Abstract
In 1985, John J. Monoco —the discoverer of LMP-2 and -7, the inducible components of the immunoprotasome— asked his advanced immunology class why we thought that the MHC region not only contained structural genes but several others as well whose functions were then unknown. As we drew a blank, he quipped: perchance because many of the MHC genes are induced by interferon-γ (IFN-γ)! The ensuing three decades has unveiled the profound fundamental and clinical implications of that tête–à–tête classroom enquiry. Amongst its multitudinous effects, the anti-tumour agent IFN-γ induces several genes to enhance antigen processing and presentation to T cells; genes encoding cellular proteases and activators of proteases are amongst them. In this issue, Ulrike Seifert and colleagues (Eur. J. Immunol. 45: pp—pp; 2015) demonstrate that the limited success of MART-1/Melan-A-targeted melanoma immunotherapy in patients could be because of inefficient MART-126—35 presentation owing to the proteolytic activities of IFN-γ-inducible β2i/MECL-1, proteasome activator 28 (PA28), and endoplasmic reticulum-associated aminopeptidases-associated with antigen processing. Specifically, whilst β2i and PA28 impede MART-126—35 liberation from the precursor protein, ERAP-1 degrades this epitope. Hence, critical to effective cancer immunotherapy is deep knowledge of multiple T cell-targeted tumour antigens and how cellular proteases generate protective epitope(s) from them or destroy them.
T cell targeted immunotherapeutics have recently emerged as new arsenal against cancers. This arsenal includes weapons such as MHC-restricted antigens —including tumour-specific antigens (reviewed in [1]), e.g., the melanocyte lineage specific antigen MART-1/Melan-A (henceforth MART-1), and neoantigens [2]— checkpoint inhibition with the aid of specific monoclonal antibodies —e.g., anti-CTLA4 or anti-PD-1 and anti-PD-L1, et cetera; [3])— and tumour-infiltrating antigen-specific T cells (TILs) [3]). MART-1 has been used in clinical trials with limited efficacy even though it contains the immunodominant HLA-A*02;01-restricted CD8+ T cell epitope MART-126(27)—35 [1,4]. Studies have shown that interference with antigen processing perhaps underlies the noted poor clinical efficacy of MART-1 immunotherapy. MART-1 contains two A*02;01-restricted epitopes —the MART-126–35 10mer and the MART-127–35 9mer— yet only the 9mer epitope is naturally processed and presented at the surface of melanoma cells [5]. The generation of these epitopes is dependent on the standard, constitutively expressed, proteasomes [6]. IFN-γ in many instances enhances antigen presentation and T cell recognition —e.g., generation of TRP2360–368 from the melanoma antigen tyrosinase-related protein 2 [7], interferes with efficient generation of MART-126–35 and other epitopes [6,8–10]. In their article, Seifert et alii, opere citato, tease out how IFN-γ interferes with MART-126—35 presentation.
CD8+ cytotoxic T lymphocytes (hereinafter CD8 T cells) play critical roles in tumour immune surveillance. T cell functions are controlled in a process termed MHC (Major Histocompatibility Complex) restriction. MHC restriction entails the processing of proteins to short peptides and their presentation at the cell surface by MHC-encoded class I and class II molecules for an appraisal by self peptide (p)/MHC-tolerant T cells. CD8 T cell functions are controlled by MHC class I molecules, which in humans are encoded by HLA (Human Leukocyte Antigen)-A, -B and -C loci. Antigen presentation by HLA class I molecules requires proteolytic processing of proteins to short peptides of 9—13 amino acid residues in the cytosplasm. Such proteolysis within the cytoplasm is accomplished by the proteasomes. Proteasomal products —short and long, i.e., longer than the typical 9—13 amino acid residue-peptides that bind to class I molecules—are further transported from the cytoplasm into the endoplasmic reticulum (ER) lumen by Transporters-associated with Antigen Processing (TAP). Within the ER, peptides are made available to peptide-receptive class I molecules within the peptide loading complex (PLC). Those that have class I binding motifs assemble with class I molecules; but those that contain the motif but are longer than can fit into the antigen-binding groove are further trimmed to size by the ER AminoPeptidases-associated with antigen processing (ERAP)-1 and 2. Once fully assembled with a bound peptide, the PLC releases class I molecules, which then egress from the ER, negotiate the Golgi Apparatus and arrive at the cell surface (reviewed in [11]).
