Abstract
Studies have suggested a decreased breast cancer risk in women with systemic lupus erythematosus. However, these studies enrolled younger patients identified primarily from lupus clinics. We compared the 5-year incidence of breast cancer among women with and without a diagnosis of SLE in a large population-based study of Medicare beneficiaries. We used a 20 % sample to create a cohort of 3,670,138 women from 2006 Medicare claims data with and without SLE at baseline. The study had 80 % power to detect whether the 5-year breast cancer incidence in the SLE cohort was 13 % higher or lower than the non-SLE cohort. Of the 18,423 women with SLE, 21 % were African American and 53 % were ≥65 years. The absolute age-adjusted risk for breast cancer in women with SLE was 2.23 (95 % CI 1.94–2.55) and 2.14 (95 % CI 1.96–2.34) in controls per 100 women. The overall absolute age and race adjusted incidence rate was 1.04 (95 % CI 0.90–1.21). Among women with SLE from “Others” (Hispanic, Native American, and/or Asian), the age-adjusted risk for breast cancer was 2.44 per 100 women (95 % CI 1.07–2.18), and age-adjusted incidence rate was 1.52 (95 % CI 1.07–2.18). In contrast to prior clinic-based studies, this population-based cohort study showed that the risk of breast cancer in women with SLE was not lower than in women without SLE. Women with SLE should follow routine breast cancer screening recommendations for their age group to avoid delay in diagnosis, because the presence of SLE may affect selection of early breast cancer therapies.
Keywords: Systemic lupus erythematosus, Breast cancer risk, Medicare claims data
Introduction
Systemic lupus erythematosus (SLE) is a multisystem inflammatory autoimmune disease with an estimated prevalence in the United States of 161,000 and 322,000 for “definite” and “definite plus probable” cases, respectively [1, 2]. SLE predominantly affects African-American women (female:male 8:1; African American:Caucasian 4:1) between their 2nd to 4th decade. As survival rates have improved steadily in recent years (5 and 10-year survival >90 and 85 %, respectively), patients are now more at risk for comorbidities including malignancies [3–5]. Although a higher than usual risk of hematologic malignancies, lung, liver, cervix, kidney, nasopharynx, and vagina/vulva cancers have been reported in patients with SLE [5–9], controversy exists about the risk for breast cancer, which is particularly salient as most SLE patients are women [5, 10, 11].
Studies evaluating breast cancer risk among SLE patients have been either retrospective clinical reviews or cohorts assembled almost entirely from rheumatology or lupus practices. Results have varied, with a recent emphasis on registry-based studies showing a lower risk for breast cancer in SLE women [5, 6, 9, 11–14]. However, earlier studies also reported an increased risk [8, 15–17], or no risk difference for breast cancer among women with SLE compared to women in general population [7, 18–21]. Small numbers of cases likely limited the ability of these studies to generalize the breast cancer risk to the entire SLE population who may or may not be cared for in specialty clinics. Despite the controversy about breast cancer risk, breast cancer is still the most commonly diagnosed cancer among women with SLE and constitutes a large portion of their overall cancer burden. Thus understanding the true breast cancer risk among all SLE women is important because it affects breast cancer prevention. Early discovery and treatment may be particularly important in women with SLE as therapy selections may be affected by the presence of the autoimmune disease. Our study aim was to overcome the limitations of the previous studies (number of cases, study design, and ascertainment) by estimating breast cancer incidence and risk ratio among women with and without SLE identified at baseline and followed forward in a large population-based older cohort at the greatest risk of breast cancer. To the best of our knowledge, this study offers the largest population-based cohort and the largest number of SLE patients for the evaluation of the risk of breast cancer.
Methods
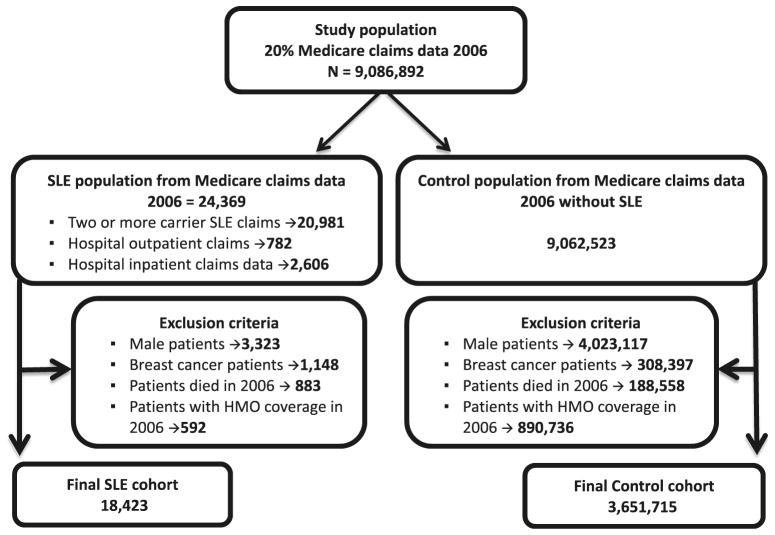
A randomly drawn 20 % sample of the Medicare claims dataset from 2006 was used as the primary study population source. The ascertained sample showed the same overall characteristics as the overall dataset. The SLE cohort was identified as Medicare beneficiaries with at least two carrier or hospital outpatient claims or a single inpatient claim with a diagnosis of SLE (ICD-9-CM 710.0x). The incidence of breast cancer was determined by the Chronic Condition Warehouse criteria for a breast cancer diagnosis. These criteria are similar to those used for identification of other studies of chronic diseases in administrative claims data [22]. The risk of beneficiary misclassification is low as validation studies using Medicare physician claims data reported 85 % sensitivity for SLE claims using the medical record as the gold standard [23, 24]. Beneficiaries were excluded if they were men, had a claim history of breast cancer in 2006 or during prior years, died during 2006, or had HMO coverage at any point during 2006. This resulted in a SLE cohort of 18,423 beneficiaries (Fig. 1). The control cohort (3,651,715) consisted of women free of SLE using the same exclusions as for the SLE cohort (Fig. 1). Given the sample sizes and the cumulative incidence of breast cancer in the non-SLE cohort, a priori, this study has 80 % power to detect whether the 5-year incidence in the SLE cohort was 13 % (RR 1.13, 0.87) higher or lower, respectively, than the non-SLE cohort, with confidence intervals that do not past unity, essentially the same as being statistically significant at the p < 0.05 level.
