Abstract
Mitochondrial disease involving complex II is rare among respiratory chain deficiencies and its genetic cause remains often unknown. Two main clinical presentations are associated with this biochemical defect: mitochondrial encephalomyopathy and susceptibility to tumors. Only one homozygous SDHB mutation has been described in a patient with mitochondrial disorder. We report here two sisters, who presented highly different phenotypes (neurological impairment with leukoencephalopathy vs. asymptomatic status) and harbored the same homozygous SDHB mutation, suggesting reduced penetrance.
1. Introduction
SDHB encodes one of four structural subunits (SDHA, SDHB, SDHC, SDHD) forming complex II (cII) of the mitochondrial respiratory chain (MRC). cII, or succinate-ubiquinone oxidoreductase (E.C. 1.3.5.1), is the only membrane-bound member of the tricarboxylic acid cycle, where it functions as a succinate dehydrogenase (SDH). By coupling this reaction to the reduction of ubiquinone to ubiquinol, cII takes part in the MRC. At least four assembly factors (SDHAF1-4) assist the formation of the holocomplex and additional proteins are required for the iron-sulfur clusters incorporation into cII.
Several heterozygous mutations in SDHA, SDHB, SDHC, SDHD, SDHAF2 are susceptibility factors for developing tumors of chromaffin-cells, such as paragangliomas (PGL) and phaechromocytomas, gastrointestinal stromal tumors and/or renal cell carcinoma [1]. On the contrary, only a few recessive mutations in SDHA [2], [3] or in SDHD [4], [5], have been reported in mitochondrial encephalomyopathy with (or without) cardiac involvement associated with cII deficiency, while SDHAF1 mutations are the most common cause of mitochondrial leukoencephalopathy associated with cII deficiency [6], [7]. The reasons determining whether cII defects lead to neurological disease or tumor are poorly understood, as well the possible link between mutations in specific cII genes and either one or the other clinical presentation.
There is only one report describing a homozygous SDHB mutation associated with mitochondrial disease in a child affected by leukoencephalopathy and cII deficiency [4]. Since no other SDHB-related mitochondrial diseases have been reported so far, this variant is classified as a variant of unknown significance because its contribution to mitochondrial complex II deficiency has not been confirmed (MIM*185470). We describe two sisters with the same homozygous mutation p.Asp48Val in SDHB, one presenting with severe hypotonia and psychomotor regression with leukoencephalopathy and the other one virtually asymptomatic.
2. Material and methods
2.1. Histochemical and biochemical analyses
Cryostatic cross sections from skeletal muscle biopsy were processed according to standard histochemical procedures. MRC complex activities were measured using standard spectrophotometric methods [8] in muscle homogenate and digitonin-treated skin fibroblasts.
2.2. Mutational analysis
Total genomic DNA was extracted by standard methods from peripheral blood of the patients and parents. A customized gene panel (TruSeq Custom Amplicon, Illumina) containing nuclear genes associated with cII deficiency (SDHA, SDHB, SDHC, SDHD, SDHAF1, SDHAF2, SDHAF3) was used for library preparation; then samples were analyzed by a Miseq system (Illumina), with 100X effective mean depth. The generated reads were aligned to human genome assembly hg19 and the identified variants were annotated (Variant-Studio, Illumina) and filtered, focusing on rare variants (minimum allele frequency < 1% in 1000 Genome Project [www.1000genomes.org] and ExAc [http://exac.broadinstitute.org] databases), causing changes potentially damaging for the protein function (Polyphen2, SIFT). Since the pedigree was suggestive for a recessive trait, we searched for genes with a homozygous variant or two heterozygous variants. Sanger sequencing was used to confirm the mutation in the patient and the segregation in the family.
2.3. Western blot analysis
Fibroblasts were pelleted and solubilized in RIPA buffer with protease inhibitors. Lymphocytes were obtained from peripheral blood using Lympholyte-H (Cedarlane Laboratories) and treated as described above. 50 μg of proteins were loaded for each sample in 12% denaturing sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Monoclonal antibodies against cII subunits SDHB and SDHA (Mitosciences), mitochondrial porin/VDAC1 (Abcam) and GAPDH (Millipore) were used.