Proteasomes are multicatalytic endoproteinase complexes composed of four rings in which each ring is made of seven related subunits. The two outer rings composed of α subunits sandwich the two inner catalytic rings of β subunits. This quartet of heptameric rings forming the core 20S proteasome assembles in such a way that they form an interior chamber. The N-terminal residues of the α rings gate the catalytic rings, the opening of which is controlled by the regulatory cap made up of the11S proteasome activators (PA) and/or the AAA+ ATPase-containing 19S unit. The N-terminus of β1, β2 and β5 subunits is exposed to the interior chamber and contains the proteolytic active sites (reviewed in [12,13]).
IFN-γ enhances MHC-restricted antigen presentation by inducing the expression of multiple structural and regulatory genes including HLA class I, β1i/LMP (Low Molecular mass Polypeptide)-2, β2i/MECL (Multicatalytic Endopeptidase Complex-Like)-1, β5i/LMP7, the regulatory cap PA28 and ERAP (ER-associated AminoPeptidase-associated with antigen Processing) amongst others especially within immune cells in healthy individuals. The induced proteasomal components occupy the place of the homologous component within the constitutive, standard proteasome creating the immunoproteasome (Figure 1A, B). Immunoproteasome formation is a highly ordered process: β2i requires β1i for efficient incorporation into preproteasomes, and preproteasomes containing β1i and β2i require pre-β5i for efficient maturation and, thereby, ensures the assembly of homogeneous immunoproteasomes for efficient generation of peptides presented by class I molecules [12,14–16].
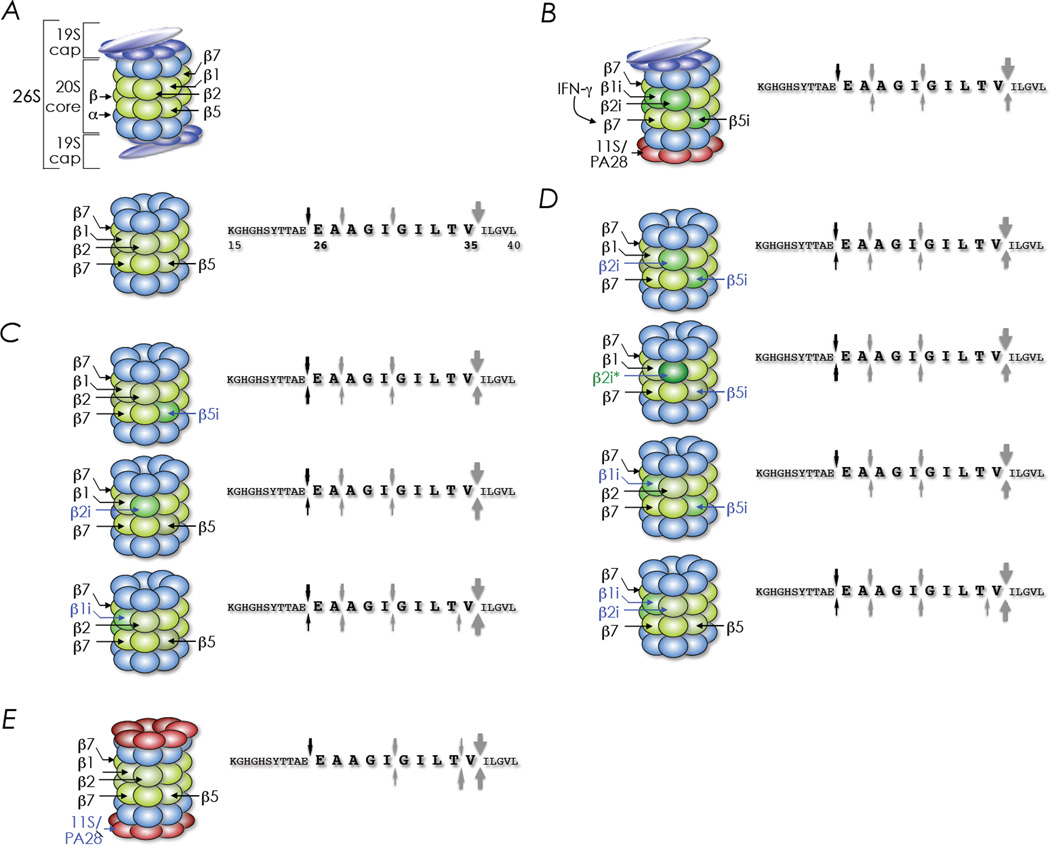
Figure 1. The making and breaking of a tumour epitope for surveillance —MART-126—35 in focus.
