Abstract
Recent reports identify the ratio between absolute neutrophil count (ANC) and absolute lymphocyte count (ALC), called neutrophil to lymphocyte ratio (NLR), as a predictor of progression-free survival (PFS) and overall survival (OS) in various malignancies. We retrospectively examined the NLR in a cohort of 309 newly diagnosed multiple myeloma (MM) patients treated upfront with novel agents. NLR was calculated using data obtained from the complete blood count (CBC) at diagnosis and subsequently correlated with PFS and OS. The median NLR was 1.9 (range 0.4–15.9). Higher NLR was independent of international staging system (ISS) stage, plasma cell infiltration or cytogenetics. The 5-year PFS and OS estimates were, respectively, 18.2 and 36.4 % for patients with NLR≥2 versus 25.5 and 66.6 % in patients with NLR<2. Among younger patients (age <65 years, N=179), NLR≥2 had a negative prognostic impact on both PFS and OS, in all ISS stages. By combining ISS stage and NLR in a model limited to young patients, we found that 19 % of the patients were classified as very low risk, 70 % standard risk and 11 % very high risk. The 5-year estimates were 39.3, 19.4 and 10.9%for PFS and 95.8, 50.9 and 23.6%for OS for very low, standard-risk and very high-risk groups. We found NLR to be a predictor of PFS and OS in MM patients treated upfront with novel agents. NLR can be combined with ISS staging system to identify patients with dismal outcome. However, larger cohorts and prospective studies are needed to use NLR as additional parameter to personalise MM therapy in the era of novel agents.
Keywords: Multiple myeloma, Granulocyte, Lymphocyte, ISS
Introduction
The introduction of very active new therapies is changing the outcomes of treatment for multiple myeloma (MM) patients. Recent reports indicate that overall survival has increased from 4.6 years for patients treated between 2001 and 2005 to unreached median for patients treated between 2006 and 2010 [1], after the introduction of novel agents including proteasome inhibitors, such as bortezomib and carfilzomib, and immunomodulator agents (IMiDs), such as thalidomide and its derivatives lenalidomide and pomalidomide.
A major prognostic system used in MM is the international staging system (ISS) which was developed using survival data from patients treated from 1981 through 2002 (in the era preceding the availability of novel agents) [2]. The ISS combines serum 2-β-microglobulin and albumin levels to classify patients into three groups with different overall survival (OS) outcomes. The ISS was validated in both autologous stem cell transplantation (ASCT) eligible and ineligible patients, but not in patients treated upfront with novel agents, except for thalidomide [3]. Thus, its validity in the era of new therapeutic regimens is unclear [4]. Recently, ISS has been evaluated in patients treated upfront with novel agents. In combination with lactate dehydrogenase levels and poor cytogenetics, ISS can identify patients with high risk of early MM-related death [5].
Moreover, ISS does not take into account the role of the tumour microenvironment in sustaining MM recurrence. Recently, the immunological impairment in MM has been investigated as an additional tool in predicting outcome [6]. Long-term survival in MM is associated with a distinct immunological profile, which is consistent with decreased immune suppression [7]. A subpopulation of myeloid cells can suppress T-cell function in both murine [8, 9] and human MM [9–13]. These cells, defined as myeloid-derived suppressor cells (MDSC), consist of both neutrophils and immature myeloid cells [14, 15]. The real in vivo activity of this subpopulation of cells on the function and balance of immune system is difficult to measure. A good surrogate in peripheral blood for MDSCs could be the absolute neutrophil count (ANC). Considering their potential negative effect of a cellular immune response, examination of the ANC to absolute lymphocyte count (ALC), known as a neutrophil to lymphocyte ratio (NLR), can have prognostic significance. In fact, NLR has previously reported as an independent prognostic marker in solid [16–24] and hematological cancers [25], including MM, where NLR≥2 is correlated to poor outcomes [26]. However, the impact of NLR on the outcome of MM patients treated upfront with novel agents has never been investigated. The aim of our study was to define in a retrospective series a simple prognostic index including NLR and ISS to predict outcome of patients from three different institutions treated upfront with novel agents.
Methods
Patients and methods
Our analysis included 309 newly diagnosed MM patients treated upfront with novel agents in two Italian centres (AOUP Vittorio Emanuele, Catania and Policlinico San Matteo, Pavia) and an American centre (Johns Hopkins University, Baltimore) enrolled in phase 2–3 trials (GIMEMA MMY-3006, GIMEMA MM03-05, RV-MM-PI209, J0231) between January 2006 and December 2012.
Details on treatment regimens and final or ongoing results of these studies have previously been reported [27–30]. Institutional review boards of each single centre approved all studies. Patients provided written informed consent before entering the studies, performed in accordance with the Declaration of Helsinki.
In all patients, complete blood count (CBC) and routine biochemical examinations were taken before treatment (including pulsed high dose of dexamethasone) and NLR was calculated using data obtained from the CBC differential count.
Statistical methods
Logistic regression was used to construct the index. The event was the progression-free survival (PFS) at 3 years from the time of inclusion until the date of progression, relapse, death or the date the patient was last known to be in remission.
Independent variables were the following: age, sex, isotype, β2-microglobulin and albumin levels, lactate dehydrogenase (LDH) relative to normal levels, ISS, adverse cytogenetics defined as t(4;14) or del(17p) by fluorescent in situ hybridization (FISH), NLR, C-reactive protein, ESR, and myelomatous bone marrow infiltration.