Fig. 1.
Study population
Descriptive statistics were generated for the ascertained entire cohort as well as for members of the cohort diagnosed with cancer during the study period. Incidence rates for breast cancer were defined for women meeting the Chronic Condition Warehouse criteria for a breast cancer diagnosis during any year from 2007 to 2011 (1 inpatient/skilled nursing facility or two hospital outpatient/carrier claims during a 1-year period). Crude incidence rates and the incidence ratio for breast cancer were calculated for women with SLE and control cohorts, women under age 65 years or 65 and above and by race (Caucasian, African American, and others). Negative binomial regression was used to calculate the incidence rate and ratios for SLE and control cohorts with adjustment for age and race.
Results
Of the total of 24,369 possible SLE patients in the cohort, 86 % met the carrier claims criteria with a small percentage included through the hospital outpatient (3.2 %) or inpatient (11 %) inclusion criteria. All male patients (14 %), patients with history for breast cancer (4.7 %), patients with HMO coverage (2.4 %), and patients who died in 2006 (3.6 %) were excluded to obtain the eligible SLE cohort of 18,423 women (Fig. 1).
Breast cancer incidence rate and risk ratio
Among women with SLE, the overall and age/race adjusted absolute breast cancer incidence was 2.26 (95 % CI 2.05–2.48) and 2.23 (95 % CI 1.94–2.55) per 100 women over 5 years, respectively. The overall and age/race adjusted absolute incidence rate of breast cancer for women without SLE was 2.59 (95 % CI 2.57–2.61) and 2.14 (95 % CI 1.95–2.34) per 100 women over 5 years, respectively. The age-adjusted breast cancer incidence rate for Caucasians, African Americans and Others (Asian, Hispanic, North American Native, and unknown) with SLE was 2.41 (95 % CI 2.16–2.69), 1.97 (95 % CI 1.55–2.50), and 2.44 (95 % CI 1.71–3.47) per 100 women over 5 years of follow-up, respectively. The overall age-adjusted breast cancer incidence for Caucasians, African Americans and Others (Asian, Hispanic, North American Native, and unknown) without SLE (control) was 2.54 (95 % CI 2.52–2.56), 2.18 (95 % CI 2.13–2.24), and 1.60 (95 % CI 1.46–1.75) per 100 women over 5 years, respectively (Table 1).
Table 1.
Study characteristics and breast cancer incidence risk by race and age groups
| All ages combined | SLE cohort (N = 18,423)
|
Control cohort (N = 3,651,715)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Total | Unadjusted incidence [95 % CI] | Age-adjusted incidence [95 % CI] | Cases | Total | Unadjusted incidence [95 % CI] | Age-adjusted incidence [95 % CI] | |
| All | 416 | 18,423 | 2.26 [2.05–2.48] | 2.23 [1.94–2.55]a | 94,572 | 3,651,715 | 2.59 [2.57–2.61] | 2.14 [1.95–2.34]a |
| Caucasians | 315 | 13,231 | 2.38 [2.13–2.66] | 2.41 [2.16–2.69] | 82,062 | 3,058,316 | 2.68 [2.66–2.70] | 2.54 [2.52–2.56] |
| African Americans | 68 | 3839 | 1.77 [1.38–2.24] | 1.97 [1.55–2.50] | 8740 | 370,533 | 2.36 [2.31–2.41] | 2.18 [2.13–2.24] |
| Others | 33 | 1353 | 2.44 [1.68–3.41] | 2.44 [1.71–3.47] | 3770 | 222,866 | 1.69 [1.64–1.75] | 1.60 [1.46–1.75] |
| Age ≥65 years | ||||||||
| All | 264 | 9795 | 2.70 [2.38–3.04] | 2.54 [2.14–3.01]a | 84,629 | 3,101,399 | 2.73 [2.71–2.75] | 2.54 [2.28–2.83]a |
| Caucasians | 215 | 8079 | 2.66 [2.32–3.04] | 2.49 [2.18–2.84] | 74,763 | 2,654,412 | 2.82 [2.80–2.84] | 2.75 [2.73–2.77] |
| African Americans | 27 | 1140 | 2.37 [1.57–3.43] | 2.18 [1.50–3.18] | 6686 | 261,264 | 2.56 [2.50–2.62] | 2.51 [2.45–2.57] |
| Others | 22 | 576 | 3.82 [2.41–5.73] | 3.58 [2.33–5.51] | 3180 | 185,723 | 1.71 [1.65–1.77] | 1.69 [1.54–1.86] |
| Age <65 years | ||||||||