3. Case reports.
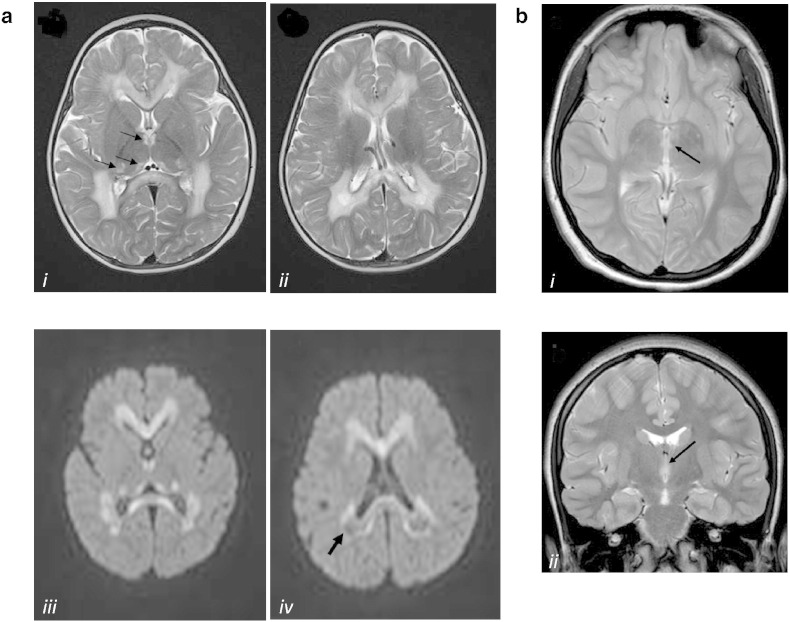
The proband (P, II-4) is a girl, fourth child of healthy related -first cousins- parents of Pakistani origin. Family and personal history were unremarkable. Psychomotor development was referred normal: head control at 3 months, sitting at 6 months, walking alone at 12 months. At 15 months, a few days after a febrile illness, she presented acute psychomotor regression, losing previously acquired psychomotor skills in about a week. She was admitted to our Institute one month later. She presented with generalized hypotonia, hyperreflexia, no postural control, poor voluntary movements, marked irritability with frequent crying. She did not present with seizures. Lactate and pyruvate were elevated in plasma: 3327 μmol/l (normal values, nv: 580–2100) and 151 μmol/l (nv: 55–145) respectively, and normal in CSF; 2-ketoglutaric aciduria (557 μg/mg creatinine; nv < 140) was detected. Brain MRI showed diffuse hyperintensity of the hemispheric white matter and corpus callosum. The subcortical U-fibers are spared. Posterior deep white matter showed evidence of rarefaction and cystic degeneration. There were also small symmetric hyperintensites in the thalami. HNMR-spectroscopy demonstrated a peak of succinate and elevate lactate (Fig. 1a).
Fig. 1.
Representative MRI images of our SDHB-mutant cases
a. Brain MRI of the proband (II-4). Axial T2-weighted images (i, ii) show diffuse hyperintensities in the hemispheric white matter and corpus callosum. Small symmetric high signal abnormalities are also present in the medial and posterior thalami (arrows in panel i). Diffusion-weighted images (iii, iv) show restricted diffusion in the corpus callosum, thalami and, partially, in the white matter. Posterior white matter has a prevalent low signal (iv) reflecting partial cystic degeneration and cavitations (arrow).
b. Brain MRI of the asymptomatic sister II-1. Axial (i) and coronal (ii) T2-weighted images show very small symmetric lesions in the medial thalami (arrows).
Fundus oculi, electroretinogram, brainstem auditory evoked potential and motor and sensory nerve conduction velocities were normal. Visual evoked potential showed central conduction abnormalities. Electroencephalography disclosed normal background activity, with a prevalence of slow activity in the right posterior regions. Electrocardiogram and echocardiogram were normal.
Informed consent for biochemical and genetic studies was obtained from patient's parents.