Qualitative results were summarised in counts and percentages. Descriptive statistics were generated for the analysis of results, and a p value under 0.05 was considered significant. The Kaplan-Meier method was used to estimate progression-free survival (PFS) and OS. PFS was defined as the maximum time from either the start of diagnosis or the start of treatment date to the occurrence of death from any cause, disease progression or relapse, or censored at the date of last contact. OS was defined as the maximum time from either the diagnosis or the treatment date to the date of death from any cause, or censored at the date of last contact. PFS and OS curves were compared by the log-rank test. All analyses were performed using Graph Pad Prism version 6.00 for Windows, Graph Pad Software, San Diego California USA, www.graphpad.com, except proportional hazards model analyses which were performed using R programming language (R 2.15.0, Vienna, Austria).
Results
Baseline characteristics of patients are listed in Table 1. Median age was 63 (range 28–88); 108 patients (35 %) were stage III according to ISS classification. FISH analysis was available for 166 (54 %) patients, and when missing was due to artefact in samples or insufficient material (in elderly patients). An abnormal karyotype was observed in 43 % of the cases, consistent with the expected findings. Adverse chromosomal abnormalities were observed in 29/166 (17 %) patients.
Table 1.
Characteristics at baseline of 309 newly diagnosed MM patients
| Characteristic | Patients N=309 (100 %) |
NLR <2 N=165 (100 %) |
NLR ≥2 N=144 (100 %) |
p value |
|---|---|---|---|---|
| Median age, years (range) | 63 (28–88) | 63 (28–88) | 63 (31–88) | 0.85 |
| Males, N (%) | 161 (52) | 88 (53) | 73 (51) | 0.81 |
| Paraproteins (isotype), N (%) | ||||
| Immunoglobulin G | 216 (70) | 120 (73) | 96 (67) | 0.30 |
| Immunoglobulin A | 58 (19) | 26 (16) | 32 (22) | 0.23 |
| Immunoglobulin D-M | 4 (1) | 2 (1) | 2 (1) | 0.56 |
| Light chain only | 31 (10) | 17 (10) | 14 (10) | 0.84 |
| WBC, cells/mmc (mean±SD) | 5730±261 | 5654±167 | 7375±294 | <0.0001 |
| ANC, cells/mmc (mean±SD) | 3370±194 | 2771±96 | 5298±239 | <0.0001 |
| ALC, cells/mmc (mean±SD) | 1736±69 | 2271±65 | 1425±63 | <0.0001 |
| NLR(range) | 1.9 (0.4–15.9) | 1.2 (0.4–1.9) | 4.5 (2–16) | |
| Cytogenetics (FISH, IWMG criteria) | ||||
| Not available | 143 (46) | 74 (45) | 69 (48) | 0.68 |
| Favourable | 137 (44) | 79 (48) | 58 (40) | 0.19 |
| Adverse | 29 (9) | 12 (7) | 17 (12) | 0.19 |
| Serum albumin, g/dL (mean±SD)beta-2 | 4.0±0.21 | 4.4±0.4 | 3.9±0.22 | 0.95 |
| Microglobulin, mg/L(mean±SD)ESR | 4.0±2.6 | 4.5±5 | 3.9±2.6 | 0.48 |
| mm/h(mean±SD) | 64±5.6 | 66.1±3.6 | 71.1±9.9 | 0.59 |
| Stage ISS, N (%) | ||||
| I | 95 (31) | 49 (30) | 46 (32) | 0.79 |
| II | 106 (34) | 59 (36) | 47 (33) | 0.66 |
| III | 108 (35) | 57 (34) | 51 (35) | 0.95 |
| Treatment upfront, N (%) | ||||
| Proteasome inhibitors—group 1 | 157 (51) | 74 (45) | 83 (58) | 0.03 |
| IMiDs—group 2 | 91 (29) | 56 (34) | 35 (24) | 0.07 |
| Proteasome inhibitors+IMiDs—group 3 | 61 (20) | 35 (21) | 26 (18) | 0.6 |
| High-dose melphalan and ASCT | 113 (37) | 61 (37) | 52 (36) | 0.94 |
| Overall response rate, N (%) | ||||
| N (%) | 275 (89) | 150 (91) | 125 (87) | 0.35 |
| CR/VGPR/PR Proteasome inhibitors—group 1 | 24/25/87 | 16/9/41 | 8/16/46 | |
| CR/VGPR/PR IMiDs—group 2 | 17/11/50 | 10/9/30 | 7/2/20 | |
| CR/VGPR/PR Proteasome inhibitors+IMiDs—group 3 | 18/4/39 | 12/4/19 | 6/0/20 |
ISS international staging system, IMiDs immunomodulatory drugs (thalidomide or lenalidomide), WBC white blood cell count, ANC absolute neutrophil count, ALC absolute lymphocyte count, SD standard deviation, CR complete remission, VGPR very good partial remission, PR partial remission
Induction regimens for patients eligible for ASCT included thalidomide and dexamethasone, with or without bortezomib, according to the GIMEMA MMY-3006 trial [27], or lenalidomide and dexamethasone, according to the GIMEMA RV-MM-PI209 [29]; 27 patients from Johns Hopkins University received bortezomib and thalidomide in a steroid-free regimen [30]. Patients not eligible for ASCT received bortezomib, melphalan, prednisone with or without thalidomide for 9 cycles, according to the GIMEMAMM-03-05 trial [28]. Thus, 157 patients (51 %) received bortezomib, 91 (29 %) received lenalidomide or thalidomide, 61 (20 %) bortezomib with lenalidomide or thalidomide; 113 (37 %) patients underwent to autologous stem cell transplantation (ASCT) as consolidation therapy.
