INTRODUCTION
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, clonal, hematopoietic stem cell disorder that manifests with a hemolytic anemia from uncontrolled complement activation, bone marrow failure, and a propensity for thrombosis.1–3 It is the chronic hemolytic anemia in PNH, largely mediated by the alternative pathway of complement (AP), from which the disease derives its descriptive moniker.2 PNH is a unique disease whose clinical manifestations have been linked to the deficiency in glycosylphosphatidylinositol-anchored proteins (GPI-APs). These manifestations include a lack of the complement regulatory proteins CD55 and CD59.4 CD55 regulates the formation and stability of the C3 and C5 convertases,1 whereas CD59 blocks the formation of the membrane attack complex (MAC).2,5
The bone marrow failure component of the disease is well-appreciated. The mechanism of the thrombophilia is less well-described. Historically, PNH is among the first diseases in which the role the complement cascade plays in the pathogenesis is well-elucidated. This review focuses on the dysregulation of the complement cascade, leading to the hemolytic anemia in PNH as well as its other clinical manifestations and the therapies available presently and possibly in the future for the disease.
THE PATHOPHYSIOLOGY OF THE COMPLEMENT DYSREGULATION IN PAROXYSMAL NOCTURNAL HEMOGLOBINURIA
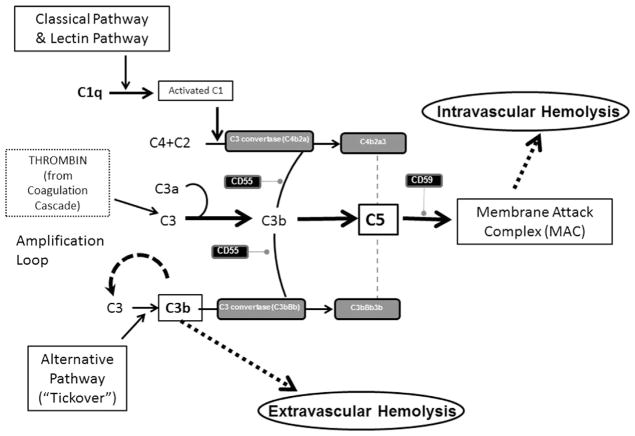
The complement system is our host defense system that protects the intravascular space through opsonizing and lysing bacteria. The complement system consists of plasma proteins that interact via 3 major pathways: the classical, alternative, and lectin binding.6,7 This system encompasses these distinct cascades with individual functions, which all converge into a common final effector mechanism—the MAC (Fig. 1). These 3 pathways independently lead to activation of C3 and C5 convertases.6 Although the classical and the lectin pathways require specific triggers to be activated—usually infection—it has been known for years that the AP exhibits low-grade continuous activation owing to spontaneous hydrolysis of C3 (called the “tick-over” phenomenon).8–10 In addition, some components of the AP constitute an amplification mechanism (the so-called AP amplification loop), which amplifies complement activation regardless of the specific pathway that initially generates the first C3b molecule (see Fig. 1). Multiple mechanisms have evolved to control the complement cascade, including membrane-bound proteins (complement receptor 1 [CR1], membrane cofactor protein, and the membrane proteins CD55 and CD59), as well as fluid-phase components, including complement factor I and factor H (FH). Among these, CD55 and CD59 are of pivotal importance in PNH, given that they are normally expressed on hematopoietic cells and attached by the GPI anchor proteins.11
Fig. 1.
The complement cascade. The complement cascade is activated via the classical, lectin or alternative pathways. C3 is activated via C3 convertases. This step is regulated by the action of CD55, a glycosylphosphatidylinositol (GPI)-anchored protein. Subsequently, C5 is cleaved into C5a and C5b. C5a mediates a number of biological processes and C5b begins the terminal pathway of complement and the assembly of the membrane attack complex (MAC). The formation of the MAC is regulated by CD59, another GPI-anchored protein. Thrombin interacts with the complement cascade where it can directly cleave C3. Thrombin can also cleave C5 into C5a, which occurs independently of C3 and therefore represents a bypass of the 3 traditional complement activation pathways.
