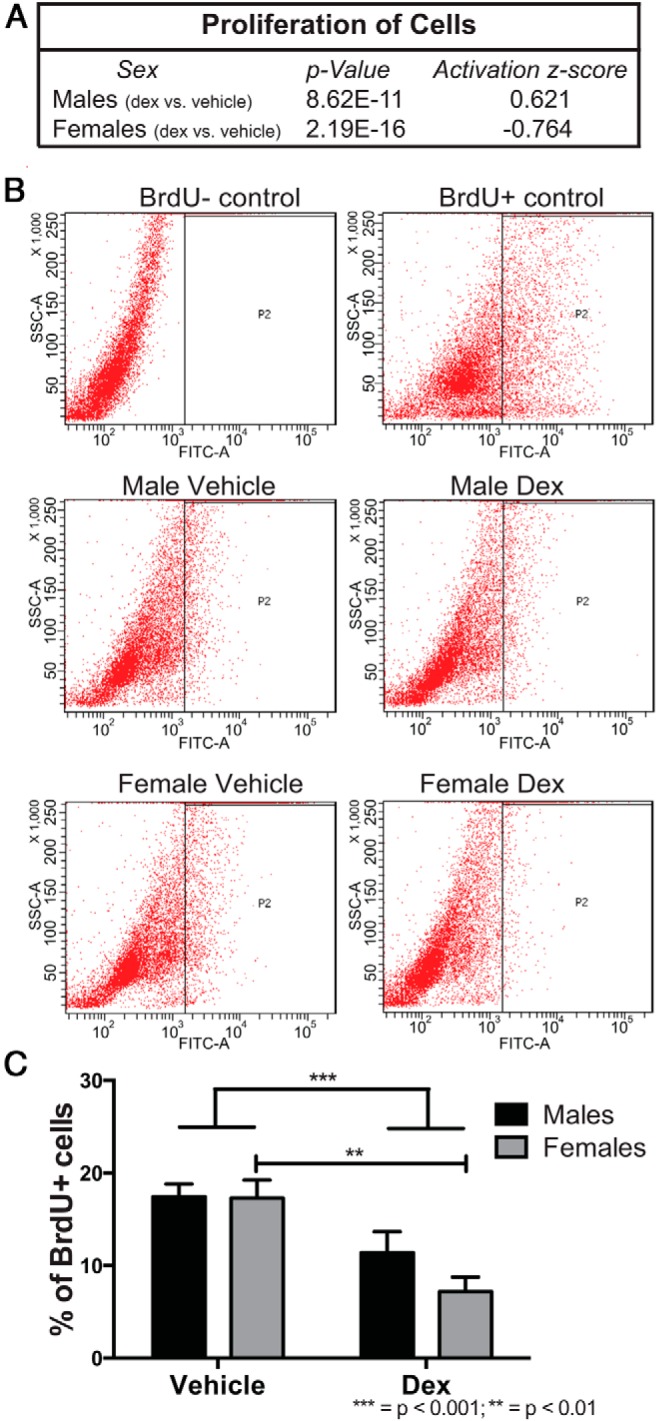
Gene ontology analysis using the list of RNA-Seq identified genes differentially expressed between vehicle and dex-exposed male and female hypothalamic NPSCs determined the top functional pathway as proliferation of cells (A). Flow cytometric analysis of BrdU incorporation in male and female hypothalamic NPSCs treated with either vehicle or dex (B). Quantification of BrdU+ cells showed a significant overall main effect in a reduction in proliferation after dex treatment in both male and female hypothalamic NPSCs (P < .001) and post hoc analysis showed a female-specific reduction in proliferation after dex treatment compared with vehicle (C) (P < .01).
Cell death and survival, cellular assembly and organization, cellular compromise along with endocrine system development and function, small molecular biochemistry, cardiovascular system development and function were among the top networks identified in dex treated NPSCs from both males and females (Supplemental Figure 3, A and B). The endocrine system and development function network is defined by a different constellation of dex-regulated genes in males and females although Akt is the central node in both and Hif3α the most highly regulated gene in this network. This suggests that physiological effects of glucocorticoids in the developing hypothalamus that do not show sexual dimorphism may be generated by gender specific-regulated gene networks and therefore differentially influenced by distinct genetic alterations or variations.