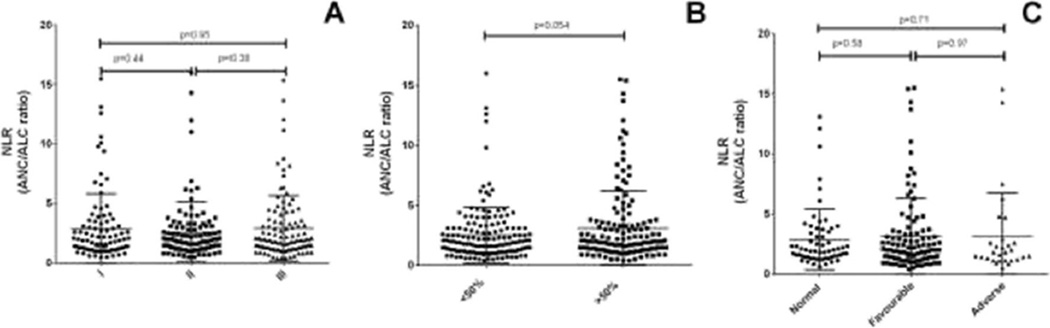
By definition, beta-2 microglobulin and albumin to assess ISS stage and NLR levels were available for all the patients. The median NLR was 1.9 (range 0.4–15.9).A higher NLR did not correlate with ISS stage, plasma cell infiltration or an adverse karyotype (Fig. 1).
Fig. 1.
NLR in newly diagnosed MM patients based on ISS stage (a), plasma cell infiltration in bone marrow (b) and cytogenetics (c)
Table 2 summarizes the correlations between the ISS stage and (i) NLR, (ii) use of high-dose therapy followed by ASCT, (iii) age, (iv) cytogenetics, and (v) single or double-novel agent-based induction therapy. As shown, ISS failed to correlate with NLR. However, ASCT was more common in patients with lower 1–2 ISS stage (p=0.0007); single novel agent use was more common in stage I ISS, while elderly patients (65 years) were more frequently stage II or III (p value 0.0003). Since the type of treatment could have impact in NLR, we considered separately differences based on the induction regimen (group 1, based on proteasome inhibitors alone; group 2, based on IMiDs alone; group 3, based on the combo proteasome inhibitor + IMiDs), but no significant differences were found, as reported in Supplementary Table 1 and Supplementary Figure 1 (Table 3).
Table 2.
Cross-correlations between NLR, high-dose chemotherapy followed by ASCT, age, cytogenetics, single-novel agent use and ISS stage
| Factor | ISS I | ISS II | ISS III | p value |
|---|---|---|---|---|
| NLR ≥2 | 44/95 (46 %) | 48/106 (45 %) | 51/108 (47 %) | 0.96 |
| High-dose therapy followed by ASCT | 44/95 (46 %) | 43/106 (41 %) | 26/108 (24 %) | 0.0007 |
| Age >65 years | 30/95 (32 %) | 43/106 (41 %) | 65/108 (60 %) | 0.0003 |
| Adverse cytogenetics | 12/51 (24 %) | 7/53 (13 %) | 10/62 (16 %) | 0.1 |
| Single novel agent | 68/95 (72 %) | 88/106 (83 %) | 92/108 (85 %) | 0.04 |
ISS international staging system, ASCT autologous stem cell transplantation
Table 3.
Progression free survival and overall survival according to ISS and NLR
| PFS | |||||||||
| ISS I | ISS II | ISS III | |||||||
| Events/N | 5-year estimate | p value | Events/N | 5-year estimate | p value | Events/N | 5-year estimate | p value | |
| NLR≥2 | 32/46 | 13.4 % | 0.0004 | 32/47 | 16.9 % | 0.57 | 27/51 | 21.8 % | 0.87 |
| NLR<2 | 21/49 | 35.3 % | 34/59 | 15.4 % | 35/57 | 23.9 % | |||
| OS | |||||||||