| All | 152 | 8628 | 1.76 [1.49–2.06] | 1.60 [1.35–1.90]a | 9943 | 550,316 | 1.81 [1.77–1.84] | 1.55 [1.47–1.64]a |
| Caucasians | 100 | 5152 | 1.94 [1.58–2.36] | 1.60 [1.31–1.95] | 7299 | 403,904 | 1.81 [1.77–1.85] | 1.50 [1.45–1.55] |
| African Americans | 41 | 2699 | 1.52 [1.09–2.06] | 1.46 [1.08–1.99] | 2054 | 109,269 | 1.88 [1.80–1.96] | 1.58 [1.50–1.67] |
| Others | 11 | 777 | 1.42 [0.71–2.52] | 1.12 [0.60–2.08] | 590 | 37,143 | 1.59 [1.46–1.72] | 1.15 [0.93–1.41] |
Race adjusted
While the overall unadjusted risk ratio for breast cancer was 0.87 (95 % CI 0.79–0.96), after adjustment for age and race, the adjusted breast cancer risk ratio was not significant, 1.04 (95 % CI 0.90–1.21) (Table 2). When stratified by race, similar trends were seen; the unadjusted risk ratio was 0.75 (95 % CI 0.59–0.95) with an age-adjusted risk ratio of 0.90 (95 % CI 0.71–1.15) among African Americans. Among Caucasians, the unadjusted breast cancer risk ratio was 0.89 (95 % CI 0.80–0.99) and age-adjusted risk was not statistically significant, 0.95 (95 % CI 0.85–1.06). We found an increased risk ratio for breast cancer for the ethnic group “Others” [Asian, Hispanic, North American Native, and unknown] unadjusted 1.44 (95 % CI 0.98–2.29) and age adjusted 1.52 (95 % CI 1.07–2.18). However, this smaller-sized sample category had only few breast cancer diagnoses (N = 33; 8 %).
Table 2.
Breast cancer incidence ratio for SLE and control cohorts
| All ages combined | Unadjusted incidence ratio | p value | Adjusted incidence ratio | p value |
|---|---|---|---|---|
| All | 0.872 [0.793–0.959] | 0.005 | 1.040 [0.896–1.208]a | 0.603 |
| Caucasians | 0.887 [0.795–0.990] | 0.032 | 0.950 [0.851–1.061] | 0.366 |
| African Americans | 0.751 [0.593–0.951] | 0.018 | 0.902 [0.710–1.146] | 0.4 |
| Others | 1.442 [0.981–2.293] | 0.034 | 1.524 [1.066–2.178] | 0.021 |
| Age ≥65 years | ||||
| All | 0.988 [0.887–1.113] | 0.839 | 1.000 [0.818–1.221]a | 0.996 |
| Caucasians | 0.945 [0.828–1.078] | 0.4 | 0.904 [0.791–1.034] | 0.141 |
| African Americans | 0.925 [0.637–1.345] | 0.684 | 0.870 [0.596–1.269] | 0.469 |
| Others | 2.231 [1.479–3.365] | <0.001 | 2.116 [1.361–3.289] | 0.001 |
| Age <65 years | ||||
| All | 0.975 [0.829–1.146] | 0.755 | 1.032 [0.874–1.218]a | 0.71 |
| Caucasians | 1.076 [0.881–1.313] | 0.474 | 1.068 [0.877–1.301] | 0.515 |
| African Americans | 0.805 [0.590–1.099] | 0.173 | 0.926 [0.679–1.261] | 0.625 |
| Others | 0.890 [0.488–1.622] | 0.703 | 0.973 [0.527–1.797] | 0.931 |
Race adjusted
We also examined breast cancer incidence rates by stratifying the data into two age groups, 65 years or older and younger than 65 years. Among women with SLE ≥ 65 years and under 65 of age, the age/race adjusted breast cancer incidence was 2.54 (95 % CI 2.14–3.01) and 1.60 (95 % CI 1.35–1.90) per 100 women over 5 years, respectively. Similarly among women without SLE (control group) ≥65 years and under 65 of age, the age/race adjusted breast cancer incidence was 2.54 (95 % CI 2.28–2.83) and 1.55 (95 % CI 1.47–1.64) per 100 women over 5 years, respectively. The incidence of breast cancer per 100 women over 5 years by race was also calculated for these two age groups (Table 1).
When women ≥65 years of age were examined, the unadjusted and age/race adjusted risk ratios for breast cancer were 0.99 (95 % CI 0.89–1.11) and 1.00 (95 % CI 0.82–1.22), respectively. Similarly, SLE women under 65 years had no difference in the risk of breast cancer than women without SLE [unadjusted risk ratio 0.98 (95 % CI 0.83–1.15); age/race adjusted risk ratio 1.03 (95 % CI 0.87–1.22)]. Interestingly, the age-adjusted higher risk ratio for breast cancer persisted for the ‘Other’ category for age group ≥65 years only (Table 2).
