Abstract
Valeriana spp. is a flowering plant that is well known for its essential oils, iridoid compounds such as monoterpenes and sesquiterpenes, flavonoids, alkaloids, amino acids, and lignanoids. Valeriana spp. exhibits a wide range of biological activities such as lowering blood pressure and heart rate, antimyocardial ischemia reperfusion injury, antiarrhythmia, and regulation of blood lipid levels. This review focuses on the chemical constituents and cardiovascular activities of Valeriana spp.
1. Introduction
Valeriana officinalis Linn, perennial herbaceous plant belonging to the Valerianaceae family, is widely distributed in temperate regions. It comprises approximately 250 species, and 11 out of the 28 (including 1 variant) Chinese varieties are used as herbal medicines [1–4]. Most research studies have focused on six species: V. officinalis L., V. jatamansi Jones, V. officinalis L. var. latifolia Miq., V. amurensis Smir. ex Kom., V. fauriei Briq., and V. alternifolia var. stolonifera Bar. et Skv.
The roots and rhizomes of Valeriana spp. are rich in essential oils, iridoids, flavonoids, alkaloids, amino acids, and lignanoids [5–9], which possess characteristic fragrance or off-flavor and are used as medicines based on their inherent bioactivities that include inducing sedation, promoting sleep, antidepression, and antianxiety [10–18]. Valeriana spp. is now listed in the European and USA pharmacopeias. It is also sold as a diet supplement in the USA and is one of the highest selling natural medicines in Europe and the USA [19]. In addition, Valeriana spp. is of high medical and economic value in the food, drink, and cosmetic industries due to its distinct flavor, and current research efforts are aimed at further exploiting other features of the plant [20, 21]. This review focuses on the chemical constituents and cardiovascular activities of Valeriana spp., aiming at providing a theoretical foundation for further research and evaluation of its medicinal value.
2. Chemical Constituents
2.1. Essential Oils
Approximately 0.5%–2.0% of Valeriana spp. consists of essential oils by gas chromatography-mass spectrometry (GC-MS), which varies with species, climate, and growing environment. Valerian plants from high-altitude fertile and sandy soil have significantly higher essential oil content and yield similar to that of biennials compared to annuals. Valerian plants that produce a higher amount of essential oil are cultivated between September and November, although the content of essential oils decreases with longer periods of propagation.
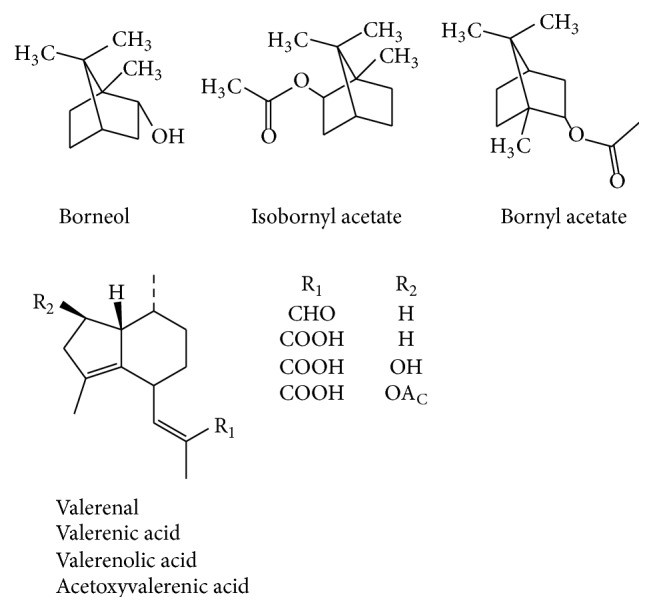
A total of 150 compounds have been identified in the essential oils of Valerian plants, mainly including monoterpenes and sesquiterpenes. Most monoterpenes, namely, borneol, bornyl acetate, and isobornyl acetate, exhibit various bioactivities. Around 30 sesquiterpenes have also been detected in the Valerian essential oils. These have been classified to be of the guaiane type and valerian type. Despite the low contents of these essential oils, their biological activities have drawn the attention of researchers around the world [22–24].
Long et al. [25], Ming et al. [26], Wang et al. [27], and Yu et al. [28] previously investigated essential oils from Valeriana by GC-MS, showing its content was 1%, and 20%–60% of it was bornyl acetate. Wang et al. [27] detected 34 compounds by GC-MS, which comprised 91.75% of the total content of the essential oil of V. officinalis L. var. latifolia Miq. (Table 1 and Figure 1). Compared to the standard spectrum, bornyl acetate showed the highest content level at 23.93%, followed by nootkatone (14.79%) and 6-isopropyl-1-methyl bicycles [3,1,0] hexane (14.19%).
Table 1.
The list of essential oil constituents from V. officinalis L. var. latifolia Miq.
| Signal | Compounds | Molecular format |
Molecular weight |
Retention time (min) |
Content (%) |
|---|---|---|---|---|---|
| 1 | Carene | C10H16 | 136 | 6.100 | 0.29 |
| 2 | α-Thujene | C10H16 | 136 | 6.473 | 4.18 |
| 3 | 6-Isopropyl-1-methyl bicycles [3,1,0] hexane | C10H16 | 136 | 6.983 | 14.19 |
| 4 | Sabinene | C10H16 | 136 | 7.736 | 2.55 |
| 5 | p-Cymene | C10H14 | 134 | 9. 143 | 0.43 |
| 6 | Limonene | C10H16 | 136 | 9.281 | 1.26 |
| 7 | Camphor | C10H16O | 152 | 12.596 | 0.19 |
| 8 | Borneol | C10H18O | 154 | 13.671 | 3.54 |
| 9 | L-Myrtanol | C11H16O | 164 | 14.584 | 0.81 |
| 10 | α-Methyl 4(1′, 1′-methyl ethyl) phenol | C11H16O | 164 | 15.432 | 2.49 |
| 11 | Bornyl acetate | C12H20O2 | 196 | 17.311 | 23.93 |
| 12 | Sabinol | C10H16O | 152 | 18.270 | 1.70 |
| 13 | α-Terpineol | C10H18O | 154 | 18.944 | 1.20 |
| 14 | β-Caryophyllene | C15H24 | 204 | 20.989 | 0.82 |
| 15 | β-Gurjunene | C15H24 | 204 | 21.343 | 1.16 |
| 16 | Humulene | C15H24 | 204 | 21.891 | 0.40 |
| 17 | Unidentified | C15H22 | 202 | 22.045 | 1.32 |
| 18 | trans-caryophyllene | C15H24 | 204 | 22.450 | 0.28 |
| 19 | Nerolidol | C15H24O | 220 | 22.838 | 0.78 |
| 20 | Elemene | C15H24 | 204 | 22.977 | 0.45 |
| 21 | Bornyl isovalerianate | C15H26O | 238 | 23.441 | 0.36 |
| 22 | Azulene furan | C15H10O2 | 222 | 23.867 | 0.58 |
| 23 | Stereoisomer of ramie enol | C10H16O | 152 | 24.485 | 1.46 |
| 24 | 4a,8-Dimethyl-α-isopropyl naphthyl ketone | C15H24O | 220 | 25.155 | 2.77 |
| 25 | Tetramethyl-4-hydroxyl cyclopropane naphthalene | C15H24O | 220 | 25.294 | 1.26 |
| 26 | Unidentified | C15H24O | 220 | 25.790 | 1.72 |
| 27 | Ledol | C15H26O | 222 | 27.011 | 1.22 |
| 28 | Guaiol | C15H26O | 222 | 27.150 | 4.73 |
| 29 | Valerone | C15H26O | 222 | 27.493 | 1.14 |
| 30 | Nootkatone | C15H22O | 218 | 29.031 | 14.79 |
| 31 | Nootkatone isomer 1 | C15H22O | 218 | 29.467 | 1.06 |
| 32 | Nootkatone isomer 2 | C15H22O | 218 | 30.333 | 0.90 |
| 33 | 1,2,3,4,4a,5,6,8,8a-Eight hydrogen-4a,8-dimethyl-α-Propenyl [α] naphthyl alcohol | C15H24O | 220 | 35.174 | 0.83 |
| 34 | Unidentified | 36.500 | 0.83 |
Figure 1.