With specific respect to the thrombosis seen in PNH, there are many direct and bidirectional interactions between the complement system and the coagulation cascade (see Fig. 1, which shows where thrombin interacts). Most notable to the clinical implications is that thrombin can cleave C5 into C5a, which occurs independent of C3 and therefore represents a bypass of the 3 traditional complement activation pathways (that is, the classical, lectin, and APs). Thrombin-activatable fibrinolysis inhibitor inactivates C3a and C5a in a negative feedback loop. The complement system also amplifies coagulation through the C5a-mediated induction of expression of tissue factor and plasminogen activator inhibitor 1 by leukocytes, the latter of which inhibits fibrinolysis.12 This activation of the complement through the generation of thrombin contributes to hypercoagulability in PNH and may explain why thrombosis in PNH often leads to an inexorable disease flare that is best interrupted by blocking terminal complement.
THE COMPLEMENT PROTEINS IN PAROXYSMAL NOCTURNAL HEMOGLOBINURIA
PNH erythrocytes are highly vulnerable to complement-mediated lysis owing to a reduction, or absence, of 2 important GPI-anchored complement regulatory membrane proteins (CD55 and CD59). CD59 is a glycoprotein that directly prevents the MAC from perforating the cell membrane by blocking the aggregation of C9.5 CD55 accelerates the rate of destruction of membrane-bound C3 convertase.13 CD55 (also called decay accelerating factor) inhibits the formation and the stability of C3 convertase (both C3bBb and C4b2a).14 CD55 was the first complement regulator reported to be absent on PNH erythrocytes.9,15 CD59 was later identified as an additional complement regulatory protein on PNH erythrocytes.16 Distinct from CD55, CD59 interferes with the terminal effector complement, blocking the incorporation of C9 onto the C5b–C8 complex, forming the MAC.17 CD59 is the more important molecule of the two—because, if it is absent, this leads to lysis,18 whereas an isolated deficiency of CD55 can be overcome. There are patients with a congenital isolated CD55 deficiency but normal CD59 expression who do not hemolyze (see Fig. 1).19
PAROXYSMAL NOCTURNAL HEMOGLOBINURIA AS A HEMOLYTIC ANEMIA
PNH erythrocytes’ in vitro susceptibility to hemolysis was initially described by Dr Ham in his pivotal studies in the 1930s.20 Dr Ham demonstrated that erythrocytes from patients who have PNH hemolyze in their own serum, especially when the AP is activated (by acidification). This hemolytic assay is called the Ham test or the acidified serum assay.21 This feature of PNH erythrocytes was subsequently described further when it was demonstrated that a distinct phenotype of PNH erythrocytes may exist, according to their specific sensitivity to complement-mediated lysis in vitro.22,23 In fact, in PNH there are erythrocytes with only modest hypersensitivity (3–5 times normal values) or a more pronounced hypersensitivity to complement-mediated lysis (15–25 times the normal one). These phenotypes are now known as PNH type II and type III erythrocytes,23 which by flow cytometry correspond with a complete (type III) or partial (type II) deficiency of GPI-APs, respectively.24 Chronic hemolysis of PNH is likely owing to a continuous steady-state complement activation coming from the low-grade spontaneous C3 tick-over, with ongoing activation of the CAP, as described. Infections or inflammatory status usually result in hemolytic crises (the paroxysms that given the disease its name), eventually as a result of substantial complement activation.