Anatomy of a 26S proteasome; it is made of a 20S core plus one or two 19S regulatory cap(s) (top structure in A). Immunoproteasomes have replaced the β1, β2 and β5 subunits of the 20S core with β1i, β2i and β5i, respectively (B) that are bound with one or two 19S cap(s) or one or two 11S αβ heteromeric PA28 (B). Proteolytic activity of the standard proteasome (20S structure in A) on the MART-115—40 substrate. This activity is compared to that of the immunoproteasome induced by IFN-γ (B) or of proteasomes containing individual or combinations of the immunoproteasome components (C, D & E). Arrows pointing down, cleavage sites of the standard 20S proteasome isolated from HeLa cells; upward pointing arrows, cleavage sites of immunoproteasomes or proteasomes containing one or two components of the immunoproteasomes; arrow thickness, cleavage sites based on yields of products identified by mass analysis; capital letters, amino acid sequence of the proteasome substrate used in the study; numbers, amino acid position; bold letters, HLA-A*02;01-restricted MART-126—35 epitope; β2i*, catalytically inactive MECL-1 mutant. An adaptation of figures 3B and 4D of Siefert et alii, opere citato
Melanomas are known to express certain, if not all, inducible components of the immunoproteasome. Seifert and colleagues, opere citato, identified an A*02;01 and MART-1 positive melanoma line —UKRV-Mel-15a, that contains very-little-to-undetectable levels of immunoproteasome components and ERAP-1 but up-regulate them in response the IFN-γ. Unexpectedly, treatment of this melanoma with IFN-γ reduced the activation of a MART-126—35-reactive CD8 T cell clone to secrete TNF-α. This result in conjunction with past findings alluded to above [6,8,9] suggested to the investigators that one or more IFNγ-induced components of the immunoproteasome affected MART-126—35 generation. To determine which IFNγ-inducible components were responsible for preventing MART-126—35 generation, HeLa cell line previously made to express A*02;01 was transduced with genes coding for individual immunoproteasome components or combinations thereof. As HeLa cells constitutively express basal levels of β5i and low basal levels of β1i, proteasome assembly containing single, double or triple inducible components in transduced cells was accomplished (Seifert et al. op. cit.; [14–17]). The resulting lines modeled intermediate proteasomes similar to those observed in some melanomas [18].
To set the stage, cleavage studies of precursor polypeptide MART-115–40 encompassing the CD8 T cell epitope MART-126–35 (bold letters in Figure 1) were initially performed using standard proteasomes and immunoproteasomes isolated from nonstimulated or IFNγ-stimulated HeLa cells, respectively. The data revealed that the immunoproteasomes were unable to generate MART-126–35 owing to inefficient cleavage at the carboxyl-terminus compared to the standard proteasomes. As well, the immunoproteasome did not cleave between residue 25 and 26 of MART-115–40 substrate to generate the epitope’s amino-terminus (Figure 1B). In additional experiments, Seifert and colleagues, found that β1i- and/or β2i-containing HeLa cells poorly activated MART-126—35-specific CD8 T cells. Accordingly, 20S proteasomes isolated from these cells yielded low-to-no MART-126—35 epitope owing to poor liberation of the carboxyl-terminus by β2i as well as the amino-terminus by both β1i and β2i. The role of β2i in MART-126—35 generation was confirmed by the T1A mutant β2i that had lost its proteolyic activity (see β2i* in Figure 1C).
IFN-γ also induces PA28α and PA28β which are required together for the assembly of the hetero-heptameric PA28 ring [19]. Forced over expression of PA28α and PA28β resulted in the loss of MART-126—35 epitope recognition by specific CD8 T cells. Accordingly, RNA interference mediated suppression of the gene encoding PA28α or PA28β therefore enhanced MART-126—35 epitope recognition by specific CD8 T cells. Furthermore, PA28-containing proteasomes do not generate MART-126—35 epitope owing to poor liberation of the carboxyl-terminus and the inability to cleave between residues 25 and 26 of the MART-115—40 substrate whilst inducing a lethal cleavage between the penultimate (V34) and ultimate (I35) residue of the MART-126—35 epitope (Figure 1D).
Normally, amino-terminus of class I binding peptides can be custom generated in the ER lumen from longer substrates by the action of ERAP1 [20–22] —another IFNγ-induced product. Incidentally, chemical inhibition of ERAP1 in UKRV-Mel-15a cells or down-regulation of ERAP1 by RNA interference enhanced MART-126–35 recognition by specific CD8 T cells suggesting that ERAP1 was destroying MART-126—35 epitope. Accordingly, recombinant ERAP1 cleaved MART-115—40 substrate to generate the MART-126–35 epitope in vitro but eventually destroyed it. But whether eventual degradation of the MART-126—35 epitope was due to the absence of peptide receptive A*02;01 in the in vitro cleavage assay remains unclear.