Discussion
Novel insights from genome-wide analysis of fetal hypothalamic NPSC transcriptome
Our study used RNA-Seq to identify the total and GR-regulated transcriptome in male and female hypothalamic NPSCs derived from E14.5 mice. A total of 13 148 transcripts with significant expression (ie, >1 FPKM) were identified from this analysis including 10 942 protein-coding, 687 long noncoding, and 1230 short noncoding RNAs. The stem/progenitor cell properties of these cultures were maintained as relatively high levels of expression was observed for stem cell markers localized in embryonic hypothalamus (eg, Ccnd1, Nes, Olig2, and Sox) (19, 22–24) and relatively low levels of expression for transcripts found in differentiated hypothalamic cells (eg, NPY, Orexin, AGRP, POMC, and GnRH) (16, 17) demonstrating hypothalamic NPSCs maintain their progenitor/stem cell phenotype in vitro.
When further examining the transcriptome of hypothalamic NPSCs, we identified genes with significantly different expression between males and females. Although most research on hypothalamic sexual differentiation focuses on the role of sex steroids, the influence of sex-dependent chromosomal genes is becoming apparent. For example, in the preoptic area of the hypothalamus, there are more cells that divide earlier in females than males (established using BrdU incorporation) independent of sex hormone exposure (27). There was limited expression (<1 FPKM) of the androgen receptor and estrogen receptors α and β, indicating that sex hormone signaling does not drive sexual dimorphic expression patterns in hypothalamic NPSC cultures. Using gene ontology analysis, there were distinct gene expression between males and females resulting in networks with functional biological relevance (Supplemental Figures 2 and 3).
Glucocorticoids impact gene expression with a subset with different responsiveness due to gender
Antenatal glucocorticoid exposure in humans and animals models has long-term behavioral and physiological consequences tied to altered hypothalamic development (2, 28). Here, we identified 175 genes significantly impacted by glucocorticoids in both males and females. Some of these were previously known GR targets (eg, Fkbp5, Cftr, and Fam107a), yet not much is known regarding their role in hypothalamic development. Fam107a (also named DRR1 and Tu3A) had the highest fold change due to dex (>300) in both male and female hypothalamic NPSCs. It was initially described as a tumor suppressor gene and is highly induced within the paraventricular nucleus of the hypothalamus after dex administration, neonatally after maternal separation, and during adulthood is response to food depravation (29). Cftr is present in human (30) and rat (31) hypothalamic neurons, but its specific function or role during development of the hypothalamus has not been investigated. Fkbp5 is an established regulator of GR and has been implicated in stress-related phenotypes (32), but its impact on glucocorticoid action in the developing hypothalamus are not known as well as the potential long-term consequences on the stress axis of Fkbp5 regulation of fetal GR function.
In addition to identifying well-established GR-regulated genes, several less well-documented or novel GR targets were identified in hypothalamic NPSC cultures. For example, Hif3α mRNA is robustly induced by dex in both male and female hypothalamic NPSCs (Figure 4, B–D) and in vivo was shown to be induced in the hypothalamus after a 24-hour dex treatment (Figure 4E). Furthermore, it is a one of strongest GR targets in both male and female “development” networks that include Akt as a central node, although identity and connectivity of components of this network are sexually dimorphic. HIF3α, is one of 3 hypoxia inducible factors, whose expression is elevated under hypoxic conditions in multiple organs including brain (cortex and hippocampus) (33) but also functions outside of its known hypoxia response role (34). It is necessary for proper lung epithelium development (35) but does not respond to dex in human fetal lung epithelial cells (36). Understanding the differential response to glucocorticoids of fetal lung vs neural stem cells and the developing hypothalamus could lead to novel combination pharmacotherapies that seek to maintain the lung maturation effects of these hormones while limiting any long-lasting detrimental effects on the brain.
Currently, there are limited studies available on sex-dependent GR actions. A genome-wide study using liver tissue revealed a sexually dimorphic GR transcriptome, particularly for genes involved in the inflammatory response (25). Our study using RNA-Seq analysis in male and female hypothalamic NPSCs detected 53 GR-target genes only in males (eg, Klf4) (Figure 5A) and 137 GR-target genes only in females (eg, Map7, H19, and miR-675) (Figure 5, D–F). Map7 is normally expressed in the rat hypothalamus and a reduction during embryonic development reduced TRH expression peptide levels (37) and in mouse embryonic stem cells a reduction directly inhibits differentiation (38). In addition to a subset of protein-coding genes, we also identified and validated the lincRNA H19 and its contained miR-675 to be regulated significantly by GR in female hypothalamic NPSCs. Previously, H19 was shown to be abundantly transcribed during development and regulate stem cell proliferation and growth through its embedded miR-675 (39). It remains to be established whether H19 or miR-675 functions similarly to regulate the proliferation of NPSCs selectively in the hypothalamus of female embryos.