All her siblings were reported in good health. The older sister (II-1), now 11 years old, was born after uncomplicated pregnancy and delivery; her neonatal period was normal. Psychomotor development was referred normal. She was in good health and neurological history was negative. After the genetic analysis, she underwent a neurological examination that resulted normal. Routine exams, lactate and pyruvate serum levels, as well as hemogasanalysis were normal. Brain MRI showed very small symmetric lesions in the medial thalami (Fig. 1b); HNMR-spectroscopy was normal. Because of the healthy status of the girl, the parents did not agree on a skin or muscle biopsy.
4. Results
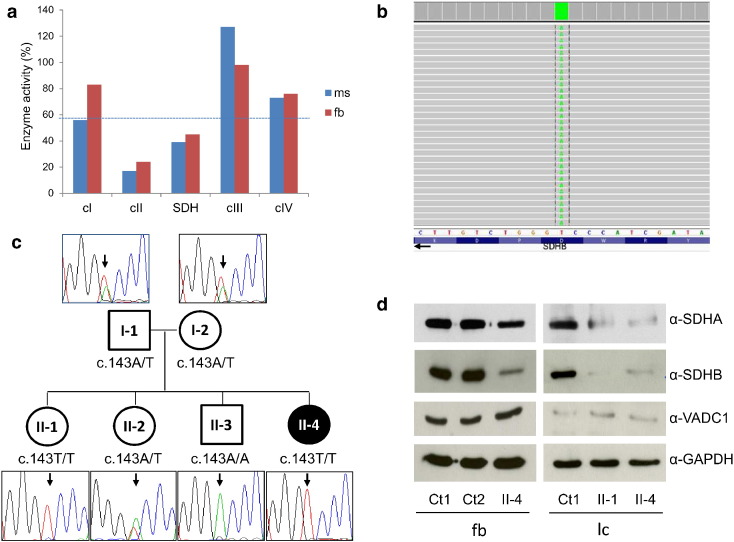
Histological analysis of proband's muscle biopsy showed few hypotrophic fibers, with normal lipids and glycogen content. Reduction of cII (succinate-ubiquinone reductase) and SDH activities were documented both on muscle tissue and skin fibroblasts (Fig. 2a). Complex I activity was at the lower range of control values in muscle. Sequence analysis of the SDHAF1 gene was negative. Targeted resequencing of a panel containing nuclear genes associated with cII deficiency revealed the presence of a homozygous variant in SDHB (NM_003000), c.143A > T p.(Asp48Val) (Fig. 2b). The mutation was found to be heterozygous in both parents (I-1, I-2: Fig. 2c); although this SDHB mutation has not been reported in association with cancer susceptibility, we preferred to refer parents for cancer surveillance and to expand analysis to siblings. In the older sister (II-1) we found the SDHB variant in homozygosity, while II-2 was heterozygous and II-3 was homozygous for the wild-type allele (Fig. 2c). The p.Asp48Val change is predicted to be damaging by different bioinformatics tools; moreover, the pathogenicity of this mutation was already experimentally validated through yeast modeling [4]. Finally, immunoblot analysis on proband's fibroblasts showed strongly decreased levels of SDHB, suggesting a deleterious effect of the identified SDHB variant on protein stability; interestingly, SDHA amount also appeared to be reduced, probably due to instability of the assembled cII (Fig. 2d). We performed the immunoblot analysis also on lymphocytes obtained from blood samples of the two mutant sisters (II-1 and II-4); notably, we observed the same results in both, with a strong reduction of SDHB and decreased levels of SDHA compared to controls (Fig. 2d).
Fig. 2.
Biochemical, genetic and protein studies
a. Biochemical analysis of MRC complex activities in muscle (ms) and fibroblasts (fb) from the proband. Enzyme activities, normalized for citrate synthase activity, are expressed as percentages of the control mean. cI, cIII, cIV: complex I, III, IV. cII: succinate-ubiquinone reductase; SDH: succinate dehydrogenase. The dotted line indicates the lower values in the control range.
b. Snapshot from IGV software of the mutation identified in the proband. SDHB is on the (−) strand thus IGV reports the reverse complementary nucleotides.
c. Pedigree of the family and electropherograms of the SDHB region containing the c.143A > T variant. The black symbol indicates the clinically affected subject (II-4).
d. Immunoblot analysis of total lysates obtained from fibroblasts (fb) or lymphocytes (lc) of controls (Ct1 and Ct2) and SDHB-mutant subjects (II-1, II-4), detected using α-SDHB, α-SDHA, α-VDAC1 and α-GAPDH antibodies. The latter was used as loading control; VDAC1, an abundant mitochondrial protein, is an index of mitochondrial content in each sample.