Major essential oil constituents from V. officinalis L. var. latifolia Miq.
Yu et al. [28] analyzed essential oils from cultivated V. officinalis L. var. latifolia Miq. by GC-MS and identified 6 compounds, bornyl acetate (60.19%), (−)-acetic acid Rhodomyrtus enol ester (3.87%), α-terpinyl acetate (1.55%), acetyl carene (1.68%), α-selinene (26.07%), and (Z,E)-α-farnesene (1.56%), comprising 94.92% of the total content. Cultivated V. officinalis L. var. latifolia Miq. consisted of a higher number of simple components, which was predominated by bornyl acetate relative to that of wild V. officinalis L.
2.2. Iridoids
Valepotriate was first isolated from V. wallichii and preliminary studies by Thies and Funke [29] have confirmed the presence of a sedation ingredient. The study drew the attention of researchers from around the world. To date, over 130 iridoids from Valeriana spp. have been identified, possibly contributing their sedative, antidepressant, and antitumor activities.
Chen et al. [30] studied the levels of valepotriate, dihydrovalepotriate, and acetyl-valepotriate from V. jatamansi Jones, V. officinalis L., and V. officinalis L. var. latifolia Miq. by using the reverse phase high-performance liquid chromatography (RP-HPLC) method. The highest levels were observed in V. jatamansi Jones, followed by V. officinalis L. and V. officinalis L. var. latifolia Miq. In addition, the content of iridoid varied significantly among different parts and habitats.
The main iridoids in Valeriana comprised didrovaltrate and valepotriates derivatives (0.5%–9.0%), including valepotriate, isovalepotriate, acetoxyvalepotriate, and isovalemxy-hydroxy-dihydrovatrate. These were characterized by a hemiacetal fragment, which leads to the decomposed productions of isopentoic acid and valerienal at a specific pH or 60°C. C-1, C-7, C-10, or C-11 of compounds were mainly substituted by acyl groups such as acetyl, isovaleryl, α-acetoxyisovaleryl, β-acetoxyisovaleryl, and β-hydroxyisovaleryl. Furthermore, iridoids could be further divided into diethenoid-type, monoethenoid-type, and other types based on the parent structure.
2.2.1. Diethenoid-Type Iridoids
Diethenoid-type iridoids were characterized by the following molecular structures: (1) two C-C double bonds often presented between C-3 and C-4, C-5, and C-6 and occasionally presented between C-4 and C-5, C-6, and C-7; (2) an oxacyclopropane was often presented between C-8 and C-10, and C-10 was usually in a β-configuration; (3) H-1 (α-configuration) and H-9 (β-configuration) were preferentially located on different sides of the ring, and the C-7 acyl group usually was determined to be in the β-configuration (Figure 2 and Table 2 and Figure 3 and Table 3).
Figure 2.
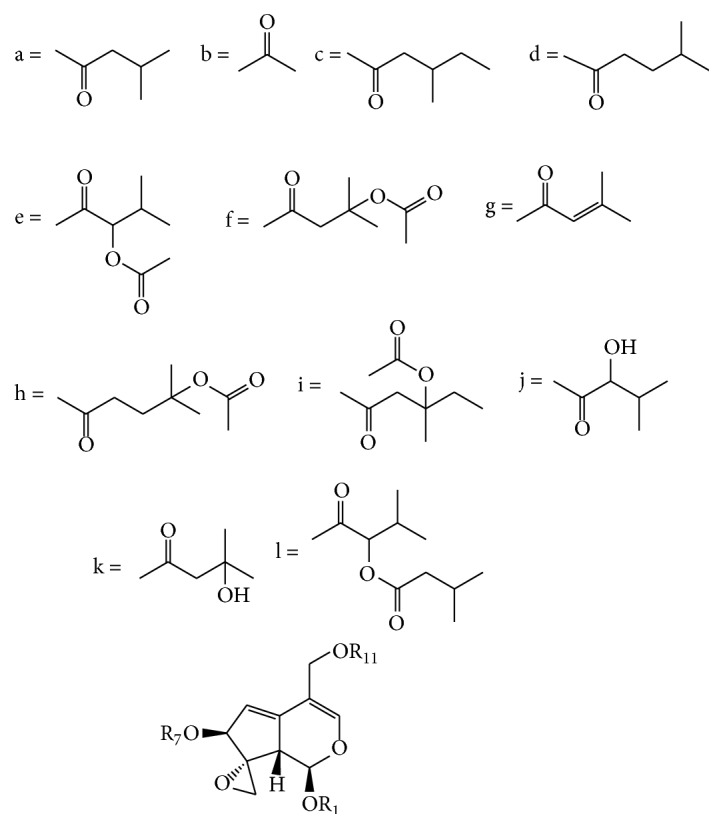
Compounds of diethenoid epoxy-type iridoids from Valeriana spp. (see Table 2).
Table 2.
| Number | R1 | R7 | R11 | Compounds | References |
|---|---|---|---|---|---|
| 1-1 | a | a | b | Valtrate | [6] |
| 1-2 | a | f | b | Acevaltrate | [6] |
| 1-3 | a | b | a | Isovaltrate | [35] |
| 1-4 | a | H | b | 7-Epi-deacetylisovaltrate | [36] |
| 1-5 | a | H | a | Deacetylisovaltrate | [37] |
| 1-6 | d | a | b | Homovaltrate 1 | [35] |
| 1-7 | a | b | d | Homovaltrate 2 | [35] |
| 1-8 | f | a | b | 1-β-Acevaltratum | [38] |
| 1-9 | g | a | b | 1-Seneciovaltrate | [39] |
| 1-10 | a | b | b | Diavaltrate | [40] |
| 1-11 | f | f | b | 1-β-Aceacevaltrate | [40] |
| 1-12 | c | b | a | Homoisovaltrate | [41] |
| 1-13 | c | c | b | 1,7-Dihomovaltrate | [41] |
| 1-14 | e | a | b | 1-α-Acevaltrate | [41] |
| 1-15 | e | c | b | Homo-A | [41] |
| 1-16 | c | b | b | Homo-B | [41] |
| 1-17 | a | c | k | Homo-Z | [41] |
| 1-18 | e | b | a | 1-α-Aceisovaltrate | [42] |
| 1-19 | a | c | b | Homovaltrate | [43] |
| 1-20 | c | f | b | 1-Homovaltrate | [44] |
| 1-21 | c | b | e | 1-Homoisoacevaltrate | [44] |
| 1-22 | g | a | a | Sorbifolivaltrate A | [45] |
| 1-23 | g | c | b | Sorbifolivaltrate B | [45] |
| 1-24 | a | a | H | Deacetlyvaltrate | [46] |
| 1-25 | a | c | b | 7-Homovaltrate | [46] |
| 1-26 | c | a | b | 1-Homovaltrate | [46] |
| 1-27 | a | b | e | 11-Acevaltrate | [46] |
| 1-28 | a | i | b | Homoacevaltrate | [46] |
| 1-29 | a | k | b | Hydroxyvaltrate | [47] |
| 1-30 | a | h | b | Isohomovaltrate | [47] |
Figure 3.