Through the use of genome wide analysis, we have identified the transcriptome of male and female hypothalamic NPSCs and how they are similarly and differentially impacted after dex. We identified known and novel candidate/target genes that can be used as a tool to further investigate the impact of elevated glucocorticoids on sexually dimorphic hypothalamic development.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Dr Teresa T. Liu for her technical guidance in these studies.
This work used the University of Pittsburgh Cancer Institute Cancer Bioinformatics Services, which is supported in part by the National Cancer Institute Award P30CA047904. This work was also supported by the Public Health Service Grant DK078394 from the National Institute of Diabetes and Digestive and Kidney Diseases (to D.B.D.), a Nuclear Receptor Signaling Atlas (NURSA) Data Resource Project from an U24 Grant DK097748 (to D.B.D. and U.R.C.), T32 Training Grant T32GM008424 from the National Institute of General Medical Sciences (to M.E.P.), and the T32 Training Grant T32DK007052 from the National Institute of Diabetes and Digestive and Kidney Diseases (to K.A.F.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- bromodeoxyuridine
- CP
- cortical plate
- dex
- dexamethasone
- E
- embryonic day
- FPKM
- fragments per kilobase of transcript per million mapped reads
- GR
- glucocorticoid receptor
- NPSC
- neural-progenitor/stem cell
- NR
- nuclear receptor
- P
- postnatal day
- P3
- third passage
- qRT-PCR
- quantitative real-time-PCR
- RNA-Seq
- RNA-Sequencing
- SVZ
- subventricular zone
- VZ
- ventricular zone.
References
- 1. Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: outcomes. Nat Rev Endocrinol. 2014;10:391–402. [DOI] [PubMed] [Google Scholar]
- 2. Peffer ME, Zhang JY, Umfrey L, Rudine AC, Monaghan AP, DeFranco DB. Minireview: the impact of antenatal therapeutic synthetic glucocorticoids on the developing fetal brain. Mol Endocrinol. 2015;29:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–335. [DOI] [PubMed] [Google Scholar]
- 4. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006:CD004454. [DOI] [PubMed] [Google Scholar]
- 5. Karemaker R, Kavelaars A, ter Wolbeek M, et al. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008;121:e870–e878. [DOI] [PubMed] [Google Scholar]
- 6. Wyrwoll CS, Holmes MC. Prenatal excess glucocorticoid exposure and adult affective disorders: a role for serotonergic and catecholamine pathways. Neuroendocrinology. 2012;95:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samarasinghe RA, Di Maio R, Volonte D, et al. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc Natl Acad Sci USA. 2011;108:16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peffer ME, Chandran UR, Luthra S, et al. Caveolin-1 regulates genomic action of the glucocorticoid receptor in neural stem cells. Mol Cell Biol. 2014;34:2611–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Altman J, Bayer SA. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- 10. Hossain A, Haiman K, Charitidi K, et al. Prenatal dexamethasone impairs behavior and the activation of the BDNF exon IV promoter in the paraventricular nucleus in adult offspring. J Endocrinol. 2008;149:6356–6365. [DOI] [PubMed] [Google Scholar]
- 11. Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. [DOI] [PubMed] [Google Scholar]
- 13. Soga T, Dalpatadu SL, Wong DW, Parhar IS. Neonatal dexamethasone exposure down-regulates GnRH expression through the GnIH pathway in female mice. Neuroscience. 2012;218:56–64. [DOI] [PubMed] [Google Scholar]
- 14. Carbone DL, Zuloaga DG, Lacagnina AF, McGivern RF, Handa RJ. Exposure to dexamethasone during late gestation causes female-specific decreases in core body temperature and prepro-thyrotropin-releasing hormone expression in the paraventricular nucleus of the hypothalamus in rats. Physiol Behav. 2012;108:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Voorn B, Wit JM, van der Pal SM, Rotteveel J, Finken MJ. Antenatal glucocorticoid treatment and polymorphisms of the glucocorticoid and mineralocorticoid receptors are associated with IQ and behavior in young adults born very preterm. J Clin Endocrinol Metab. 2015;100:500–507. [DOI] [PubMed] [Google Scholar]