5. Discussion
We identified the second case of inherited biallelic SDHB mutation associated with mitochondrial disorder; like the previous patient, she was characterized by leukodystrophy and cII deficiency. Unexpectedly, the same mutation was present also in an unaffected sister of our proband. The c.143A > T variant (rs202101384) has been reported only in South Asian subjects, but with a very low frequency (0.036% in ExAc database; 0 homozygotes out of 8256, in this ethnic group). Notably, both our patients and the other described SDHB-mutant case were Asian. Nevertheless, the pathogenicity of the p.Asp48Val amino acid change was previously demonstrated in a yeast model [4]. In addition, the Western blot analysis showed that SDHB level is strongly affected on proband's fibroblasts as well as in the lymphocytes from both SDHB-mutant sisters. Decreased amount of SDHB has been observed with mutations not only in SDHB but also in other SDHx genes [4], [9]; however, the extensive genetic analysis we performed, including all genes encoding cII structural subunits and known assembly factors, detected only the c.143A > T variant in SDHB. Moreover, all the described patients with the c.143A > T variant (both sisters of the present paper and the previously reported patient [4]) showed a strong decrease in SDHB level making unlikely the hypothesis that a common deleterious variant in another SDHx gene is responsible for the SDHB reduction. All these data strongly suggested the causative role of the identified SDHB variant.
Despite having the same mutation, the older sister did not present any clinical symptom and showed only minimal lesions at MRI. However, at the protein level, the two siblings displayed the same defect affecting cII subunits. We speculate that the mutant SDHB allows a residual activity of the cII, enough for maintaining a minimal proficiency of the complex in most of the physiological conditions or during the majority of life periods. This hypothesis is in agreement with the symptom-free period of 15 months observed in the proband and the onset of the disease after a febrile illness; it is possible that the unaffected sister II-1 overpassed the critical periods without triggering stimuli, thus preventing the onset of an overt clinical presentation.
Incomplete or reduced penetrance of phenotypes has been rarely associated with recessive mutations [10] and is infrequent in infantile mitochondrial disorder. However, for instance, we recently described two sisters harboring a homozygous non-sense mutation in a gene associated with another mitochondrial leukoencephalopathy, who showed very different clinical pictures: the older sibling developed severe motor and cognitive impairment at an early age (after a symptom-free period) while the second, now 16 years old, never developed neurological signs [11]. The white matter has probably specific energy request and may be more prone to metabolic dysfunctions and damages than other cerebral districts, at least in certain phases of infancy. In addition, although common for heteroplasmic mutations in mitochondrial DNA, incomplete penetrance has been described also for diverse homoplasmic mutations causing even severe infantile mitochondrial diseases [12], [13].
It is not possible to define if the observed phenotypes (ranging from leukoencephalopathy to an asymptomatic status) are strictly related to the disease-gene or are mutation-specific; additional SDHB-mutant patients should be examined to achieve a better definition of clinical presentations, genotype/phenotype correlations and prognostic clues. After the genetic diagnosis, treatment with riboflavin and coenzyme Q10 was started in the proband but, differently from the patient reported by Alston and colleagues [4], the baby did not improve. Three months later, the child presented important sweating and feeding difficulty and a gavage became necessary. At neurological examination, she presented spastic tetraparesis and severe cognitive impairment.