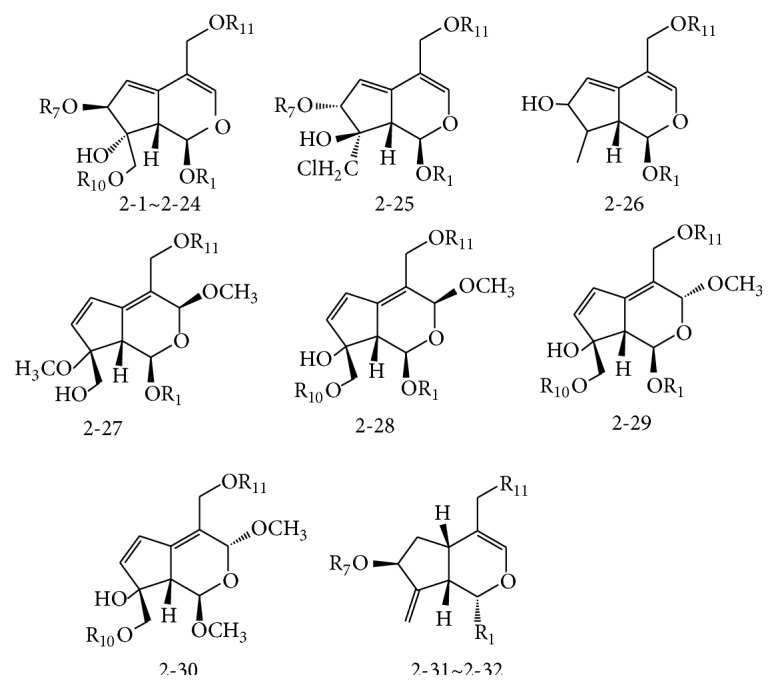
Compounds of diethenoid open ring-type iridoids from Valeriana spp. (see Table 3).
Table 3.
| Number | R1 | R7 | R10 | R11 | Compounds | References |
|---|---|---|---|---|---|---|
| 2-1 | a | a | a | b | Valtrate-isovaleroxyhydrin | [37] |
| 2-2 | a | a | a | b | Valtrate hydrin B1 | [47] |
| 2-3 | a | a | b | b | Valtrate hydrin B2 | [47] |
| 2-4 | j | a | a | b | Valtrate hydrin B3 | [47] |
| 2-5 | a | b | a | a | Valtrate hydrin B4 | [39] |
| 2-6 | a | a | c | b | Valtrate hydrin B5 | [39] |
| 2-7 | a | b | c | b | Valtrate hydrin B6 | [39] |
| 2-8 | g | a | a | b | Valtrate hydrin B7 | [39] |
| 2-9 | e | a | a | b | Valtrate hydrin B8 | [39] |
| 2-10 | e | b | b | a | Acetoxydesiovaleroxy-1-α-acetoxy-isovaleroxy isovaltratehydrine | [42] |
| 2-11 | c | a | b | b | 10-Acetoxy-1-homovaltrate hydrin | [44] |
| 2-12 | f | a | b | b | 10-Acetoxy-1-acevaltrate hydrin | [44] |
| 2-13 | k | a | a | a | Sorbifolivaltrate C | [45] |
| 2-14 | g | c | a | b | Sorbifolivaltrate D | [45] |
| 2-15 | a | e | l | b | Valeriandoid F | [48] |
| 2-16 | a | b | X | a | Jatamanvaltrate I | [49] |
| 2-17 | a | H | a | b | Jatamanvaltrate J | [49] |
| 2-18 | a | a | H | a | Jatamanvaltrate K | [49] |
| 2-19 | a | a | b | b | 10-Acetoxyvaltrahedrin | [49] |
| 2-20 | a | b | Cl | a | Rupesin B | [50] |
| 2-21 | b | H | Cl | a | Valeriandoids A | [51] |
| 2-22 | a | f | Cl | b | Valeriandoids B | [51] |
| 2-23 | a | b | a | a | Isovaltrate isovaleroyloxyhydrin | [51] |
| 2-24 | a | a | Me | b | Valeriandoids F | [52] |
| 2-25 | a | a | — | b | Volechlorine | [36] |
| 2-26 | a | — | — | a | Nardostachin | [53] |
| 2-27 | a | — | — | b | Jatamanvaltrate N | [50] |
| 2-28 | a | — | l | b | Jatamanvaltrate O | [50] |
| 2-29 | a | — | l | b | Valeriandoids D | [52] |
| 2-30 | — | — | l | b | Valeriandoids E | [52] |
| 2-31 | a | b | — | a | 8,11-Desoidodidrovaltrate | [37] |
| 2-32 | d | b | — | a | 8,11-Desoidohomoddidrovaltrate | [37] |
2.2.2. Monoethenoid-Type Iridoids
Monoethenoid-type iridoids were predominantly aglycones, which are characterized by the following structures: (1) a carbon double bond occurring mostly between C-3 and C-4; (2) H-1 (α-configuration), H-5 or 5-OH (β-configuration), H-7 (β-configuration), and H-9 (β-configuration); (3) a triatomic heterocyclic structure occurring between C-8 and C-10, and C-10 is usually a β-methylene, which is called monoene closed-loop iridoids. When C-8 and C-10 were not in ring formation, the structure is classified as a monoene open-loop iridoid (Figure 4 and Table 4).
Figure 4.
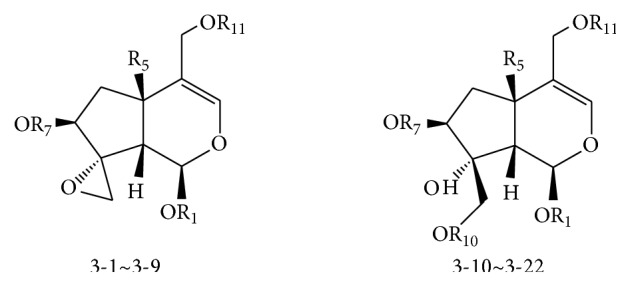
Compounds of monoethenoid-type iridoids from Valeriana spp. (see Table 4).