Taken together, the report by Seifert and co-workers (op. cit.) indicates that a combination of proteolytic activities induced by IFN-γ can result in immunoediting of tumours and promote immune evasion. This form of immunoediting might explain the curious case of a patient VMM5 who had a melanoma recur twice over a 12-year period. Tumour-infiltrating T cells (TILs) at the first recurrence reacted to MART-1 but TILs from the second recurrence failed to react to it but instead reacted to the epitope369YMDGTMSQV377 from tyrosinase [23]. As MART1-reactive TILs attended to most of VMM5’s melanoma at first recurrence, soluble mediators [24] may have turned on the immunoproteasome activity in the tumour thereby preventing the display of the MART-126—35 epitope. As the MART-126—35 epitope-reactive TILs waned, the subdominant TILs against the Tyr369—377 epitope became dominant at second recurrence by repeated stimulation through interactions with the slow smoldering immune escapees. Such a mechanism could be tested by determining whether Try369—377 epitope generation required or is enhanced by, or alternatively resistant to, immunoproteasome activity as is the case with the tyrosinase-related protein-2 derived epitope TRP2360—368 [25,26]. Nonetheless, the display of multiple CD8 T cell epitopes derived from tumour-specific antigens [27] and neoantigens [28–31] might make immune evasion difficult.
Polymorphisms in genes that encode proteins involved in HLA class I-restricted antigen processing and presentation are linked to susceptibility to certain cancers and various immunologic diseases. How these polymorphisms impact diseases remains unclear. A recent study revealed that polymorphisms impacting the enzymatic activity of ERAP1 altered the repertoire of peptides presented by a HLA class I molecule [32]. Hence, to fully realize the protective power of T cell targeted immunotherapeutics against cancers, the roles of heritable polymorphisms, somatic mutations within evolving tumors, and tumour responses to immune mediators that lead to immunoediting and susceptibility to cancers needs to be fully explored.
The risk for developing a cancer increases with age and generally burdens those beyond their prime fecund years. Hence, it is generally assumed that cancers contribute little to selection pressure for the survival of the species. Although the evolutionary implications of Seifert and colleagues’ findings are unclear, one could imagine how IFNγ-induced protease activities associated with antigen processing may facilitate the control of microbial infections that cause fatal disease. From this evolutionary vantage, escape from tumour immunity and immunotherapy are perchance happenstance.
Acknowledgments
Supported by VA Merit Award (BX001444) and NIH grants (AI042284, HL121139)
Literature Cited
- 1.Slingluff JCL, Chianese-Bullock KA, Bullock TNJ, Grosh WW, Mullins DW, Nichols L, Olson W, Petroni G, Smolkin M, Engelhard VH. Advances in Immunology. Vol. 90. Academic Press; 2006. Immunity to Melanoma Antigens: From Self-Tolerance to Immunotherapy; pp. 243–295. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 4.Romero P, Gervois N, Schneider J, Escobar P, Valmori D, Pannetier C, Steinle A, Wolfel T, Lienard D, Brichard V, et al. Cytolytic T lymphocyte recognition of the immunodominant HLA-A*0201-restricted Melan-A/MART-1 antigenic peptide in melanoma. J Immunol. 1997;159:2366–2374. [PubMed] [Google Scholar]
- 5.Skipper JC, Gulden PH, Hendrickson RC, Harthun N, Caldwell JA, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Mass-spectrometric evaluation of HLA-A* 0201-associated peptides identifies dominant naturally processed forms of CTL epitopes from MART-1 and gp100. Int J Cancer. 1999;82:669–677. doi: 10.1002/(sici)1097-0215(19990827)82:5<669::aid-ijc9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Morel S, Levy F, Burlet-Schiltz O, Brasseur F, Probst-Kepper M, Peitrequin AL, Monsarrat B, Van Velthoven R, Cerottini JC, Boon T, et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000;12:107–117. doi: 10.1016/s1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Sijts AJ, Song M, Janek K, Nussbaum AK, Kral S, Schirle M, Stevanovic S, Paschen A, Schild H, et al. Expression of the proteasome activator PA28 rescues the presentation of a cytotoxic T lymphocyte epitope on melanoma cells. Cancer Res. 2002;62:2875–2882. [PubMed] [Google Scholar]
- 8.Guillaume B, Stroobant V, Bousquet-Dubouch MP, Colau D, Chapiro J, Parmentier N, Dalet A, Van den Eynde BJ. Analysis of the processing of seven human tumor antigens by intermediate proteasomes. J Immunol. 2012;189:3538–3547. doi: 10.4049/jimmunol.1103213. [DOI] [PubMed] [Google Scholar]