PAROXYSMAL NOCTURNAL HEMOGLOBINURIA AS A DISEASE OF MARROW FAILURE
Patient with PNH obviously suffer from anemia, but often they also have other cytopenias in the setting of their marrow failure owing to impaired hematopoiesis. The marrow failure component of PNH can vary from subclinical disease to severe aplastic anemia (AA) and may be categorized as such.2 It has been demonstrated that PNH patients have a reduced number of hematopoietic progenitors assessed by cultures assays regardless of the categorization.25,26 There is considerable overlap between PNH and AA both in disease presentation; in addition, they have long been viewed as distinct presentations of the same disorder.27 The mechanism by which PNH clones expand has been an area of active research. Immunoselection is considered to be essential for the selective expansion of these clones.28 The expansion is not simply attributable to PIGA mutations alone.29 Also, PNH clonal populations can be detected frequently in patients with the other marrow failure syndromes, such as AA and myelodysplastic syndrome.30,31 This may suggest that GPI− cells survive immune-mediated bone marrow injury putatively caused by cytotoxic cells such as natural killer cells.32 Human leukemic K562 cells become relatively resistant to natural killer cell-mediated cytotoxicity after acquisition of PIGA mutations in vitro.33 This relative survival advantage may be owing to deficiency of stress-inducible GPI-linked membrane proteins upregulation of UL16-binding protein (ULBP)1 and ULBP2 on PNH cells. ULBPs activate natural killer and T cells and are detected on GPI-expressing but not on GPI-deficient K562 cells. Thus, in the setting of an immune attack on the bone marrow, the lack of ULBPs may contribute to immunoselection of the PNH clone over normal cells.34 There also is the view that the patients with PNH clones and the autoimmune phenomenon of AA have an attack against the hematopoiesis at the level of the stem cell, and this allows the clonal expansion for the clinical PNH phenotype.35 More recently, it has been suggested that the GPI-AP could be the target of the immune attack and thus the PNH cells are spared naturally, again allowing their clonal outgrowth over the normal hematopoiesis.36 The PNH clone is often considered a marker of an immune form of marrow failure because it may predict response to immunosuppressive therapy in AA and patients with inherited forms of AA lack the PNH clone.37 The size of the PNH clone may vary over time and this is the best determinant of the hemolytic component of the disease.38–41 Therapies directed at this hemolysis will not improve the patients’ marrow failure.
PAROXYSMAL NOCTURNAL HEMOGLOBINURIA AS A DISEASE OF THROMBOSIS
Thrombosis is another typical manifestation of PNH. It is the leading cause of death in the disease.42 Thrombosis may occur at any site in PNH: venous or arterial. Common sites include intraabdominal (hepatic, portal, splenic or mesenteric) and cerebral (cavernous or sagittal sinus) veins, with hepatic vein thrombosis (also known as Budd–Chiari syndrome) being the most common. Deep venous thrombosis, pulmonary emboli, and dermal thrombosis are also prevalent. In contrast with the mechanisms of the hemolysis or the marrow failure, less is definitively known about the pathophysiology and mechanism of the thrombophilia in PNH, especially in patients not treated with eculizumab. Clinically, the complication of thrombosis is more prevalent in patients as the PNH clone increases in size.42–44 Thrombosis may occur in any PNH patient, but those with a large percentage of PNH cells (>50% granulocytes) are at greatest risk.44,45 This may suggest that the ultimate etiology of the thrombophilia in PNH is related to the hemolysis with complement activation. As discussed, there are also clear interactions between the complement system and the coagulation cascade, namely thrombin and C3, which contribute to the thrombosis in PNH. There are currently several hypothesized mechanisms and ultimately the pathophysiology may be multifactorial. The thrombophilia may directly result from the hemolytic anemia as the free hemoglobin is released by the erythrocytes into the serum causing nitric oxide (NO) scavenging and thus preventing the inhibition by NO on platelet aggregation and adhesion to endothelium.46 Next, the uncontrolled complement regulation on platelet surface could be hypothesized to lead to platelet activation and aggregation, enhancing the formation of thrombi.47 Another known issue is that the absence of GPI-APs on PNH platelets leads to thrombotic microparticles.48 Another possible mechanism of thrombosis in PNH could be a disruption of the fibrinolytic system, owing to the lack of membrane-bound urokinase-type plasminogen activator receptor, another GPI-anchored protein, leaving excess of its soluble form.49,50 Complement activation also contributes to the prothrombotic tendency of PNH patients. Specifically, C5a may result in proinflammatory and prothrombotic processes by generating inflammatory cytokines such as interleukin-6, interleukin-8, and tumor necrosis factor.51 It is unclear which of these mechanisms contribute most to thrombosis in PNH; however, complement inhibition with eculizumab is the most effective means to stop thrombosis in PNH.52,53 Anticoagulation and eculizumab are indication for acute thrombotic events; however, primary prophylactic anticoagulation has not been proven to be beneficial in PNH.42 Anticoagulation after the acute event in a PNH patient well-maintained on eculizumab may not be necessary.54