Table 4.
| Number | R1 | R5 | R7 | R10 | R11 | Compounds | References |
|---|---|---|---|---|---|---|---|
| 3-1 | a | H | a | — | b | Didrovaltrate | [6, 37, 53] |
| 3-2 | a | OH | b | — | l | Isovaleroxyhydroxydihydrovaltrate | [43] |
| 3-3 | a | H | b | — | a | Isodidrovaltrate | [54] |
| 3-4 | a | OH | b | — | e | AHD-valtrate | [40] |
| 3-5 | a | OH | b | — | c | 11-Homohydroxyldihydrovaltrate | [44] |
| 3-6 | c | H | b | — | a | Homodidrovaltrate | [37] |
| 3-7 | a | OH | H | — | l | Jatamanvaltrate L | [49] |
| 3-8 | a | OH | b | — | Et | Jatamanvaltrate M | [49] |
| 3-9 | a | OH | b | — | a | 5-Hydroxydidrovaltrate | [49] |
| 3-10 | a | OH | H | b | l | Valeriotriate B | [55] |
| 3-11 | a | OH | b | l | l | Valeriotertrate A | [56] |
| 3-12 | a | OH | b | f | l | Jatamanvaltrate A | [49] |
| 3-13 | a | OH | b | a | l | Jatamanvaltrate B | [49] |
| 3-14 | a | OH | b | b | l | Jatamanvaltrate C | [49] |
| 3-15 | a | OH | b | X | l | Jatamanvaltrate D | [49] |
| 3-16 | a | OH | b | Me | l | Jatamanvaltrate E | [49] |
| 3-17 | a | H | b | f | a | Jatamanvaltrate F | [49] |
| 3-18 | a | H | H | b | a | Jatamanvaltrate J | [49] |
| 3-19 | a | H | b | H | a | Jatamanvaltrate K | [49] |
| 3-20 | a | H | b | b | a | Didrovaltrate acetoxyhydrin | [49] |
| 3-21 | a | OH | b | Cl | l | Volvatrate B | [57] |
| 3-22 | a | OH | H | Cl | l | Jatamandoid A | [58] |
2.2.3. Other Types of Iridoids
Iridoids from Valeriana spp. were mostly of the two above-mentioned types (Figure 5). In addition, other types were also identified: (1) most of one type having free hydroxyl groups and ester groups, with a lactone structure between C-1 and C-3 and a double bond between C-4 and C-1, (2) an oxygen bridge between C-3 and C-8, C-3 and C-10, or C-8 and C-11, (3) cleaved Ring-A of other types forming a free hydroxyl or aldehyde group between C-1 and C-3 (e.g., see Lin et al. [31]).
Figure 5.
Compounds of other types of iridoids from Valeriana spp.
2.3. Lignanoids
Recent researches have indicated that lignanoids are 7,9-monoepoxy lignin and a glycoside or bisepoxy lignin. Britta Schumacher isolated eight lignanoids from Valeriana officinalis, namely, pinoresinol-4-O-D-glucoside, lignans 8′-hydro-xypinoresinol, 7,9′-monoepoxylignans massoniresinol-4′-O-beta-D-glucoside, berchemol-4′-O-D-glucoside, 8′-hydroxy-pinoresinol-4′-O-D-glucoside, and 8-hydroxypinoresinol-4′-O-D-glucoside [32]. Piccinelli et al. isolated two novel lignan glycosides from Valeriana prionophylla, including fraxireslnol-4′-O-D-glucopyranoside and prinse-piol-4-O-D-glucopy-ranoside [33].
2.4. Alkaloids
Alkaloids in Valeriana spp. included chatinine, nordelporphine, norphoebine, thaliperphine, nantenine, phenanthrene, phoebine, dehydroaphine, valerine, valeriane, and oxoaporphine, which occupy the low level of 1% [3, 34].
2.5. Flavonoids
Flavonoids in Valeriana spp. were mainly acacetin, apigenin, diosmetin, luteolin, quercetin, kaempferol, linarin, and luteolin [10, 33, 34], which occurred at low levels.
2.6. Amino Acids
Free amino acids in the water extracts of Valeriana spp. included γ-amino butyric acid (GABA), tyrosine, refined ammonia acid, glutamine, caffeic acid, chlorogenic acid, tannins, and sitosterol. GABA, a well-studied inhibitory neurotransmitter, is involved in lots of metabolic activities [59–62].
3. Research Advances on the Cardiovascular Activities of Valeriana
3.1. Reduction in Blood Pressure Level and Heart Rate
The increase of peripheral resistance in blood circulation was the common characteristic for primary hypertension, whose pathological mechanism was related to an increase of peripheral vascular tone and structural change of vessel walls. Additionally, structure and function disorders of vascular smooth muscle cells (VSMC) also contributed largely to this abnormal change. Therefore, improving the contract status of VSMC, expending the peripheral vessels, and inhibiting abnormal growth of VSMC preventing or alleviating vessel reconstruction at the same time were the keys to treating hypertension. Wang et al. [63] cultured aortic medial smooth muscle cells from a 6-month-old aborted fetus and examined the migration of cultured cells by Boyden Chamber. They found essential oil (VOL) could significantly inhibit the migration of human VSMC in a dose-dependent manner. Yang et al. [64] observed the effect of VOL and L-nitro arginine methyl ester (L-NAME) on the contraction of VSMC through the analogous experiment and investigated changes of 3H-thymidine (3H-TdR) and 3H-Leucine caused by angiotensin II (Ang II) and different concentrations of VSMC. VOL markedly inhibited the Ang II-stimulated contraction and growth of VSMC, which was not affected by L-NAME. In addition, VOL inhibited the incorporation of 3H-TdR and 3H-leucine. Zhou et al. [65] found that VOL could decrease the heart rate and blood pressure (priority to diastolic pressure) of rabbit and prolonged the duration of ST segment and T wave in a dose-dependent manner. VOL could decrease heart rate and blood pressure stimulated by adrenaline, which might be related to relaxing VSMC, enlarging vessel diameter, and decreasing blood resistance. VOL also observably inhibited contraction of VSMC stimulated by adrenaline, dilated coronary arteries, and decreased myocardial oxygen consumption. The vasorelaxant effects of the EtOH extract (1 mg·mL−1) and 8-hydroxypinoresinol (100 µm) from the roots of Valeriana prionophylla have been already shown [66]. Fields et al. [66] reported that VOL could dilate pulmonary vessels in felines via a nonselective GABA mechanism and inhibited contraction of isolated frog hearts stimulated by cardenolide. It has already been shown that hexanic extracts (HEVe) from V. edulis ssp. procera enriched in valepotriates present vasorelaxant properties by blocking calcium channels. HEVe induced a significant concentration-dependent and endothelium-independent relaxation on isolated rat aorta precontracted with noradrenaline (0.1 µm). HEVe, the most potent extract (0.15–50 µg/mL), induced relaxation in aortic rings precontracted with KCl (80 mm), with IC50 value of 34.61 µg/mL and E max value of 85.0% [67].
3.2. Antimyocardial Ischemia Reperfusion Injury
As early as the 1980s, Zhang et al. [68] reported that the ethanol extract of valerian could dilate the coronary artery and reduce myocardial oxygen consumption in anesthetized cats. Yang et al. [69] reported that its essential oil and iridoids enhance microcirculation perfusion of the heart and kidney. The valerian extract can prevent injuries to myocardial ischemia reperfusion model in the rabbit by decreasing the levels of xanthine oxidase (XOD), malondialdehyde (MDA), and tumor necrosis factor-α (TNF-α), thereby increasing the 6-keto-prostaglandin F1α/thromboxane B2 (6-keto-PGF1α/TXB2) ratio. Huang et al. [70] conducted a study to investigate myocardial protection mechanism of monoterpene oxide of valerian (VMO). Compared to the control group, VMO showed a maximum change rate of left ventricular pressure, with a maximal rate of the increase of the left ventricular pressure (+d ip/t max) and maximal rate of the decrease of the left ventricular pressure (−d ip/t max) by 25.1% and 25.3%, respectively. Adenosine triphosphate (ATP) and energy charge (E C) increased by 72.8% and 20.9%, respectively, whereas myocardial creatine kinase-myocardial band (CK-MB) decreased by 20.7%. These results demonstrated the analogical performance between VMO and ischemic preconditioning pretreatment on cardio protection, which indicated a mobilizing myocardial endogenesis protective mechanism and an exoteric ATP-sensitive potassium channel. Yang et al. [71] set up an isolated rat ischemia reperfusion (I/R) heart model using a Langendorff-perfusion system, observing the effects of VOL pretreatment on I/R injury and related biochemical factors and cytosolic free calcium. The results indicated that VOL pretreatment markedly prevented I/R injury, weakened vasospasm perfusion, sustained the heartbeat, and reduced ventricular arrhythmic events in a dose-dependent manner. Simultaneously, VOL significantly lowered lactate dehydrogenase (LDH), creatine phosphokinase (CK), and MDA levels. The activities of superoxide dismutase (SOD), adenosine triphosphatase (ATPase), and glutathione peroxidase (GSH-Px) were enhanced. VOL reduced intracellular calcium in a concentration-dependent manner. The mechanism of action for VOL's aforementioned activities potentially involved preventing the increase in concentration of free Ca2+ and decrease in lipid peroxidation.