- 9.Chapatte L, Ayyoub M, Morel S, Peitrequin AL, Levy N, Servis C, Van den Eynde BJ, Valmori D, Levy F. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006;66:5461–5468. doi: 10.1158/0008-5472.CAN-05-4310. [DOI] [PubMed] [Google Scholar]
- 10.Cardinaud S, Consiglieri G, Bouziat R, Urrutia A, Graff-Dubois S, Fourati S, Malet I, Guergnon J, Guihot A, Katlama C, et al. CTL escape mediated by proteasomal destruction of an HIV-1 cryptic epitope. PLoS Pathog. 2011;7:e1002049. doi: 10.1371/journal.ppat.1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomko RJ, Jr, Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem. 2013;82:415–445. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inobe T, Matouschek A. Paradigms of protein degradation by the proteasome. Curr Opin Struct Biol. 2014;24:156–164. doi: 10.1016/j.sbi.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, Monaco JJ, Colbert RA. Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingsbury DJ, Griffin TA, Colbert RA. Novel propeptide function in 20 S proteasome assembly influences beta subunit composition. J Biol Chem. 2000;275:24156–24162. doi: 10.1074/jbc.M001742200. [DOI] [PubMed] [Google Scholar]
- 16.De M, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. Beta 2 subunit propeptides influence cooperative proteasome assembly. J Biol Chem. 2003;278:6153–6159. doi: 10.1074/jbc.M209292200. [DOI] [PubMed] [Google Scholar]
- 17.Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci U S A. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillaume B, Chapiro J, Stroobant V, Colau D, Van Holle B, Parvizi G, Bousquet-Dubouch MP, Theate I, Parmentier N, Van den Eynde BJ. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc Natl Acad Sci U S A. 2010;107:18599–18604. doi: 10.1073/pnas.1009778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Krutchinsky A, Endicott S, Realini C, Rechsteiner M, Standing KG. Proteasome activator 11S REG or PA28: recombinant REG alpha/REG beta hetero-oligomers are heptamers. Biochemistry. 1999;38:5651–5658. doi: 10.1021/bi990056+. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Parekh VV, Mendez-Fernandez Y, Olivares-Villagomez D, Dragovic S, Hill T, Roopenian DC, Joyce S, Van Kaer L. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J Exp Med. 2006;203:647–659. doi: 10.1084/jem.20052271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shastri N, Cardinaud S, Schwab SR, Serwold T, Kunisawa J. All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol Rev. 2005;207:31–41. doi: 10.1111/j.0105-2896.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 22.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–483. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 23.Yamshchikov GV, Mullins DW, Chang CC, Ogino T, Thompson L, Presley J, Galavotti H, Aquila W, Deacon D, Ross W, et al. Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma. J Immunol. 2005;174:6863–6871. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 24.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Upregulation of PD-L1, IDO, T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sijts A, Sun Y, Janek K, Kral S, Paschen A, Schadendorf D, Kloetzel PM. The role of the proteasome activator PA28 in MHC class I antigen processing. Mol Immunol. 2002;39:165–169. doi: 10.1016/s0161-5890(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 26.Cho HI, Lee YR, Celis E. Interferon gamma limits the effectiveness of melanoma peptide vaccines. Blood. 2011;117:135–144. doi: 10.1182/blood-2010-08-298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slingluff CL, Jr, Cox AL, Henderson RA, Hunt DF, Engelhard VH. Recognition of human melanoma cells by HLA-A2.1-restricted cytotoxic T lymphocytes is mediated by at least six shared peptide epitopes. J Immunol. 1993;150:2955–2963. [PubMed] [Google Scholar]
- 28.Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 31.Granados DP, Sriranganadane D, Daouda T, Zieger A, Laumont CM, Caron-Lizotte O, Boucher G, Hardy MP, Gendron P, Cote C, et al. Impact of genomic polymorphisms on the repertoire of human MHC class I-associated peptides. Nat Commun. 2014;5:3600. doi: 10.1038/ncomms4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Navarro C, Martin-Esteban A, Barnea E, Admon A, Lopez de Castro JA. Endoplasmic Reticulum Aminopeptidase 1 (ERAP1) Polymorphism Relevant to Inflammatory Disease Shapes the Peptidome of the Birdshot Chorioretinopathy-Associated HLA-A*29:02 Antigen. Mol Cell Proteomics. 2015;14:1770–1780. doi: 10.1074/mcp.M115.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]