Discussion
In this large population-based cohort identified from Medicare claims data, heavily skewed toward older women who are at greater risk for breast cancer, we found that the 5-year risk of incident breast cancer among women with SLE is similar to women in the general population, in contrast to published results from recent specialty clinic-based and SLE registry cohorts. We further found that when examined across racial groups, the risk of breast cancer in women with SLE is similar to women without SLE in both Caucasian and African women. Surprisingly, we found that the risk of breast cancer may be higher in women with SLE from Hispanic, Native American, and/or Asian ethnicities. To our knowledge this is the largest population-based study that has examined the risk of breast cancer among women from Hispanic, Native American, and/ or Asian ethnicities with SLE compared to women without SLE, although this comparison is low power with few cases and may not be as robust as the other subgroup analyses.
Several factors may be responsible for the discordant results between our study and previously reported clinic-based and registry studies. The clinic-based cohorts were assembled both prospectively and retrospectively by enrolling SLE patients from outpatient clinics or from hospital discharge registries. SLE patients were generally younger and not draw a priori from a general population making comparisons difficult. Valid comparable control groups for this population are notoriously difficult to define and likely come from a different underlying population distribution than the SLE patients. The largest assembled clinic-based registry cohort has consistently reported a lower risk of breast cancer in women with SLE [9], which could have profound implications for breast cancer screening among women with lupus, or may affect an index of suspicion for breast cancer among clinicians. Interestingly results from these clinical-based studies [9] underestimated the breast cancer risk by excluding in situ breast cancers cases from the clinical cohort [25] analysis and did not note this exclusion. This made it difficult to calculate the correct expected number of cases for standardized incidence ratio (SIR) analysis. Notably, however, clinic-based SLE cohorts are limited by selection bias wherein patients (or treating physicians) self-select themselves for follow-up at a specialty clinic and may thus have different exposures or disease characteristics than those found along all SLE patients who do not seek such care. Such differences may include differences in socioeconomic status, general health care, or possibly even greater use of immunomodulators.
As noted, a major limitation of clinic-based or registry cohort is the lack of a true control group within the study population itself. Most such studies have used standardized populations with the assumption that the exposure-profile of SLE women enrolled from clinics is similar to any standardized population. Lack of ethnic diversity and utilization of data from non-population-based (non-representative) registries are among other limitations of the prior smaller clinical cohort studies.
Similar to our study, the only population-based cohort study with a sufficient number of cases of breast cancer cases reported to date (The Taiwan National Cohort) [8] did not report a protective effect of SLE on breast cancer incidence rates. They actually reported an increased risk of breast cancer (standardized incidence ratio: 1.55, 95 % 1.51–1.6) in women with SLE as compared to women without SLE [8]. The increased risk noted in Taiwan National Cohort appears to be consistent with our finding of an increased risk in women from the “Others” category in our analysis, which includes women from Asian ethnic background.
Findings from our study are also consistent with some earlier prior studies that have shown no difference in the risk of breast cancer among women with SLE as compared to general population (See Table 3) [7, 18–21]. However, these studies reported their results based on small numbers (less than 6) of breast cancer cases [7, 18, 20, 21].
Table 3.
Studies reporting risk of overall cancer and breast cancer among women with SLE
| Study author and years | Study design | Study population and size | Number of breast cancer cases | Results | Study limitations/strength/comments |
|---|---|---|---|---|---|
| Pettersson et al. [16] | Finnish cancer registry—clinical cohort consists of SLE patients from medicine clinics, who were diagnosed with ARA 1971 criteria. These patients were followed over time (total person-years at risk 2340), and breast cancer was ascertained though Finnish Cancer Registry. The comparison group for the study was sex and age-specific cancer incidence rate for overall Finish Cancer Registry | 205 total SLE patients (182 women and 23 men)—age range for the registry not provided | 4 breast cancer cases out of total 13 cancer cases in women (two breast cancers were diagnosed in the same patient). Mean age of women at cancer diagnosis 55 years and overall age range 39–80 years | Overall cancer RR 2.6 (1.5–4.4) Breast cancer RR 2.7 (0.7–6.8) |
Ability to evaluate the influence of cytotatic drugs on cancer limited due to small number of cases. Only relative risk was reported |
| Dupla ML et al. [21] | Clinical Cohort with retrospective analysis of SLE patients at La paz Hospital, Madrid. Using ARA 1982 criteria for SLE diagnosis. Only number of cases reported without risk evaluation | 96 SLE patients (84 were women). Mean age at SLE onset 30 years | One breast cancer diagnosed out of 5 total female cancer cases. Mean age of women 42 years at cancer diagnosis with age range (33–56 years) | Conclusion SLE patients may have the same malignancies as general population after adjustment for age and sex | Information on cytotoxic medicine reported (cyclophosphamide, azathioprine, and vincristine). Small number of cases |