3.3. Antiarrhythmia
Arrhythmia is a common disease that involves various pathological mechanisms. Although western medicines have considerable efficiency, the adverse reactions at different levels and the development of arrhythmia caused by the drug itself have been reported. Therefore, it is imperative to discover an antiarrhythmic drug that features efficiency, stability, and the absence of adverse effects; these properties are inherent to traditional Chinese medicine, which are also of scientific and societal significance.
Arrhythmia induced by aconitine might be caused by myocardium excitability, which opens Na+ channel of the cardiac muscle and promotes sodium currents, resulting in a ventricular and supraventricular ectopic rhythm and ventricular tachycardia. Ventricular fibrillation induced by chloroform could be related to the release of neurotransmitters or adrenaline secretion in the adrenal medulla, as well as stimulation of β receptors [72, 73].
Jia and Zhang [74] found that chloroform extract of ethanol extract (v3d) could effectively prevent atrial fibrillation in mice induced by acetylcholine-calcium chloride and ventricular fibrillation induced by chloroform. It also protected rats from ischemia arrhythmia induced by ligation of the left anterior descending coronary artery. In addition, it effectively prevents dog auricular and renal vessels contraction induced by high K+ levels. Therefore, v3d prevents arrhythmia in various animal species partly by inhibiting Ca2+ channel from opening, which was induced by high K+ level.
Huang [75] found valerian extract (monoterpene and sesquiterpene oxides from essential oils) could dose-dependently reduce the duration of an action potential and inhibit Na current (I Na), L-type calcium current (I Ca-L), and transient outward potassium current (I to). It interacts with inactivated I Na and I Ca-L, although various concentrations of v3d had no detectable effect on the delayed rectifier potassium current (I K) or inward rectifier potassium current (I KI) or direct interference with adenosine triphosphate sensitive potassium current (I KATP). The impacts of the valerian extract on these ion pathways might have contributed to its antiarrhythmia activity.
Wen et al. [76] reported the water, essential oil, and other fractions of valerian could protect a rat model from arrhythmia caused by aconitine or chloroform. Water extract at a dose of 50 and 25 g·kg−1 (calculated as raw herb) effectively decreased the occurrence of ventricular fibrillation, delayed the occurrence of arrhythmia, and decreased the mortality rate. The essential oil at a dose of 50 and 25 g·kg−1 (calculated as raw herb) effectively inhibited arrhythmia that was induced by chloroform; other fractions also demonstrated antiarrhythmia activities at different levels. Duan [77] found two active compounds from V. officinalis L., prinsepiol-4-O-β-D-glucoside and 8-hydroxy pinoresinol-4-O-β-D-glucoside; both showed antiarrhythmia activities. The former imparted an inhibitory effect on the Kv1.5 channel, which is the key mechanism for antiarrhythmia activity.
It was shown that didrovaltrate blocks L-type calcium current in a concentration-dependent manner and probably inhibited these currents in its inactive state. Didrovaltrate at concentrations of 30 μg/L and 100 μg/L significantly decreased peak I Ca-L (I Ca-Lmax) from 6.01 to 3.45 pA/pF and 2.16 pA/pF, respectively. Didrovaltrate shifted upwards the current-voltage curves of I Ca-L without changing their active, peak, and reverse potentials. Didrovaltrate affected the steady-state inactivation of I Ca-L. The half activation potential (V 1/2) was significantly shifted from −26 to −36 mV, with a significant change in the slope factor (k) (from 8.8 to 11.1) [78].
Liu et al. [79] studied antiarrhythmia effective substances in serum of V. officinalis L. The study showed that borneol and bornyl acetate from Valerian essential oils and another unidentified compound from ethyl acetate extract could be absorbed into the blood in its original form, which indicated that this unidentified compound might be the main substance that contributes to the antiarrhythmia activity of the ethyl acetate extract.
3.4. Regulation of Blood Lipid Levels
Reports on V. officinalis L. var. latifolia Miq. (VOL) and its constituents in lipid regulation are limited. Hu et al. [80] examined the effects of VOL on blood lipid metabolism in rabbits with hyperlipidemia. VOL imparts a remarkable antilipid peroxidation effect, reduces the levels of serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and MDA, and elevates the levels of high-density lipoprotein cholesterol (HDL-C) and SOD. The results prove that it is imperative to further investigate the underlying mechanisms in regulating lipid metabolism. Si et al. [81] also demonstrated VOL could reduce the serum levels of total cholesterol, low-density lipoprotein, urinary albumin, and serum creatinine. Light microscopy and immunohistochemical stain revealed that, in the same time of lowering serum lipid, mesangial matrix index was significantly reduced, accompanied by decreased expression of TGF-β 1 and type IV collagen.
4. Conclusions
Valeriana spp. possesses a wide range of bioactivities, which have been conferred by its complex and diverse active ingredients. Although the effects of Valeriana spp. mainly affected the cardiovascular system in Section 3, its mechanism of action needs to be further investigated.
Acknowledgment
This study was supported by a research grant from the National Natural Science Foundation of China (no. 81503421).
Abbreviations
- +dip/tmax:
Maximal rate of the increase of the left ventricular pressure
- 3H-TdR:
3H-Thymidine
- 6-keto-PGF1α/TXB2:
6-Keto-prostaglandin F1α/thromboxane B2
- Ang II:
Angiotensin II
- ATP:
Adenosine triphosphate
- CK:
Creatine phosphokinase
- CK-MB:
Creatine kinase-myocardial band
- −dip/tmax:
Maximal rate of the decrease of the left ventricular pressure
- EC:
Energy charge
- GABA:
γ-Amino butyric acid
- GC-MS:
Chromatography-mass spectrometry
- GSH-Px:
Glutathione peroxidase
- HDL-C:
High-density lipoprotein cholesterol
- HEVe:
Hexanic extracts
- I/R:
Ischemia reperfusion
- ICa-L:
L-type calcium current
- IK:
Delayed rectifier potassium current
- IKATP:
Adenosine triphosphate sensitive potassium current
- IKI:
Inward rectifier potassium current
- INa:
Na current
- Ito:
Transient outward potassium current
- LDH:
Lactate dehydrogenase
- LDL-C:
Low-density lipoprotein cholesterol
- L-NAME:
L-nitro arginine methyl ester
- MDA:
Malondialdehyde
- RP-HPLC:
Reverse phase high-performance liquid chromatography
- SOD:
Superoxide dismutase
- TC:
Total cholesterol
- TG:
Triglyceride
- TNF-α:
Tumor necrosis factor-α
- v3d:
Chloroform extract of ethanol extract
- VMO:
Monoterpene oxide of valerian
- VOL:
Essential oil
- VSMC:
Vascular smooth muscle cells
- XOD:
Xanthine oxidase.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Heng-Wen Chen and Ben-Jun Wei contributed equally to this work.