PAROXYSMAL NOCTURNAL HEMOGLOBINURIA AND CONSEQUENCES OF NITRIC OXIDE
Many manifestations of PNH result from intravascular hemolysis and are explained by hemoglobin-mediated NO scavenging after free hemoglobin is released from hemolyzed cells.46 NO is a major regulator of vascular physiology. NO acts on the vascular wall to maintain normal tone and limit platelet activation. Free hemoglobin has enormous affinity for NO and can reduce the plasma level of NO to the point of causing symptoms. This reduction has been demonstrated in clinical trials where the administration of cell-free hemoglobin solutions to healthy people is associated with development of abdominal pain and esophageal spasm.55 Under normal conditions, hemoglobin is sequestered by the erythrocyte membrane, which minimizes the scavenging of NO. In PNH, the intravascular hemolysis results in release of large amounts of free hemoglobin into the plasma. This release leads to scavenging of NO and degradation of the substrate for NO synthesis.56,57 This depletion of NO at the tissue level manifests clinically as fatigue, abdominal pain, esophageal spasm, erectile dysfunction, and possibly thrombosis. These clinical symptoms are more common in patients with PNH who have larger populations of PNH cells (>60% of granulocytes).44 Additionally, chronic kidney disease and pulmonary hypertension are complications that may go unrecognized, but also result from scavenging of NO. For example, in pulmonary arterial hypertension the symptoms are usually mild and are often nonspecific (eg, tiredness, breathlessness). Chronic kidney disease stages 1 through 3 are also described and can be quite common in PNH patients.58
DIAGNOSIS AND CLASSIFICATION OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA
The diagnosis of PNH is both a laboratory and a clinical diagnosis. The laboratory measures include a reticulocyte count, lactate dehydrogenase (LDH) levels, complete blood count indicative of hemolysis, and peripheral blood flow cytometry to detect the deficiency of the GPI (Box 1).59 This absence of GPI-APs is detected after staining cells with monoclonal antibodies and a reagent known as fluorescein-tagged proaerolysin variant that binds the glycan portion of the GPI anchor.60 The erythrocytes may be classified as types I, II, or III PNH cells, as noted. Type I cells have normal levels of CD55 and CD59, whereas type II have reduced levels and type III have complete absence.2 Hematopathologists have recently published guidelines for diagnosis of PNH using flow cytometry.61
Box 1. Clinical care of PNH patients.
Diagnosis
PNH by FLAER assay
LDH
Reticulocyte count
CBC
Therapy
-
Eculizumab intravenously
Loading: 600 mg weekly × 4 weeks
Maintenance (followed 1 week later): 900 mg every 2 weeks thereafter
Consideration of HSCT in suboptimal responders
Monitoring while on therapy
-
At least monthly
LDH, reticulocyte count, CBC, chemistries
-
At least yearly
PNH by FLAER assay
-
If concern for extravascular hemolysis
Direct antiglobulin test
Abbreviations: CBC, complete blood count; FLAER, fluorescein-tagged proaerolysin variant; HSCT, hematopoietic stem cell transplantation; LDH, lactate dehydrogenase; PNH, paroxysmal nocturnal hemoglobinuria.
Laboratory testing for diagnosis and monitoring during treatment. Standard therapy regimen with eculizumab.
The classification of PNH has been proposed by the International PNH Interest Group (IPIG)2 and includes 3 subtypes: classical PNH, which includes hemolytic and thrombotic patients who have evidence of PNH in the absence of another bone marrow failure disorder; PNH in the context of other primary bone marrow disorders, such as AA or myelodysplastic syndrome; and subclinical PNH, in which patients have small PNH clones but no clinical or laboratory evidence of hemolysis or thrombosis.2
CURRENT THERAPIES FOR PAROXYSMAL NOCTURNAL HEMOGLOBINURIA DIRECTED AGAINST COMPLEMENT
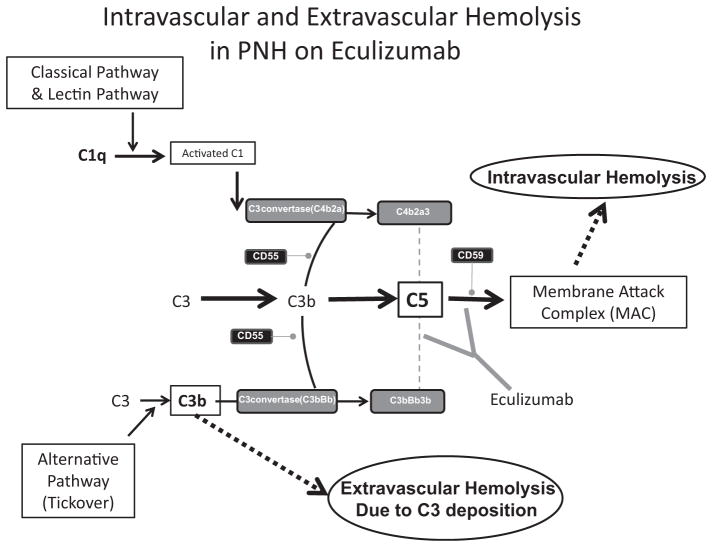
Eculizumab is a humanized monoclonal antibody that binds to C5 and inhibits its further cleavage into C5a and C5b. The drug decreases intravascular hemolysis, reduces thrombosis risk, and improves quality of life in PNH52,62 by inhibiting the formation of the MAC (Fig. 2).63 It is the only therapy approved by the US Food and Drug Administration for PNH.