| Sweeney et al. [17] | Clinical cohort assembled from University medical center (Allegheny county Clinical) using revised criteria ARA 1982 for SLE diagnosis. Cancer cases ascertained through postal survey and non-respondent to the survey the cancer ascertainment was done via university medical records. Expected number calculated by regional age-specific incidence from 1985 Allegheny county census data and 1987 Pennsylvania cancer incidence registry data. Person-year risk cohort was multiplied by regional age-specific incidence | 219 SLE patients with age range 29–69 years | 3 out of 6 cases detected with breast cancer, mean age of women with cancer 54 years, age range at dx of cancer (36–65 years) | Overall cancer SIR 1.36 (0.5–2.96) Breast cancer SIR 2.05 (0.42–5.99) |
Small number but evaluated the immunosuppressive agents |
| Abu-Shakra et al. [18] | Clinical cohort assembled from chart review where SLE was diagnosed using ARA 1982 criteria. Patients followed prospectively for 24 years at University of Toronto Lupus Clinic for cancer. Multiple comparison groups used including (i) General population of Ontario, (ii) 1426 patient with RA and (iii) 248 patients with scleroderma | Total SLE patients 724, out of which 627 were females. Mean age at SLE 33.3 years (range 8–83.1 years) with 10 years mean follow-up | 4 out of 21 cancer in women were breast cancer with mean age at cancer diagnosis 57.2 years (range 27–84 years) | Overall cancer SIR 1.08 (0.7–1.62) Breast cancer SIR 0.7 (0.19–1.8) |
Small number of cases not adjusted for potential variables that could influence the disease incidence |
| Mellemkjaer et al. [19] | Clinical cohort of SLE patients from Danish Hospital discharge register linked to cancer registry in Denmark for detection of breast cancer cases. Expected number calculated using national cancer incidence—ICD code used for SLE diagnosis | SLE patients 1585 (83 % women—1315), 71 % women were under age 60 years at entry into cohort | 14 out of 102 total cancer were in the breast. No information provided for mean age of women at diagnosis of cancer | Overall cancer RR 1.3 (1.06–1.58) Breast cancer RR 1 (0.5–1.7) |
Selection bias—hospital discharge patients and limited number |
| Ramsey-Goldman et al. [15] | Chicago Lupus Cohort Clinical using ARA 1982 linked to tumor registry used to ascertain cancer cases. SIR estimated using age, gender, and race stratified cancer incidence from Cook County IL | 616 women with lupus with mean age at SLE 35.3 years (±17.7 SD) | 8 breast cancer cases—mean age of cancer women 51.6 years (±14.5 SD) | Overall cancer SIR 2 (1.4–2.9) Breast cancer SIR 2.9 (1.4–6.4) [among Caucasian women] |
Small no of cases, single-state registry data |
| Sultan et al. [20] | UK Clinical cohort of SLE patients assembled using ARA 1982 that was followed prospectively. Cancer cases were detected using medical notes. SIR calculated using Southeast England population annualized age/gender specific cancer incidence rate | 276 SLE patients (258 were women—93.5 % females)Mean age at SLE 34.7 years and mean time to occurrence of cancer 7.4 years | 3 breast cancer out of total 10 cancer cases. No information provided for age of the patients at cancer diagnosis | Overall cancer SIR 1.16 (0.55–2.13) Breast cancer SIR 1.06 (0.21–5.9) |
Treatment information provided for cyclosporine and cyclophosphamide. Out of ten cases 6 had prednisolone, 4 received azathioprine, 5 were on hydroxychloroquine and 1 had cyclophosphamide. Limited number of cases |
| Nived et al. [24] | Clinical cohort of SLE patients assembled form Swedish National Population registry (1981–1996) and linked to National Cancer registry. Patients were prospectively followed for development of cancer till 1998. SLE diagnosis was based on typical symptoms in at least two organ systems, the presence of typical immunological findings and lack of better diagnosis. SMR calculated based on observed frequencies from age and gender matched general population data from national cancer registry | 116 SLE patients (99 were women), off these only 110 full filled four or more ACR criteria. Median age at diagnosis of SLE was 48 years | No breast cancer detected. Total 11 cancer detected (7 cases were in women).Mean age for women cancer 66 years (range 53–78 years) | Overall cancer SMR for women 1.02 (0.4–2.1) | Treatment information provided. 19 % patients used azathioprine and or cyclophosphamide and 69 % were treated at least once with steroids during the study period. No breast cancer case detected |
| Cibere et al. [7] | Lupus clinical cohort assembled from Saskatchewan (Canada) University-based clinic and hospital data using ARA 1982 criteria. Provincial cancer statistics was used to calculate the expected number. Tumor registry was utilized to ascertain cancers | 297 SLE patients (84 % women). Mean age at SLE 36 years (range 10–80 years) | 4 breast cancer cases. Mean age for all cancer 60 years (range 27–89 years) | Overall cancer SIR 1.59 (1.05–2.32) Breast cancer SIR 1.15 (0.31–2.95) |
Small number of cases |
| Bjornadal et al. [11] | Clinical cohort based on hospitalized SLE patients from Swedish Hospital discharge register data base using ICD codes. Cases ascertained via National Swedish Cancer Register. Expected number obtained by National Swedish Cancer Register rates | 5715 SLE patients (4201 were women). 59 % of women were under age 60 years | 52 breast cancer cases out of total 443 cancer cases. No information provided for age at diagnosis | Overall cancer SIR 1.25 (1.14–1.37) Breast cancer SIR 0.72 (0.54–0.95) |
Small number of cases, selection bias (cohort assembled from hospital discharged registry matched to Swedish Cancer Registry), acute care setting causing detection bias. No validated study for diagnostic code in hospital discharge Register. Low specificity of the SLE would resulted in underestimation of true risk |