References
- 1.Chen H.-B., Cheng J.-R. Taxonomic revision of the relative species of Valeriana officinalis Linn. from China. Bulletin of Botanical Research. 1991;3:29–40. [Google Scholar]
- 2.Chen H. B., Cheng J. R. Studies on the medicinal plants of Valerianaceae in China. China Journal of Chinese Materia Medica. 1994;2(3):67–70. [PubMed] [Google Scholar]
- 3.Houghton P.-J. The biological activity of valerian and related plants. Journal of Ethnopharmacology. 1988;22(2):121–142. doi: 10.1016/0378-8741(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 4.Houghton P. J. The scientific basis for the reputed activity of Valerian . The Journal of Pharmacy and Pharmacology. 1999;51(5):505–512. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 5.Muller D., Pfeil T., von den Driesch V. Valeriana officinalis (monograph) Alternative Medicine Review. 2004;9(4):438–441. [PubMed] [Google Scholar]
- 6.Thies P.-W. Zur Konstitution der isovalerian saureester valepotriat, acetoxyvalepotriat und dihydrovalepotriat. Tetrahedron Letters. 1966;7(11):1163–1170. [Google Scholar]
- 7.Hendriks H., Geertsma H.-J., Malingre T.-M. The occurrence of valeranone and crytofauronol in the essential oil of Valeriana officinalis cinalis L. collected in the northern part of the Netherlands. Pharmaceutisch Weekblad Scientific Edition. 1981;116:1316–1320. [Google Scholar]
- 8.Leathwood P. D., Chauffard F., Heck E., Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacology, Biochemistry and Behavior. 1982;17(1):65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto T., Mitani Y., Nakajima K. Psychotropic effects of Japanese Valerian root extract. Chemical and Pharmaceutical Bulletin. 1992;40(3):758–761. doi: 10.1248/cpb.40.758. [DOI] [PubMed] [Google Scholar]
- 10.Santos M.-S., Ferreira F., Faro C., et al. The amount of GABA present in aqueous extracts of valerian is sufficient to account for [3H] GABA release in synaptosomes. Planta Medica. 1994;60(5):475–476. doi: 10.1055/s-2006-959538. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z.-X., Yao X.-S. The advance of chemical study on the medicinal plant Valeriana officinalis L. Chinese Journal of Medicinal Chemistry. 2000;(3):226–229. [Google Scholar]
- 12.Liu X.-G., Gao P.-Y., Wang G.-S., et al. In vivo antidepressant activity of sesquiterpenes from the roots of Valeriana faurieiBriq. Fitoterapia. 2012;83(3):599–603. doi: 10.1016/j.fitote.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q.-H., Wang C.-F., Zuo Y.-M., Wang Z.-B., Yang B.-Y., Kuang H.-X. Compounds from the roots and rhizomes of Valeriana amurensis protect against neurotoxicity in PC12 cells. Molecules. 2012;17(12):15013–15021. doi: 10.3390/molecules171215013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam S. M., Choi J. H., Yoo D. Y., et al. Valeriana officinalis extract and its main component, valerenic acid, ameliorate d-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Experimental Gerontology. 2013;48(11):1369–1377. doi: 10.1016/j.exger.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Felgentreff F., Becker A., Meier B., Brattström A. Valerian extract characterized by high valerenic acid and low acetoxy valerenic acid contents demonstrates anxiolytic activity. Phytomedicine. 2012;19(13):1216–1222. doi: 10.1016/j.phymed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q.-H., Wang C.-F., Shu Z.-P., et al. Valeriana amurensis improves Amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. Journal of Ethnopharmacology. 2014;153(2):318–325. doi: 10.1016/j.jep.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Sridharan S., Mohankumar K., Jeepipalli S. P., et al. Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology. 2015;51:172–183. doi: 10.1016/j.neuro.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Letchamo W., Ward W., Heard B., Heard D. Essential oil of Valeriana officinalis L. cultivars and their antimicrobial activity as influenced by harvesting time under commercial organic cultivation. Journal of Agricultural and Food Chemistry. 2004;52(12):3915–3919. doi: 10.1021/jf0353990. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y.-D., Gu C.-H. Study on the chemical composition of essential oil from Valeriana Officinalis L. Chemistry and Industry of Forest Products. 1989;9:59–64. [Google Scholar]
- 20.Gu C.-H., Gu L., Zhang Y.-K. Comparison study on essential oil from wild and cultivated Valeriana pseudofficinalis by GC/MS anaylsis. Chemistry and Industry of Forest Products. 1999;19:64–69. [Google Scholar]
- 21.Huang B.-K., Qin L.-P., Chu Q.-C., Zhang Q.-Y., Gao L.-H., Zheng H.-C. Comparison of headspace spme with hydrodistillation and sfe for analysis of the volatile components of the roots of Valeriana officinalis var. latifolia . Chromatographia. 2009;69(5-6):489–496. doi: 10.1365/s10337-008-0921-y. [DOI] [Google Scholar]
- 22.Shi J.-L., Liu Y., Xiao P.-G. The chemical constituents and bioactivities of Valeriana officinlais L. World Phytomedicines. 2003;18(6):231–239. [Google Scholar]
- 23.Pavlovic M., Kovacevic N., Tzakou O., Couladis M. The essential oil of Valeriana officinalis L. s.l. growing wild in Western Serbia. Journal of Essential Oil Research. 2004;16(5):397–399. doi: 10.1080/10412905.2004.9698753. [DOI] [Google Scholar]
- 24.Bos R., Hendriks H., Pras N., Stojanova A.-S., Georgiev E. V. Essential oil composition of Valeriana officinalis ssp. collina cultivated in Bulgaria. Journal of Essential Oil Research. 2000;12(3):313–316. doi: 10.1080/10412905.2000.9699524. [DOI] [Google Scholar]
- 25.Long C.-Z., Xiao H.-L., Peng J.-Q. Chemical constituents of the volatile oil from Valeriana officinalis Linn. var. Latifolia Miq grown in guizhou province. Acta Botanica Yunnanica. 1987;9(1):109–112. [Google Scholar]
- 26.Ming D.-S., Guo J.-X., Shun Q.-S. Determination of chemical composition of the essential oil from four kinds of Valeriana officinalis L. by GC/MS. Chinese Traditional Patent Medicine. 1994;16(1):41–42. [Google Scholar]
- 27.Wang L.-Q., Xiong Y.-T., Tao F.-H., Li N.-Q. Chemical constituents of essential oil from Valeriana officinalis Linn. var. Latifolia Miq . China Journal of Chinese Materia Medica. 1999;22(6):298–299. [Google Scholar]