Fig. 2.
The complement cascade, paroxysmal nocturnal hemoglobinuria (PNH), and eculizumab. PNH cells have a deficiency in glycosylphosphatidylinositol-anchored proteins on their cell surface. Absence of CD55 and CD59 leads to uncontrolled complement activation on the surface of PNH cells. Deficiency of CD59 increases MAC formation and induces intravascular hemolysis, which is central to the pathophysiology of PNH. Deficiency of CD55 leads to increased C3 convertase activity and C3d-associated extravascular hemolysis. Eculizumab therapy for PNH is a humanized monoclonal antibody that targets C5. By preventing C5 activation, eculizumab prevents the formation of the MAC, leading to a significant reduction in intravascular hemolysis of PNH cells. Use of eculizumab can lead to increased extravascular hemolysis.
Eculizumab was studied originally in a pilot trial published in the New England Journal of Medicine, which showed that it was safe and well-tolerated in PNH patients. This pilot study demonstrated also that LDH levels in these patients with transfusion-dependent anemia from their PNH decreased as intravascular hemolysis was blocked with the drug.64 These principles were demonstrated further in a larger, multicenter, randomized, placebo-controlled, blinded study in 86 PNH patients. Eculizumab was administered intravenously at 600 mg weekly for 4 weeks, followed 1 week later by 900 mg every 2 weeks thereafter (see Box 1).65 Again, therapy with eculizumab resulted decreased intravascular hemolysis, as measured by LDH, and transfusion independence in about one-half of the patients. There was also the disappearance of many of the clinical symptoms of intravascular hemolysis, including fatigue, esophageal spasm, and erectile dysfunction in the PNH patients on eculizumab arm in comparison with placebo. This second study again proved that eculizumab treatment was safe with few adverse events, even in comparison with the placebo. A third study of eculizumab (open-label phase III study SHEPHERD62) was conducted with broader inclusion criteria for the PNH patients, allowing for minimally transfused patients as well as those with more pronounced thrombocytopenia. In the 96 patients enrolled in the study, treatment with eculizumab resulted again in intravascular hemolysis, regardless of the severity of disease before therapy. Transfusion independence was achieved in about one-half of the patients and improvement in fatigue and quality of life were demonstrated as well.62 The study of eculizumab continued in the final open-label extension study. This extension included 187 patients who have previously been treated on the parent clinical trials.52 The extension study confirmed the safety and efficacy of eculizumab as well. More recently, additional follow-up data has been published, again with same findings.66
Patients require close monitoring while on eculizumab treatment (see Box 1). Standardly, peripheral blood work should include a reticulocyte count, LDH, complete blood count, and chemistries (including bilirubin) weekly during induction therapy. Thereafter, the same laboratory tests should be checked every 4 weeks. Also a direct antiglobulin test (Coombs’ test) should be obtained in patients with evidence of persistent hemolysis while on therapy. This may alert the clinician to ongoing extravascular hemolysis that the eculizumab and its downstream C3 deposition are causing. PNH flow cytometry should be obtained at least every 6 to 12 months because the clone size may vary over time. The measure of hemolysis (LDH) in a therapy responder usually fall within the normal range within days to weeks after starting eculizumab; however, the reticulocyte count usually remains elevated and the hemoglobin response can vary by patient and time.