| Bernatsky et al. [6] | International clinical cohort of SLE patients from 23 centers where cases were determined through linkage to regional tumor registries. Expected cases determined by multiplying person-years in the cohort by geographically matched age, sex and calendar year-specific cancer rates, and summing over all person-year. Both ARA 1982/1997used for SLE diagnosis | 9547 patients (90 % female 8607) | 73 breast cancer cases detected out of 431 total cancer cases. Out of 431 cases 86 % cancer were diagnosed among women and 52 % of all cancer were diagnosed among women under age 60 years | Overall cancer SIR 1.15 (1.05–1.27) Overall women cancer SIR 1.17 (1.05–1.29) Breast cancer SIR 0.76 (0.60–0.95) |
Largest study cohort for its time. Breast cancer was the most commonly diagnosed cancer |
| Parikh-Patel et al. [5] | Retrospective clinical cohort study assembled from California patient discharge data using ICD codes. Cancer cases detected via California Cancer Registry. Expected number calculated by sex, race, and 5 years age group using rates from general California population | 30,478 patients. 89 % (27,133) were women and 28 % were age 60 years and above | 237 breast cancer cases out of 1053 total cases among women—51 % cases of all cancer and 43 % of breast cancer cases were diagnosed among women age 60 years and above | Overall cancer risk for both genders 1.14 (1.07–1.2) Breast cancer SIR: 0.76 (0.67–0.86) No difference by race Risk was lower for whites Women age less than 30 SIR 5 (1.91–11.3) and 30-44 years SIR 1.18 (0.86–1.58) |
Single-state (CA) Cohort assembled from hospitalized state discharge data—acute care setting causing detection bias Temporality cannot be established in close cohort between the sequence of events No information about treatment or risk factors. A large study enriched with Race/ethnicity. High quality cancer data from state cancer registry |
| Bernatsky et al. [12] | Multicenter international clinical cohort of SLE from 24 centers. Cases determined via regional tumor registries and expected number determined by multiplying person-years in the cohort by geographically matched age, sex, and calendar year-specific cancer rates | 13,492 study population | 112 breast cancer cases out of 632 total cancer. No Information provided for age (only abstract available) | Overall cancer SIR 1.15 (1.06–1.24) Breast cancer SIR 0.70 (0.58–.085) |
Abstract only no additional information available |
| Chen et al. [8] | Taiwan national cohort study a Population-based cohort assembled from National Health Insurance Research Database and represents 99 % of Taiwan’s entire population. Using ARA 1982, cases detected by ICD-710. SLE patients with a history of cancer were excluded from 1996 to 2005. Cancer was detected by Cancer Registry and expected number of cancer cases were calculated using national age, gender, and period specific cancer rate | 11,763 SLE patients (10,394 female—88.4 %).Mean age 34.7 years (SD 15.61), 67 % younger than age 40 years and 88 % female | 45 breast cancer cases out of 228 solid tumors (total cancer 259). Out of total 85 % cases among women, 71 % of cancer diagnosed among patients age 40 years and above | Overall cancer SIR 1.54 (1.52–1.56) Breast cancer SIR 1.55 (1.51–1.60) |
Population-based large cohort that showed enhanced risk of breast cancer among women with SLE. No explanatory variable information provided or adjusted |
| Bernatsky et al. [13] | Five large SLE cohorts—Bernatsky [6], Bjomadal [11], Kang [30], Mellemkjaer [19], Paarikh-Patel [5]—all clinical cohorts | 42,171 female SLE patients | 376 breast cancer, 66 endometrial, 44 ovarian | Overall cancer SIR 0.76 (0.69–0.85) Breast cancer SIR 0.71 (0.55–0.91) |
No information about treatment or risk factors that can influence breast cancer incidence. Lack of subgroup analysis by race or age |
| Dey et al. [14] | Nested Case-cohort design—Lupus clinic cohort London assembled using ARA 1997 criteria. Control SLE patient without cancer age, sex, race, and SLE duration matched. Cases identified through hospital medical records and confirmed histology or autopsy. UK National cancer registry used for cancer detection. Expected number calculated from Thames Cancer Registry 5-year-annualized rated age and gender specific. Control group was assembled to evaluate different marker (serological/clinical) between cases and controls | 595 SLE patients followed up for 32 years | 5 breast cancer cases out of 33 total cancer—91 % cases diagnosed in women and 27 % of cancer diagnosed age 60 years and above. Mean age for these cases at diagnosis of SLE was 33.5 years (SD 12.7) and at diagnosis of cancer was 50.3 years (SD 14.8) | Overall cancer SIR 1.05 (0.52–1.58) female overall cancer SIR 1.03 (0.66–1.40) Breast cancer SIR 0.48 (0.35–0.64)—91 % women were Caucasian |
Overall risk statistically insignificant and limited number of breast cancer cases. Evaluated serologic and immunosuppressive medicine with clinical cases. Reported anti-thyroid antibodies and SLICC damage was associated with cases |
| Bematsky et al. [9] | Multicenter (30) international SLE cohort—linked to regional tumor registries-almost all clinical cohorts no population-based registry | 16,409 SLE patients (90 % females) | 114 breast cancer cases out of 559 female cancers (Overall total cancer cases 644–84 % cancer in women, 41 % cancer among patients age 60 plus) | Overall cancer SIR 3.02 (2.48–3.63) Overall female cancer SIR 1.15 (1.05–1.24) Breast cancer SIR 0.73 (0.61–0.88) |
Large study with the largest lupus patient population. No data on the race or treatment effect evaluated. Breast carcinoma in situ excluded from the cases, no such exclusion reported for control group |
Interestingly, and as expected, most (64 %) of the incident breast cancer cases observed were diagnosed among women age 65 years or above. This is to be anticipated as breast cancer risk is well known to increase with age. Medicare participants are mostly 65 years of age or older. However, even when stratifying the cohort by age, the breast cancer incidence and risk ratio were similar for women ≥65 and for those <65 years. Only one study has reported an increased risk of breast cancer in the younger age group especially among Caucasian women that may be attributed possibly to SLE disease severity [5]. Conversely we did not observe this in our younger women <65 years of age.