- 28.Yu Z.-W., Yang Z.-N., Yi Y. Analysis of chemical constituents of essential oil from cultured Valeriana officinalis L. Chinese Journal of Spectroscopy Laboratory. 2011;28(4):1672–1674. [Google Scholar]
- 29.Thies P.-W., Funke S. Active principles of baldrian. I. Detection and isolation of the sedative active isovalerianic acid esters from roots and rhizomes of various Valerian and Centranthus species. Tetrahedron Letters. 1966;7(11):1155–1162. doi: 10.1016/s0040-4039(00)72388-4. [DOI] [PubMed] [Google Scholar]
- 30.Chen L., Qin L.-P., Zheng H.-C. Chemical constituents, plant resource and pharmacology-activity on the Valeriana officinalis L. Journal of Pharmaceutical Practice. 2000;18(5):277–279. [Google Scholar]
- 31.Lin S., Chen T., Liu X.-H., et al. Iridoids and lignans from Valeriana jatamansi . Journal of Natural Products. 2010;73(4):632–638. doi: 10.1021/np900795c. [DOI] [PubMed] [Google Scholar]
- 32.Schumacher B., Scholle S., Hölzl J., Khudeir N., Hess S., Müller C. E. Lignans isolated from valerian: identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors. Journal of Natural Products. 2002;65(10):1479–1485. doi: 10.1021/np010464q. [DOI] [PubMed] [Google Scholar]
- 33.Piccinelli A. L., Arana S., Caceres A., di Villa Bianca R. D., Sorrentino R., Rastrelli L. New lignans from the roots of Valeriana prionophylla with antioxidative and vasorelaxant activities. Journal of Natural Products. 2004;67(7):1135–1140. doi: 10.1021/np049879c. [DOI] [PubMed] [Google Scholar]
- 34.Nazarova I.-P., Glushenkova A.-I., Umarov A.-U. Gossypol-like compounds of the cotton plant. Methods of determining gossypol. Chemistry of Natural Compounds. 1981;17(2):87–102. doi: 10.1007/bf00634720. [DOI] [Google Scholar]
- 35.Thies P.-W., Finner E., Rosskopf F. Über die wirkstoffe des baldrians—X: die konfiguration des valtratum und anderer valepotriate. Tetrahedron. 1973;29(20):3213–3226. doi: 10.1016/s0040-4020(01)93469-6. [DOI] [Google Scholar]
- 36.Popov S., Handjieva N., Marekov N. A new valepotriate, 7-epi-deacetylisovaltrate from Valeriana officinalis . Phytochemistry. 1974;13(12):2815–2818. doi: 10.1016/0031-9422(74)80247-5. [DOI] [Google Scholar]
- 37.Thies P.-W. Constitution of valepotriates. Report on active agents of valerian. Tetrahedron. 1968;24(1):313–347. [Google Scholar]
- 38.Holzl J., Koch U. The compounds of Valeriana alliariifolia. 1-β-acevaltratum, a new valepotriate. Planta Medica. 1984;50(5):p. 458. doi: 10.1055/s-2007-969771. [DOI] [PubMed] [Google Scholar]
- 39.Koch U., Hoelzl J. Constituents of Valeriana alliariifolia. 2. Valepotriathydrines. Planta Medica. 1985;51(2):172–173. doi: 10.1055/s-2007-969443. [DOI] [Google Scholar]
- 40.Salles L.-A., L. Silva A., Rech S. B., Zanatta N., Von Poser G. L. Constituents of Valeriana glechomifolia Meyer. Biochemical Systematics and Ecology. 2000;28(9):907–910. doi: 10.1016/s0305-1978(99)00124-6. [DOI] [PubMed] [Google Scholar]
- 41.Becker H., Chavadej S. Tissue cultures of Valerianaceae. 7. Valepotriate production of normal and colchicine-treated cell-suspension cultures of Valeriana Wallichii . Journal of Natural Products. 1985;48(1):17–21. doi: 10.1021/np50037a003. [DOI] [PubMed] [Google Scholar]
- 42.Amanzadeh Y., Ghassemi-Dehkordi N., Sadat-Ebrahimi S. E., Pirali-Hamedani M. Two new valepotriates from the roots of Valeriana sisymbriifolia . DARU Journal of Pharmaceutical Sciences. 2002;10(2):63–66. [Google Scholar]
- 43.Fuzzati N., Wolfender J. L., Hostettmann K., Msonthi J. D., Mavi S., Molleyres L. P. Isolation of antifungal valepotriates from Valeriana capense and the search for valepotriates in crude Valerianaceae extracts. Phytochemical Analysis. 1996;7(2):76–85. [Google Scholar]
- 44.Tang Y., Liu X., Yu B. Iridoids from the rhizomes and roots of Valeriana jatamansi . Journal of Natural Products. 2002;65(12):1949–1952. doi: 10.1021/np0203335. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y.-M., McLaughlin S.-P., Gunatilaka A. A. L. Sorbifolivaltrates A-D, diene valepotriates from Valeriana sorbifolia . Journal of Natural Products. 2007;70(12):2045–2048. doi: 10.1021/np0704553. [DOI] [PubMed] [Google Scholar]
- 46.Thies P.-W., Finner E., David S. On the active agents of valerian.14. assignment of type and location of the acyloxy substituents in valepotriates via 13C-NMR spectroscopy. Planta Medica. 1981;41(1):15–20. doi: 10.1055/s-2007-971667. [DOI] [PubMed] [Google Scholar]
- 47.Holzl J., Chari V.-M., Seligmann O. Structure of 3 genuine valtrate hydrines from Valeriana tiliaefolia . Tetrahedron Letters. 1976;17(15):1171–1174. doi: 10.1016/s0040-4039(00)78009-9. [DOI] [Google Scholar]
- 48.Wang R., Xiao D., Bian Y.-H., et al. Minor iridoids from the roots of Valeriana wallichii . Journal of Natural Products. 2008;71(7):1254–1257. doi: 10.1021/np070598p. [DOI] [PubMed] [Google Scholar]
- 49.Lin S., Shen Y.-H., Li H.-A., et al. Acylated iridoids with cytotoxicity from Valeriana jatamansi . Journal of Natural Products. 2009;72(4):650–655. doi: 10.1021/np800716f. [DOI] [PubMed] [Google Scholar]
- 50.Xu J., Guo P., Fang L.-Z., Li Y.-S., Guo Y.-Q. Iridoids from the roots of Valeriana jatamansi . Journal of Asian Natural Products Research. 2012;14(1):1–6. doi: 10.1080/10286020.2011.618804. [DOI] [PubMed] [Google Scholar]
- 51.Xu J., Zhao P., Guo Y.-Q., et al. Iridoids from the roots of Valeriana jatamansi and their neuroprotective effects. Fitoterapia. 2011;82(7):1133–1136. doi: 10.1016/j.fitote.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Xu J., Guo Y.-Q., Jin D.-Q., et al. Three new iridoids from the roots of Valeriana jatamansi . Journal of Natural Medicines. 2012;66(4):653–657. doi: 10.1007/s11418-012-0631-5. [DOI] [PubMed] [Google Scholar]
- 53.Bach K.-K., Ghia F., Torssell K.-B.-G. Valtrates and lignans in Valeriana microphylla . Planta Medica. 1993;59(5):478–479. doi: 10.1055/s-2006-959740. [DOI] [PubMed] [Google Scholar]
- 54.Kucaba W., Thies P.-W., Finner E. Isodidrovaltratum, ein neues valepotriat aus Valeriana vaginata . Phytochemistry. 1980;19(4):575–577. doi: 10.1016/0031-9422(80)87018-x. [DOI] [Google Scholar]