| ISS I | ISS II | ISS III | |||||||
| Events/N | 5-year estimate | p value | Events/N | 5-year estimate | p value | Events/N | 5-year estimate | p value | |
| NLR≥2 | 16/46 | 41.5 % | 0.0026 | 22/47 | 35.3 % | 0.10 | 20/51 | 33.7 % | 0.03 |
| NLR<2 | 4/49 | 89.2 % | 18/59 | 51.9 % | 12/57 | 58.5 % |
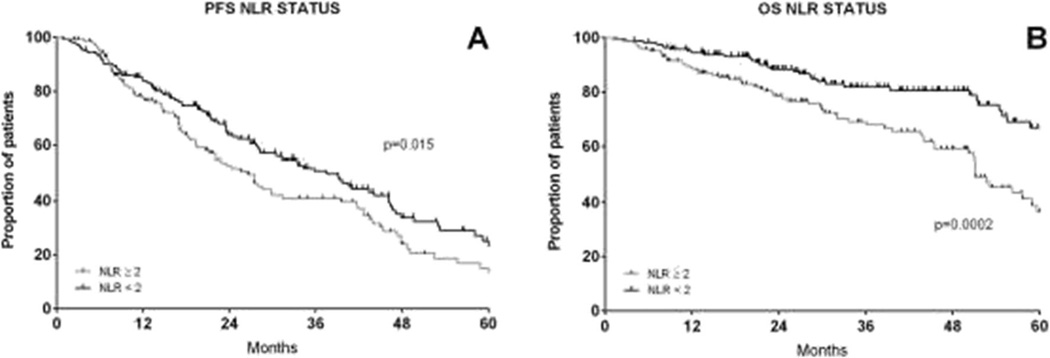
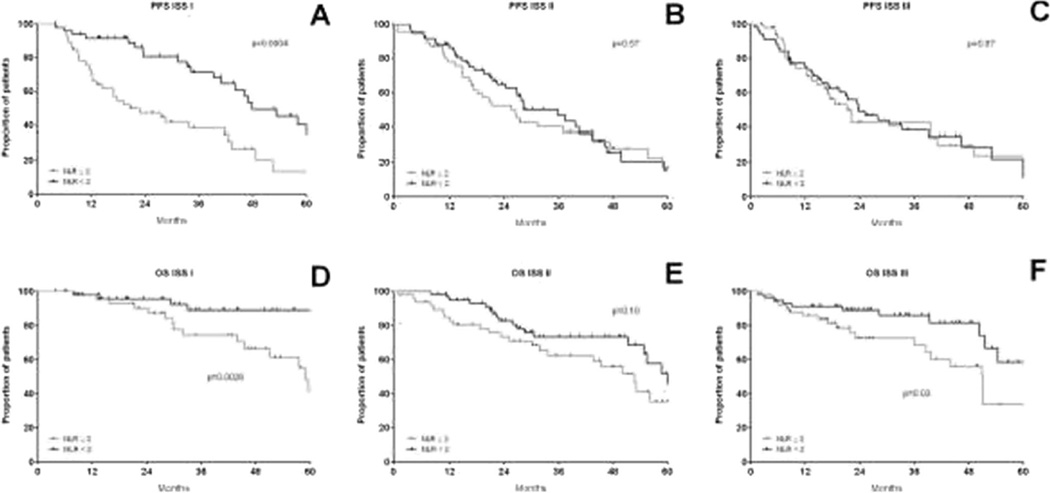
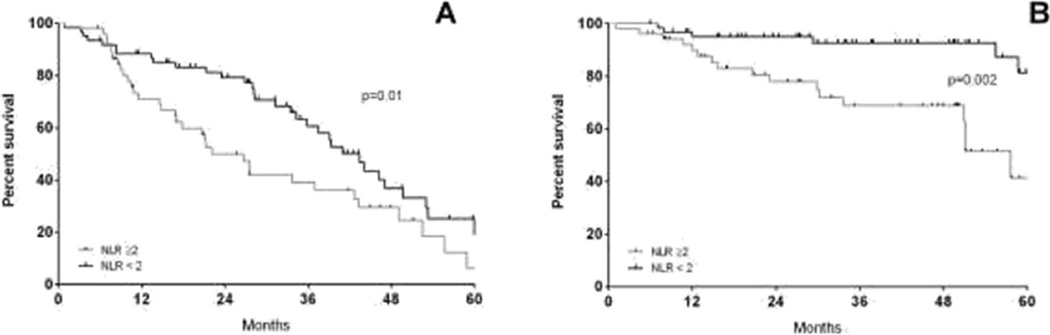
We chose an NLR cut-off of 2 based on previously published reports [26]. The 5-year PFS and OS estimates were, respectively, 18.2 and 36.4% for patients with NLR≥2 versus 25.5 and 66.6%in patients with NLR<2 (p value respectively 0.015 and 0.0002, Fig. 2). NLR was assessed separately for ISS stages I, II and III, as shown in Fig. 3. NLR≥2 reduced the PFS for stage I (Fig. 3a) and OS significantly for stages I and III (Fig. 3d–f). Conversely, for stage I patients with an NLR <2, the outcomes were excellent with 5-year OS estimates of 89.2 %.
Fig. 2.
Progression-free survival (a) and overall survival (b) by NLR≥2 (grey line) or NLR<2 (black line)
Fig. 3.
Progression-free survival (a–c) and overall survival (d–f) by ISS stages I, II and III with NLR≥2 (grey line) and NLR<2 (black line). Abbreviations: ISS international staging system, NLR neutrophil to lymphocyte ratio
Among younger patients, NLR≥2 had a negative prognostic impact on both PFS and OS, in all ISS stages (Fig. 4), while it could not add any prognostic information in the setting of older patients (data not shown).
Fig. 4.
Progression-free survival (a–c) and overall survival (d–f) by ISS stages I, II and III with NLR≥2 (grey line) and NLR<2 (black line) in the setting of younger patients (age <65 years). Abbreviations: ISS international staging system, NLR neutrophil to lymphocyte ratio
The NLR impact was also assessed on the outcomes for different therapies including induction therapy using one- versus two-novel agents based as well as the use or not of high-dose therapy (Fig. 5). Outcomes for upfront treatment with either single- or double-novel agents was independent of NLR status (Table 1). However, the prognostic significance of NLR was seen when examining clinical outcomes from ASCT (Fig. 5). The median PFS was 22.1 for an NLR≥2 versus 43.4 months for an NLR<2 (p=0.017) and the median OS was 57.6 months versus undefined respectively in patients with or without NLR≥2 at diagnosis (p=0.002).
Fig. 5.