In the past it was suggested a strict connection between cII mutated genes and phenotypes: SDHB, SDHC, SDHD, SDHAF2 associated with PGL4, PGL3, PGL1, PGL2 respectively; SDHA associated with Leigh syndrome; SDHAF1 associated with leukoencephalopathy [14]. Conversely, recent data [4], [5], [15] and our findings indicate that, irrespective of the mutated gene, recessive mutations impairing cII may cause infantile mitochondrial disease whereas germline heterozygous mutations may determine or predispose to hereditary PGL or other tumors; a second mutation in the other allele, occurring somatically, determines the elimination of the functionally active protein and the consequent induction of the neoplastic transformation. Thus, all genes encoding SDH subunits or assembly factors should be assessed in cases of isolated complex II deficiency in pediatric patients.
6. Conclusions
Our report confirms the pathogenic role of SDHB mutations in mitochondrial leukoencephalopathy associated with cII deficiency, in addition to their established link with hereditary PGL. We thus recommend to add SDHB screening in the genetic diagnostic procedure for cII deficiency related-leukoencephalopathy. However, the presence of an unaffected sister harboring the same SDHB mutation, suggests a broad range of clinical presentations associated with this genetic defect. This observation has important consequences in the genetic counseling, indicating that reduced penetrance should be considered also in infantile mitochondrial disorders, in particular leukoencephalopathies, caused by nuclear gene mutations.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Telethon Foundation Grant GGP11011, the Ministry of Health, Italy (GR2010–2316392), the Pierfranco and Luisa Mariani Foundation (CM23). We acknowledge the “Cell Lines and DNA Bank of Pediatric Movement Disorders and Neurodegenerative Diseases” of the Telethon Network of Genetic Biobanks (grant GTB12001J) and the Eurobiobank Network.
References
- 1.Hoekstra A.S., Bayley J.P. The role of complex II in disease. Biochim. Biophys. Acta. 1827;2013:543–551. doi: 10.1016/j.bbabio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Bourgeron T., Rustin P., Chretien D. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995;11:144–149. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- 3.Levitas A., Muhammad E., Harel G. Familial neonatal isolated cardiomyopathy caused by a mutation in the flavoprotein subunit of succinate dehydrogenase. Eur. J. Hum. Genet. 2010;18:1160–1165. doi: 10.1038/ejhg.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alston C.L., Davison J.E., Meloni F. Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J. Med. Genet. 2012;49:569–577. doi: 10.1136/jmedgenet-2012-101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson C.B., Nuoffer J.M., Hahn D. Mutations in SDHD lead to autosomal recessive encephalomyopathy and isolated mitochondrial complex II deficiency. J. Med. Genet. 2014;51:170–175. doi: 10.1136/jmedgenet-2013-101932. [DOI] [PubMed] [Google Scholar]
- 6.Ghezzi D., Goffrini P., Uziel G. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat. Genet. 2009;41:654–656. doi: 10.1038/ng.378. [DOI] [PubMed] [Google Scholar]
- 7.Ohlenbusch A., Edvardson S., Skorpen J. Leukoencephalopathy with accumulated succinate is indicative of SDHAF1 related complex II deficiency. Orphanet J. Rare Dis. 2012;7:69. doi: 10.1186/1750-1172-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugiani M., Invernizzi F., Alberio S. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta. 2004;1659:136–147. doi: 10.1016/j.bbabio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Pantaleo M.A., Astolfi A., Urbini M. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur. J. Hum. Genet. 2014;22:32–39. doi: 10.1038/ejhg.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper D.N., Krawczak M., Polychronakos C., Tyler-Smith C., Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum. Genet. 2013;132:1077–1130. doi: 10.1007/s00439-013-1331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melchionda L., Haack T.B., Hardy S. Mutations in APOPT1, encoding a mitochondrial protein, cause cavitating leukoencephalopathy with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 2014;95:315–325. doi: 10.1016/j.ajhg.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland R., Clark K.M., Morris A.A., Taylor R.W., Macphail S., Lightowlers R.N., Turnbull D.M. Multiple neonatal deaths due to a homoplasmic mitochondrial DNA mutation. Nat. Genet. 2002;30:145–146. doi: 10.1038/ng819. [DOI] [PubMed] [Google Scholar]
- 13.Tuppen H.A., Blakely E.L., Turnbull D.M., Taylor R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 1797;2010:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Rutter J., Winge D.R., Schiffman J.D. Succinate dehydrogenase — assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnichon N., Brière J.J., Libé R. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]