Women with SLE have earlier menopause (even when not exposed to cytotoxic medications), as compared with women without SLE [26]. This change in hormonal exposure could be the reason that the risk of breast cancer among women with SLE at younger ages equilibrates early to that of the general population. In specialty clinics versus population-based studies, earlier exposure to SLE modulating agents such as hydroxychloroquine (an anti-malarial drug) may theoretically inhibit growth of breast cancer cells and result in lower rates of cancer in prior studies of younger populations [27]. However, studies investigating the relation of SLE treatment with cancer risk failed to identify/quantify such treatment effect on cancer incidence [10, 28, 29].
The main strength of our study is a very large robust population-based cohort that was ethnically enriched and large enough to evaluate breast cancer risk among women with lupus. We also believe this study provides a real-world contemporary control group. Our study also has potential limitations. Whereas we used a population-based cohort for women ≥65 years of age, women younger then this age were limited and were likely not representative of all US women in that age category. To address this limitation, we compared SLE women <65 years of age with controls <65 years, which should control for various Medicare-qualifying demographic, social, and clinical factors. It can be argued that as SLE is a disease of relatively younger women who are lower risk of breast cancer, a seeming protective effect of SLE on breast cancer risk may be purely a function of age distributions in lupus groups studied to date. Further, as age is such a strong predictor of breast cancer risk, theoretically it is possible that older age overwhelms any possible protective effect of SLE. However, this is an unlikely explanation because 46 % of our lupus cohort consisted of women less than age 65 and we found that younger SLE women had breast cancer risk similar to younger non-SLE women. Another limitation is that we did not have information on patient’s SLE severity index or specific SLE treatment to adjust for disease severity or therapy.
In conclusion, our results support the hypothesis that the risk of breast cancer among women with SLE is not lower than the risk of breast cancer in the general population, and this is also likely to be case for younger women. These results are of great importance to both the patients and their physicians who have in recent years been exposed primarily to literature that promotes the belief that lupus has a protective effect on the breast cancer risk among women with SLE. Therefore, awareness of equivalent breast cancer risk should guide appropriate screening, follow-up care, and regular surveillance to all women, directed by clinical judgment in conjunction with population-based guidelines and regardless of any underlying diagnosis of SLE. Further evaluation is needed on whether there is a difference in the type of breast cancer and stage at diagnosis between women with and without SLE, and the extent to which specific SLE treatments may potentially reduce the incidence of breast cancer.
Acknowledgments
Sponsored in part by the Harvey research fund of the Johns Hopkins University School of Medicine, Department of Medicine.
Appendix
See Table 3.
Footnotes
Financial support and disclosure No Competing financial interests exist.
Presented in part at the American Society of Clinical Oncology (ASCO), May 29th–June 2nd, 2015.
Conflict of interest All authors have no conflict of interest.