- 55.Yu L., Huang R., Han C., Lv Y., Zhao Y., Chen Y. New iridoid triesters from Valeriana jatamansi . Helvetica Chimica Acta. 2005;88(5):1059–1062. doi: 10.1002/hlca.200590077. [DOI] [Google Scholar]
- 56.Yu L.-L., Han C.-R., Huang R., Lv Y.-P., Gui S.-H., Chen Y.-G. A new iridoid tetraester from Valeriana jatamansi . Pharmazie. 2006;61(5):486–488. [PubMed] [Google Scholar]
- 57.Wang P.-C., Hu J.-M., Ran X.-H., et al. Iridoids and sesquiterpenoids from the roots of Valeriana officinalis . Journal of Natural Products. 2009;72(9):1682–1685. doi: 10.1021/np9003382. [DOI] [PubMed] [Google Scholar]
- 58.Xu J., Guo P., Guo Y., et al. Iridoids from the roots of Valeriana jatamansi and their biological activities. Natural Product Research. 2011;26(21):1996–2001. doi: 10.1080/14786419.2011.636747. [DOI] [PubMed] [Google Scholar]
- 59.Huang B.-K., Zheng H.-C., Qin L.-P. Material basis of the sedative and hypnotic activities of Valeriana officinalis . Pharmaceutical Care and Research. 2006;6(3):165–168. [Google Scholar]
- 60.Wu B., Fu Y.-M., Huang A.-H., Ma Y.-J. Changes of GABA and Glu content in hippocampus of PTZ-induced epileptic rats treated with volatile oil of Valeriana . Chinese Archives of Traditional Chinese Medicine. 2008;26(11):2476–2477. [Google Scholar]
- 61.Chen J.-S., Wu J.-K., Liu L., Zhang Y., Wang F.-J., Du X.-W. Studies on improving sleep function and relative mechanism of mice by petroleum extract of Valeriana amurensis . Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(24):245–249. [Google Scholar]
- 62.Zhang Z.-X., Yao X.-S. The development and research advances in bio-activity for the medicinal plant Valeriana officinalis . Journal of Shenyang Pharmaceutical University. 2000;(1):222–225. [Google Scholar]
- 63.Wang J.-F., Yang G.-Y., Wang J.-N. Effects of Valeriana officinalis var Latifolia Miq on the migration of cultured human vascular smooth muscle cells. Journal of Yunyang Medical College. 1999;18(4):196–197. [Google Scholar]
- 64.Yang G.-Y., Xu Q., Wang J.-F. Valeriana officinalis var. Latifolia Miq regulates vascular smooth muscle cell contraction and growth. Journal of Yunyang Medical College. 2002;21(6):324–326. [Google Scholar]
- 65.Zhou X.-Z., Kang L., Kang Y., Li L., Xiong S.-H. Effect of Valeriana Officinalis Var Latifolia Miq on heart rat and arterial blood pressure of rabbit. Journal of Liaoning University of TCM. 2009;11(12):188–189. [Google Scholar]
- 66.Fields A.-M., Richards T.-A., Felton J.-A., et al. Analysis of responses to valerian root extract in the feline pulmonary vascular bed. Journal of Alternative and Complementary Medicine. 2003;9(6):909–918. doi: 10.1089/107555303771952253. [DOI] [PubMed] [Google Scholar]
- 67.Estrada-Soto S., Rivera-Leyva J., Ramírez-Espinosa J.-J., Castillo-España P., Aguirre-Crespo F., Hernández-Abreu O. Vasorelaxant effect of Valeriana edulis ssp. procera (Valerianaceae) and its mode of action as calcium channel blocker. Journal of Pharmacy and Pharmacology. 2010;62(9):1167–1174. doi: 10.1111/j.2042-7158.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang B.-H., Meng H.-P., Wang T., et al. Effects of Valeriana officinalis L. extract on cardiovascular system. Acta Pharmaceutica Sinica. 1982;17(5):382–384. [PubMed] [Google Scholar]
- 69.Yang J., Xue C.-K., Zhu X.-Z., et al. Evaluate the effect of some TCMs extracts on improving micro circulation reperfusion volume of both cardiac and renal tissues by 86Rb tracer. Chinese Journal of Microcirculation. 1998;8(1):15–17. [Google Scholar]
- 70.Huang Z.-R., Tang Q.-Z., Li W.-H., Zhang L.-J., Xie Q., Wu G. Study of monoterpene oxide pretreatment on donor heart preservation. Chinese Heart Journal. 2006;18(2):182–184. [Google Scholar]
- 71.Yang S.-H., Chen F., Ma H.-M., Wang T. Protection of Valeriana officinalis L. extract preconditioning on ischemia-reperfusion injury in rat hearts in vitro. Medical Journal of Wuhan University. 2012;33(5):639–643. [Google Scholar]
- 72.Wang H., Luo S.-D., Cai H.-S., Wang F., Yang J. Antiarrhythmic effect of diacetyl-linesinine. Chinese Journal of Hospital. Pharmacy. 2001;21(6):326–330. [Google Scholar]
- 73.Gong D.-M., Shan H.-L., Dong D.-L., Zhou H.-Y., Yang B.-F. Study of ion targets in Ouabain-induced rat arrhythmia. Journal of Harbin Medical University. 2002;36(2):87–90. [Google Scholar]
- 74.Jia J.-N., Zhang B.-H. Effects of extract of Valeriana officinalis L. (V3d) on cardiovascular system. Journal of Guangxi University of Chinese Medicine. 1999;16(1):40–42. [Google Scholar]
- 75.Huang Z.-R. Effect of Valerian Extract on Ionic Channels of Rabbit Ventricular Myocytes. Wuhan, China: Wuhan University; 2004. [Google Scholar]
- 76.Wen L., Zhou Y., Zhou W., Duan X.-Y., Fang Y. Effect of extracts of Valeriana officinalis L. on cardiac arrhythmias. Chinese Journal of Hospital Pharmacy. 2009;29(3):191–194. [Google Scholar]
- 77.Duan X.-Y. Study on the Drug Effect Substances and the Mechanism of Arrhythmic Effects of Valeriana officinalis L. Wuhan, China: Hubei College of TCM; 2009. [Google Scholar]
- 78.Xie Q., Li W.-H., Huang Z. R., Zhang Z. Effect of didrovaltrate on l-calcium current in rabbit ventricular myocytes. Journal of Traditional Chinese Medicine. 2012;32(3):442–445. doi: 10.1016/S0254-6272(13)60052-7. [DOI] [PubMed] [Google Scholar]
- 79.Liu J.-F., Fang Y., Gong Z.-F., Liu Y.-W., Jiu J.-F. Study on the anti-arrhythmia effective substances and serum pharmacochemistry of Valeriana officinalis L. Hubei Journal of Traditional Chinese Medicine. 2013;35(1):72–73. [Google Scholar]
- 80.Hu C.-X., Zhang D.-B., Li H., et al. Effects of Valeriana officinalis L. var Latifolia Miq on blood-lipid metabolism in rabbits with hyperlipidemia. Journal of Nanjing Military Medical College. 1999;21(2):65–68. [Google Scholar]
- 81.Si X.-Y., Jia R.-H., Huang C.-X., Ding G.-H., Liu H.-Y. Effects of Valeriana officinalis var. latifolia on expression of transforming growth factor β 1 in hypercholesterolemic rats. China Journal of Chinese Materia Medica. 2003;28(9):845–848. [PubMed] [Google Scholar]