Progression-free survival (a) and overall survival (b) by ASCT with NLR≥2 (grey line) and NLR<2 (black line). Abbreviations: ASCT autologous stem cell transplantation, NLR neutrophil to lymphocyte ratio
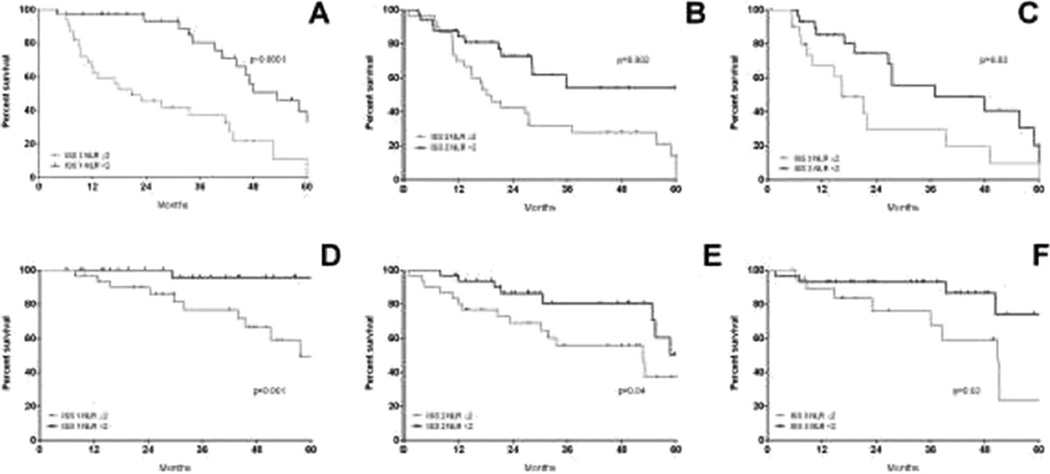
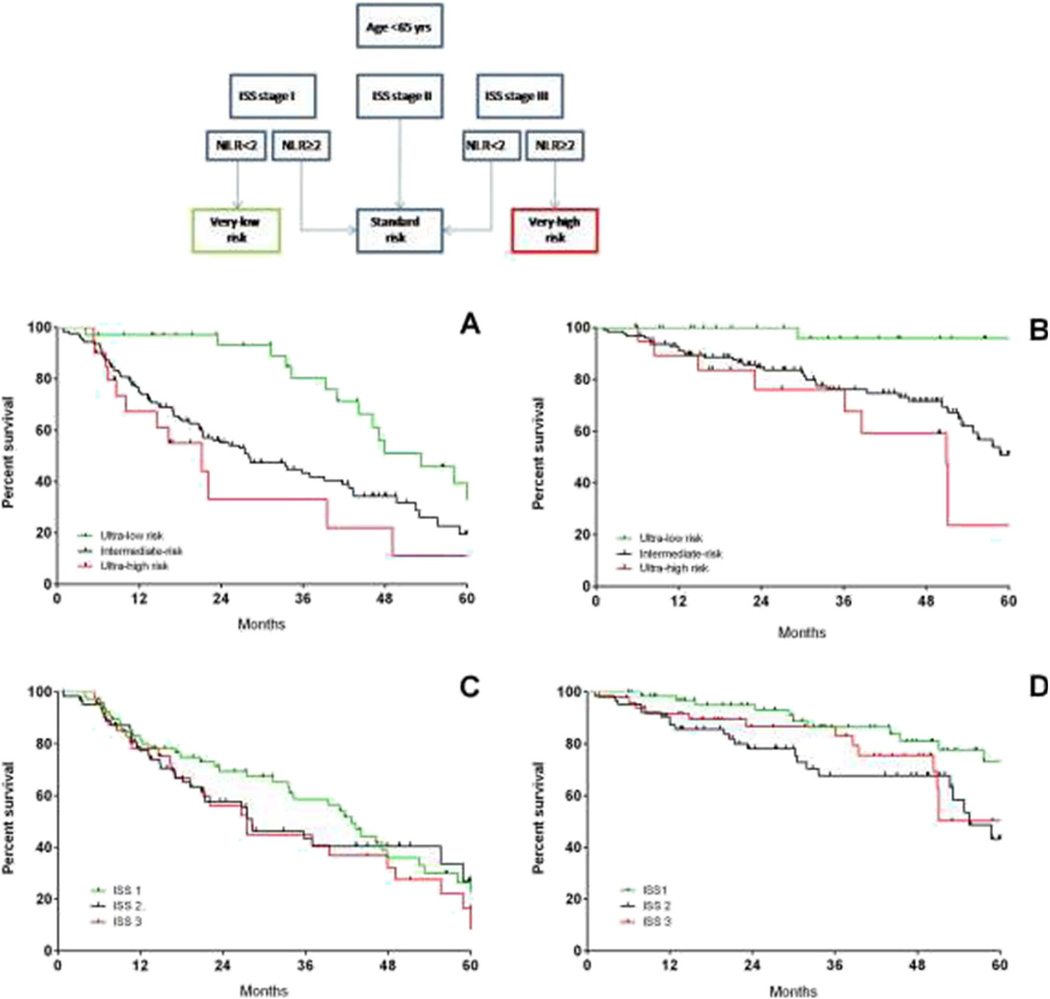
Thus, we limited further deeper analysis to the young patients (N=179) and we combined ISS stage and NLR (Fig. 6). The ISS-NLR very low-risk group was defined as ISS stage I and NLR <2; ISS-NLR standard risk was defined by either ISS stage II, ISS stage I and NLR≥2, or ISS stage III and NLR <2. Finally, ISS-NLR very high-risk group was defined by ISS stage III and NLR≥2. In all, 19 % of the patients were classified in the very low-risk group, 70 % standard risk and 11 % very high risk. The 5-year PFS estimates were 39.3, 19.4 and 10.9 % for ISS-NLR very low, standard-risk and very high-risk groups, respectively (Fig. 6a). Figure 6b shows the 5-year OS estimates, which were 95.8, 50.9 and 23.6% for ISS-NLR very low, standard-risk and very high-risk groups, respectively. In panels C and D, we reported the PFS and OS for the same cohort based only on ISS. In contrast to the ISS-NLR classification, ISS alone was unable to separate out cohorts based on either PFS or OS. In order to overcome statistical bias due to the retrospective analysis in a limited series, we split randomly 50:50 the cohort into a training set (N=155) and a validation set (N=154, Supplementary Table 2) to test the ISS-NLR risk stratification in the whole cohort (Supplementary Figure 2, A1 through B2) and in the subset of patients <65 years (training test N=85, validation set, N=94, Supplementary Figure 2, A3 through B4). In both training and validation sets, the larger part of patients belonged to intermediate-risk category (Supplementary Table 2). In the training set, when analysis was limited to young patients, the 5-year PFS estimates were 76.3, 42.9 and 25 % for ISS-NLR very low, standard-risk and very high-risk groups, respectively, confirmed in the validation set (47.8, 24.1 and 17.1 %, Fig. 6a). The 5-year OS estimates were 82.1, 42.8 and 50 % for ISS-NLR very low, standard-risk and very high-risk groups, respectively, and 100, 61.5 and 22.3 % confirmed in the validation set (Supplementary Figure 2).
Fig. 6.
Progression-free survival (a) and overall survival (b) by ISS-NLR risk stratification in the setting of younger patients (age <65 years). ISS-NLR very low-risk group was defined as ISS stage I and NLR <2; ISS-NLR standard-risk was defined by either ISS stage II, ISS stage I and NLR≥2, or ISS stage III and NLR <2. Finally, ISS-NLR very high-risk group was defined by ISS stage III and NLR≥2. Abbreviations: ISS international staging system, NLR neutrophil to lymphocyte ratio
Discussion
A high neutrophil-to-lymphocyte ratio (NLR) has been reported to have a negative prognostic impact in several solid tumours [31] and lymphomas [25]. A recent report indicates that a NLR>2 can be considered a bad prognostic factor for both PFS and OS also in MM patients. However, in that report, treatment details were not provided and most patients were treated upfront with regimens that did not include novel agents (melphalan and prednisone, PAD) [26].
Our study retrospectively evaluated the prognostic significance of NLR in newly diagnosed patients treated upfront with novel agents, and our findings confirmed the importance of NLR in predicting PFS and OS. The prognostic relevance of NLR was even more evident in the non-elderly patients and in those treated with ASCT upfront.