References
- 1.Ward MM. Prevalence of physician-diagnosed systemic lupus erythematosus in the United States: results from the third national health and nutrition examination survey. J Womens Health. 2004;13:713–718. doi: 10.1089/jwh.2004.13.713. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthr Rheum. 2008;58:15–251. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Doria A, Iaccarino L, Ghirardello A, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med. 2006;119:700–706. doi: 10.1016/j.amjmed.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Bematsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthr Rheum. 2006;54:2550–2557. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 5.Parikh-Patel A, White RH, Allen M, Cress R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control. 2008;19:887–894. doi: 10.1007/s10552-008-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernatsky S, Boivin JF, Joseph L, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthr Rheum. 2005;52:1481–1490. doi: 10.1002/art.21029. [DOI] [PubMed] [Google Scholar]
- 7.Cibere J, Sibley J, Haga M. Systemic lupus erythematosus and the risk of malignancy. Lupus. 2001;10:394–400. doi: 10.1191/096120301678646128. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Chang YT, Wang CB, Wu CY. Malignancy in systemic lupus erythematosus: a nationwide cohort study in Taiwan. Am J Med. 2010;123:1150.e1–1150.e6. doi: 10.1016/j.amjmed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Bernatsky S, Ramsey-Goldman R, Labrecque J, et al. Cancer risk in systemic lupus : an updated international multi-centre cohort study. J Autoimmun. 2013;42:130–135. doi: 10.1016/j.jaut.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontos M, Fentiman IS. Systemic lupus erythematosus and breast cancer. Breast J. 2008;14:81–86. doi: 10.1111/j.1524-4741.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 11.Bjornadal L, Lofstrom B, Yin L, Lundberg I, Ekbom A. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand J Rheumatol. 2002;31:66–71. doi: 10.1080/03009740252937568. [DOI] [PubMed] [Google Scholar]
- 12.Bernatsky SR, Clarke AE, Petri MA, et al. Further defining cancer risk in systemic lupus: updated results in an expanded intemational multi-centre cohort [abstract] Arthr Rheum. 2010;62:731. [Google Scholar]
- 13.Bernatsky S, Ramsey-Goldman R, Foulkes WD, Gordon C, Clarke AE. Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: a meta-analysis. Br J Cancer. 2011;104:1478–1481. doi: 10.1038/bjc.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey D, Kenu E, Isenberg DA. Cancer complicating systemic lupus erythematosus: a dichotomy emerging from a nested case-control study. Lupus. 2013;22:919–927. doi: 10.1177/0961203313497118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey-Goldman R, Mattai SA, Schilling E, et al. Increased risk of malignancy in patients with systemic lupus erythematosus. J Investig Med. 1998;46:217–222. [PubMed] [Google Scholar]
- 16.Pettersson T, Pukkala E, Teppo L, Friman C. Increased risk of cancer in patients with systemic lupus erythematosus. Ann Rheum Dis. 1992;51:437–439. doi: 10.1136/ard.51.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney DM, Manzi S, Janosky J, et al. Risk of malignancy in women with SLE. J Rheumatol. 1995;22:1478–1482. [PubMed] [Google Scholar]
- 18.Abu-Shakra M, Gladman DD, Urowitz MB. Malignancy in systemic lupus erythematosus. Arthr Rheum. 1996;39:1050–1054. doi: 10.1002/art.1780390625. [DOI] [PubMed] [Google Scholar]
- 19.Mellemkjaer L, Andersen V, Linet MS, et al. Non-Hodgkin’s lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus. Arthr Rheum. 1997;40:761–768. doi: 10.1002/art.1780400424. [DOI] [PubMed] [Google Scholar]
- 20.Sultan SM, Ioannou Y, Isenberg DA. Is there an association of malignancy with systemic lupus erythematosus? An analysis of 276 patients under long-term review. Rheumatology. 2000;39:1147–1152. doi: 10.1093/rheumatology/39.10.1147. [DOI] [PubMed] [Google Scholar]
- 21.Dupla ML, Khamashta M, Garcia VP, et al. Malignancy in systemic lupus erythematosus: a report of five cases in a series of 96 patients. Lupus. 1993;2:377–380. doi: 10.1177/096120339300200608. [DOI] [PubMed] [Google Scholar]
- 22.https://www.ccwdata.org/cs/groups/public/documents/document/ccw_conditioncategories.pdf
- 23.Katz JN, Barrett J, Liang MH, et al. Sensitivity and positive predictive value of Medicare part B physician claims for rheumatologic diagnoses and procedures. Arthr Rheum. 1997;40:1594–1600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA. The sensitivity of Medicare claims data for case ascertainment of six common. Med Care. 1999;37(5):436–444. doi: 10.1097/00005650-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 25.TessierCloutier B, Clarke AE, Ramsey-Goldman R, et al. Breast cancer in systemic lupus erythematosus. Oncology. 2013;85(2):117–121. doi: 10.1159/000353138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Hormonal and reproductive risk factors for development of systemic lupus erythematosus: results of a population-based, case: control study. Arthr Rheum. 2002;46:1830–1839. doi: 10.1002/art.10365. [DOI] [PubMed] [Google Scholar]
- 27.Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009;20:736–745. doi: 10.1097/CAD.0b013e32832f4e50. [DOI] [PubMed] [Google Scholar]
- 28.King J, Costenbader K. Characteristics of patients with systemic lupus erythematosus (SLE) and non-Hodgkins lymphoma (NHL) Clin Rheumatol. 2007;26:1491–1494. doi: 10.1007/s10067-006-0532-7. [DOI] [PubMed] [Google Scholar]
- 29.Bernatsky S, Boivin JF, Gordon C, et al. The relationship between cancer and medication exposure in SLE. Ann Rheum Dis. 2008;67:74–79. doi: 10.1136/ard.2006.069039. [DOI] [PubMed] [Google Scholar]
- 30.Kang KY, Kim HO, Yoon HS, Lee J, Lee WC, Ko HJ, Ju JH, Cho CS, Kim HY, Park SH. Incidence of cancer among female patients with systemic lupus erythematosus in Korea. Clin Rheumatol. 2010;29:381–388. doi: 10.1007/s10067-009-1332-7. [DOI] [PubMed] [Google Scholar]