- 16. Salvi R, Arsenijevic Y, Giacomini M, et al. The fetal hypothalamus has the potential to generate cells with a gonadotropin releasing hormone (GnRH) phenotype. PLoS One. 2009;4:e4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sousa-Ferreira L, Alvaro AR, Aveleira C, et al. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and orexin-A and differentiate to functional neurons. PLoS One. 2011;6:e19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayoub AE, Oh S, Xie Y, et al. Transcriptional programs in transient embryonic zones of the cerebral cortex defined by high-resolution mRNA sequencing. Proc Natl Acad Sci USA. 2011;108:14950–14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimogori T, Lee DA, Miranda-Angulo A, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu TT, Arango-Argoty G, Li Z, et al. Noncoding RNAs that associate with YB-1 alter proliferation in prostate cancer cells. RNA. 2015;21:1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diez-Roux G, Banfi S, Sultan M, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caqueret A, Boucher F, Michaud JL. Laminar organization of the early developing anterior hypothalamus. Dev Biol. 2006;298:95–106. [DOI] [PubMed] [Google Scholar]
- 24. Cariboni A, Conti L, Andre V, Aprile D, Zasso J, Maggi Establishment of a radial glia-like mouse fetal hypothalamic neural stem cell line (AC1) able to differentiate into neuroendocrine cells. Neurogenesis. 2014;1:e29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal. 2010;3:ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tobet SA, McClellan K. Development of the Hypothalamus. In: McCarthy M.M, ed. Colloquium Series in the Developing Brain #10. San Rafael, CA: Morgan and Claypool Life Sciences; 2013:1–57. [Google Scholar]
- 27. Knoll JG, Wolfe CA, Tobet SA. Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus. Eur J Neurosci. 2007;26:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldstein JM, Handa RJ, Tobet SA. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front Neuroendocrinol. 2014;35:140–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt MV, Schülke JP, Liebl C, et al. Tumor suppressor down-regulated in renal cell carcinoma 1 (DRR1) is a stress-induced actin bundling factor that modulates synaptic efficacy and cognition. Proc Natl Acad Sci USA. 2011;1008:17213–17218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo Y, Su M, McNutt MA, Gu J. Expression and distribution of cystic fibrosis transmembrane conductance regulator in neurons of the human brain. J Histochem Cytochem. 2009;57:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mulberg AE, Resta LP, Wiedner EB, Altschuler SM, Jefferson DM, Broussard DL. Expression and localization of the cystic fibrosis transmembrane conductance regulator mRNA and its protein in rat brain. J Clin Invest. 1995;96:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Touma C, Gassen NC, Hermann L, et al. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Bio Psychiatry. 2011;70:928–936. [DOI] [PubMed] [Google Scholar]
- 33. Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third α-class hypoxia inducible factor subunit, HIF3α. Gene Expr. 1998;7:205–213. [PMC free article] [PubMed] [Google Scholar]
- 34. Heidbreder M, Qadri F, Jöhren O, et al. Nonhypoxic induction of HIF-3α by 2-deoxy-D-glucose and insulin. Biochem Biophys Res Commun. 2007;352:437–443. [DOI] [PubMed] [Google Scholar]
- 35. Huang Y, Kapere Ochieng J, Kempen MB, et al. Hypoxia inducible factor 3α plays a critical role in alveolarization and distal epithelial cell differentiation during mouse lung development. PLoS One. 2013;8:e57695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wade KC, Guttentag SH, Gonzales LW, et al. Gene induction during differentiation of human pulmonary type II cells in vitro. Am J Respir Cell Mol Biol. 2006;34:727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pérez-Monter C, Martínez-Armenta M, Miquelajauregui A, et al. The Krüppel-like factor 4 controls biosynthesis of thyrotropin-releasing hormone during hypothalamus development. Mol Cell Endocrinol. 2011;333:127–133. [DOI] [PubMed] [Google Scholar]
- 38. Aksoy I, Guidice V, Delahaye E, et al. Klf4 and Klf5 differentially inhibit medoderm and endocerm differentiation in embryonic stem cells. Nat Commun. 2014;5:3719. [DOI] [PubMed] [Google Scholar]
- 39. Keniry A, Oxley D, Monnier P, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Bio. 2012;14:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.