The biological significance of NLR and the mechanisms that relate this finding with a poor outcome remain to be defined. It probably reflects the immunosuppressive capacity of a subpopulation of myeloid cells, the so-called myeloid-derived suppressor cells (MDSC) that has been found to be elevated in the peripheral blood and bone marrow of MM patients [9, 10, 12] and that have many functional and phenotypic similarities with neutrophils [32], but this will have to be fully explored in a prospective manner. Myeloid dysfunction could be an actor in the immune surveillance imbalance that only high-dose therapy (ASCT) can modify. This could explain why NLR is still significantly different in young versus old patients, and in particular in those receiving ASCT. To avoid bias in patient selection, NLR should be investigated in a larger prospective series.
Considering the significance of NLR in correlating with clinical outcomes, we also examined whether the NLR could improve the prognostic impact of the international staging system that is based on levels of albumin and beta-2 microglobulin in peripheral blood and distinguishes patients in three prognostic groups, with median survival variable from 62 months (stage I) to 29 months (stage III) [2]. The ISS staging was retrospectively validated on patients treated prior to the advent of new drugs/strategies. Interestingly, in our series, the ISS was unable to provide any clear distinction in the outcomes for either PFS and OS. In contrast, the addition of NLR to the ISS further defined prognosis particularly in stage I and non-elderly patients (<65 years). We thus propose an algorithm that combines these two variables to define three groups of patients with different prognosis, which could possibly improve the predictive value of the ISS staging system and assist in individualising therapies.
For ASCT candidates, a new index based on ISS stage III, high LDH levels and adverse cytogenetics has been recently released which identifies a small subgroup comprising approximately 5–8 % of patients who die early as a result of disease progression [5]. The addition of fluorescent in situ hybridization (FISH) in the same set of patients used to validate ISS staging system can add prognostic information resulting in three different iFISH-ISS groups with different prognosis [33]. The Intergroupe Francophone du Myelome has tested the combination of t(4;14), del(17p) and high β2- microglobulin levels obtaining similar results [34].
In our series, ISS-NLR was able to identify a subset of very high-risk patients (11 %) and very low-risk patients (19 %) and if confirmed in further series could further improve risk stratification in MM treated with novel agents. Other variables such as age, cytogenetics and FISH have a tremendous impact on survival and should be taken in account in developing novel prognostic score. However, in our series, FISH was available only for half of the patients examined, and thus, no correlations with FISH to the ISS_NLR staging can be examined. It is highly probably that the use of FISH and other more sophisticated techniques such as gene-expression profile or next-generation sequencing has the potential of further define the prognostic profile of every single patient. However, such techniques are not broadly available. In contrast, the ISS and NLR are easily available for all patients and appear to have profound prognostic significance which could be used worldwide to design and tailor therapeutic strategies.
Acknowledgments
This work was supported in part by a grant from Ministero della Salute (Ricerca Finalizzata), by Associazione Italiana contro le Leucemie (AIL) of Catania and Fondazione Catanese per lo Studio e la Cura delle Malattie Neoplastiche del Sangue (FON.CA.NE.SA).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00277-015-2462-4) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have conflict of interest.
References
- 1.Kumar A, Hozo I, Wheatley K, Djulbegovic B. Thalidomide versus bortezomib based regimens as first-line therapy for patients with multiple myeloma: a systematic review. Am J Hematol. 2011;86(1):18–24. doi: 10.1002/ajh.21904. doi: 10.1002/ajh.21904. [DOI] [PubMed] [Google Scholar]
- 2.Greipp PR, SanMiguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Harousseau JL, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol : Off J Am Soc Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 3.Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, Michali E, Zomas A, Katodritou E, Gika D, Pouli A, Christoulas D, Roussou M, Kartasis Z, Economopoulos T, Dimopoulos M. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG) Leukemia. 2009;23(6):1152–1157. doi: 10.1038/leu.2008.402. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda J, Shimura Y, Ohta K, Tanaka H, Shibayama H, Kosugi S, Fuchida S, Kobayashi M, Kaneko H, Uoshima N, Ishii K, Nomura S, Taniwaki M, Takaori-Kondo A, Shimazaki C, Tsudo M, Hino M, Matsumura I, Kanakura Y. Limited value of the international staging system for predicting long-term outcome of transplant-ineligible, newly diagnosed, symptomatic multiple myeloma in the era of novel agents. Int J Hematol. 2014;99(4):441–449. doi: 10.1007/s12185-014-1539-5. doi: 10.1007/s12185-014-1539-5. [DOI] [PubMed] [Google Scholar]
- 5.Moreau P, Cavo M, Sonneveld P, Rosinol L, Attal M, Pezzi A, Goldschmidt H, Lahuerta JJ, Marit G, Palumbo A, van der Holt B, Blade J, Petrucci MT, Neben K, San Miguel J, Patriarca F, Lokhorst H, Zamagni E, Hulin C, Gutierrez N, Facon T, Caillot D, Benboubker L, Harousseau JL, Leleu X, Avet-Loiseau H, Mary JY. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol : Off J Am Soc Clin Oncol. 2014;32(20):2173–2180. doi: 10.1200/JCO.2013.53.0329. doi: 10.1200/jco.2013.53.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pessoa de Magalhaes RJ, Vidriales MB, Paiva B, Fernandez-Gimenez C, Garcia-Sanz R, Mateos MV, Gutierrez NC, Lecrevisse Q, Blanco JF, Hernandez J, Delas Heras N, MartinezLopez J, Roig M, Costa ES, Ocio EM, Perez-Andres M, Maiolino A, Nucci M, De La Rubia J, Lahuerta JJ, San-Miguel JF, Orfao A. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2013;98(1):79–86. doi: 10.3324/haematol.2012.067272. doi: 10.3324/haematol.2012.067272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant C, Suen H, Brown R, Yang S, Favaloro J, Aklilu E, Gibson J, Ho PJ, Iland H, Fromm P, Woodland N, Nassif N, Hart D, Joshua DE. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013;3:e148. doi: 10.1038/bcj.2013.34. doi: 10.1038/bcj.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Valckenborgh E, Schouppe E, Movahedi K, De Bruyne E, Menu E, De Baetselier P, Vanderkerken K, Van Ginderachter JA. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia. 2012;26(11):2424–2428. doi: 10.1038/leu.2012.113. doi: 10.1038/leu.2012.113. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran IR, Martner A, Pisklakova A, Condamine T, Chase T, Vogl T, Roth J, Gabrilovich D, Nefedova Y. Myeloid-derived suppressor cells regulate growth of multiple myeloma by inhibiting T cells in bone marrow. J Immunol. 2013;190(7):3815–3823. doi: 10.4049/jimmunol.1203373. doi: 10.4049/jimmunol.1203373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121(15):2975–2987. doi: 10.1182/blood-2012-08-448548. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased level of both CD4+ FOXP3+ regulatory T cells and CD14+HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72(6):540–547. doi: 10.1111/j.1365-3083.2010.02463.x. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 12.Romano A, Conticello C, Cavalli M, Vetro C, La Fauci A, Parrinello NL, Di Raimondo F. Immunological dysregulation in multiple myeloma microenvironment. BioMed Res Int. 2014;2014:198539. doi: 10.1155/2014/198539. doi: 10.1155/2014/198539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botta C, Gulla A, Correale P, Tagliaferri P, Tassone P. Myeloid-derived suppressor cells in multiple myeloma: preclinical research and translational opportunities. Front Oncol. 2014;4:348. doi: 10.3389/fonc.2014.00348. doi: 10.3389/fonc.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Semin Cancer Biol. 2013;23(3):171–182. doi: 10.1016/j.semcancer.2013.02.007. doi: 10.1016/j.semcancer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, Lang S. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2010;89(2):311–317. doi: 10.1189/jlb.0310162. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 16.Absenger G, Szkandera J, Stotz M, Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H, Samonigg H, Gerger A. Preoperative neutrophil-to-lymphocyte ratio predicts clinical outcome in patients with stage II and III colon cancer. Anticancer Res. 2013;33(10):4591–4594. [PubMed] [Google Scholar]
- 17.Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, Guglielmo A, Pirtoli L, Sperlongano P, Gridelli C, Caraglia M, Tassone P, Tagliaferri P, Correale P. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther. 2013;14(6):469–475. doi: 10.4161/cbt.24425. doi: 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho IR, Park JC, Park CH, Jo JH, Lee HJ, Kim S, Shim CN, Lee H, Shin SK, Lee SK, Lee YC. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17(4):703–710. doi: 10.1007/s10120-013-0330-2. doi: 10.1007/s10120-013-0330-2. [DOI] [PubMed] [Google Scholar]
- 19.Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2013;6:1605–1612. doi: 10.2147/OTT.S52501. doi: 10.2147/ott.s52501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer J Int Cancer. 2013;134(10):2403–2413. doi: 10.1002/ijc.28536. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 21.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2013;23(1):31–39. doi: 10.1016/j.suronc.2013.12.001. doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Santoni M, De Giorgi U, Iacovelli R, Conti A, Burattini L, Rossi L, Luca Burgio S, Berardi R, Muzzonigro G, Cortesi E, Amadori D, Cascinu S. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer. 2013;109(7):1755–1759. doi: 10.1038/bjc.2013.522. doi: 10.1038/bjc.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol. 2013;20(13):4330–4337. doi: 10.1245/s10434-013-3227-8. doi: 10.1245/s10434-013-3227-8. [DOI] [PubMed] [Google Scholar]
- 24.Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, Cramer DW. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132(3):542–550. doi: 10.1016/j.ygyno.2014.01.026. doi: 10.1016/j.ygyno.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porrata LF, Ristow K, Habermann T, Inwards DJ, Micallef IN, Markovic SN. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol. 2010;85(11):896–899. doi: 10.1002/ajh.21849. doi: 10.1002/ajh.21849. [DOI] [PubMed] [Google Scholar]
- 26.Kelkitli E, Atay H, Cilingir F, Guler N, Terzi Y, Ozatli D, Turgut M. Predicting survival for multiple myeloma patients using baseline neutrophil/lymphocyte ratio. Ann Hematol. 2013;93(5):841–846. doi: 10.1007/s00277-013-1978-8. doi: 10.1007/s00277-013-1978-8. [DOI] [PubMed] [Google Scholar]
- 27.Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo A, Offidani M, Corradini P, Narni F, Spadano A, Pescosta N, Deliliers GL, Ledda A, Cellini C, Caravita T, Tosi P, Baccarani M. Lancet. Vol. 9758. England: Elsevier Ltd; 2010. 2010. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study; pp. 2075–2085. vol 376. doi: 10.1016/s0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 28.Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, Offidani M, Patriarca F, Nozzoli C, Guglielmelli T, Benevolo G, Callea V, Baldini L, Morabito F, Grasso M, Leonardi G, Rizzo M, Falcone AP, Gottardi D, Montefusco V, Musto P, Petrucci MT, Ciccone G, Boccadoro M. In: J Clin Oncol. Vol. 34. United States: 2010. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial; pp. 5101–5109. vol 28. doi: 10.1200/jco.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 29.Cavallo F, Di Raimondo F, Harda I, Lupo B, Romano A, Catalano L, Baldini L, Guglielmelli T, Masini L, Rossi D, Ria R, Benevolo G, Falcone AP, Pescosta N, Galli M, Baraldi A, Redogia V, Boccadoro M, Palumbo A. A phase III study of enoxaparin Vs aspirin as thromboprophylaxis for newly diagnosed myeloma patients treated with lenalidomide-based regimen. Blood. 2010;116(21):476–477. [Google Scholar]
- 30.Ghosh N, Ye X, Ferguson A, Huff CA, Borrello I. Bortezomib and thalidomide, a steroid free regimen in newly diagnosed patients with multiple myeloma. Br J Haematol. 2011;152(5):593–599. doi: 10.1111/j.1365-2141.2010.08534.x. doi: 10.1111/j.1365-2141.2010.08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-tolymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6) doi: 10.1093/jnci/dju124. dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 32.Giallongo C, Parrinello N, Tibullo D, La Cava P, Romano A, Chiarenza A, Barbagallo I, Palumbo GA, Stagno F, Vigneri P, Di Raimondo F. Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS One. 2014;9(7):e101848. doi: 10.1371/journal.pone.0101848. doi: 10.1371/journal.pone.0101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, Goldschmidt H, Hajek R, Lee JH, Sezer O, Barlogie B, Crowley J, Fonseca R, Testoni N, Ross F, Rajkumar SV, Sonneveld P, Lahuerta J, Moreau P, Morgan G. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project. Leukemia. 2013;27(3):711–717. doi: 10.1038/leu.2012.282. doi: 10.1038/leu.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neben K, Jauch A, Bertsch U, Heiss C, Hielscher T, Seckinger A, Mors T, Muller NZ, Hillengass J, Raab MS, Ho AD, Hose D, Goldschmidt H. Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica. 2010;95(7):1150–1157. doi: 10.3324/haematol.2009.016436. doi: 10.3324/haematol.2009.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]