PREDICTORS OF RESPONSE TO ECULIZUMAB THERAPY
The majority of classical PNH patients respond to eculizumab; however, the hemoglobin response is highly variable and may depend on underlying bone marrow failure, concurrent inflammatory conditions, genetic factors, and the size of the PNH red cell clone after therapy.39 However, there are limitations to this therapy and not all patients have their disease-specific needs met by eculizumab.39 There have been observed clinical scenarios that seem to predict for either breakthrough hemolysis or a poor response to eculizumab. As previously reported, eculizumab does not improve underlying bone marrow failure.67 There are also reports of patients who have a coexistent autoimmune disease (2 with Crohn’s disease, 1 with Graves’ disease, and 1 with rheumatoid arthritis) with ongoing activation of complement from their underlying disease, which lead to suboptimal responses from eculizumab.39 Breakthrough hemolysis is a challenge in these patients. Although the mechanism for this potential association is unclear, it is conceivable that chronic inflammatory states lead to increased complement activation that requires high dosages of eculizumab because standard doses resulted in incomplete C5 blockade. It is also known that transient breakthrough intravascular hemolysis is observed after viral or bacterial infections.39
Another group of suboptimal responders to eculizumab has been described recently. A single missense C5 heterozygous mutation, c.2654G→A, prevents binding and blockade by eculizumab while retaining the functional capacity to cause hemolysis. The polymorphism accounts for the poor response to eculizumab in patients carrying the mutation. The c.2654G→A mutation is present in 3.5% of the Japanese population and has not yet been described in other ethnic groups.68 Pharmacogenetics has also been shown to influence response to therapy. Polymorphisms in the CR1 gene are associated with response to eculizumab. CR1, through binding C3b and C4b, enhances the decay of the C3 and C5 convertases. The density of CR1 on the surface of red cells modulates binding of C3 fragments to the GPI-negative red cells when C5 is inhibited. PNH patients with polymorphisms in CR1 that lead to low CR1 levels (L/L genotype) are more likely to be suboptimal responders to eculizumab than patients with intermediate (H/L genotype) or high (H/H genotype) levels of CR1.69
Pregnancy can be another limitation on the efficacy of eculizumab. Pregnancy is a hypercoagulable state itself and there have been concerns both about the potential for increased maternal and fetal morbidity in a pregnant patient as well as the safety of eculizumab therapy in pregnancy. There are multiple case reports in the literature of successful pregnancies in female patients on eculizumab.70–73 However, what has been shown is that these pregnant patients tend to experience increased breakthrough hemolysis as they progress through the trimesters and often require reduced dosing interval (to 12 or even 7 days between doses) by the third trimester. This may be owing to increased activation of the complement cascade with increase terminal complex formation in the third trimester of pregnancy and/or increased volume of distribution of the drug during the latter stage of pregnancy.73
CURE FOR PAROXYSMAL NOCTURNAL HEMOGLOBINURIA: HEMATOPOIETIC STEM CELL TRANSPLANTATION
Hematopoietic stem cell transplantation (HSCT) is the only curative therapy for PNH. However, it is not recommended as initial therapy in the eculizumab era, given the risks of transplant-related morbidity and mortality. HSCT is a reasonable therapeutic option in patients who do not respond to therapy with eculizumab39,74 or those patients who have severe pancytopenia owing to underlying bone marrow failure. The transplant paradigm pursued is often with reduced intensity conditioning regimens, because myeloablation is not required to eradicate the PNH clone.75 The use of HSCT may be studied more in the future as patients and their health care providers determine that the cost–benefit ratio of HSCT outweighs a lifetime of eculizumab therapy.
FUTURE THERAPIES FOR PAROXYSMAL NOCTURNAL HEMOGLOBINURIA DIRECTED AGAINST COMPLEMENT
There continue to be challenges in therapies for PNH patients, both when eculizumab results in suboptimal response as well as with new drugs. The reasons for this are 2-fold. One is that, by blocking the terminal pathway of complement, an arm of immunity is simultaneously blocked, which prevents the formation of the MAC, which is needed to protect against infections, especially Neisseria. Therefore, patients who are on eculizumab therapy are usually more susceptible to infections caused by meningococcus or gonococcus. However, this can be overcome by way of a vaccine against meningococci or prophylactic antibiotics such as a fluroquinolone. The other challenge is that the therapy only influences a certain part of complement activities. It allows the immunoprotection and immunoregulation functions mediated by C3b to be retained. This function can be beneficial for patients, because it allows them to maintain their immune defense.58 Eculizumab compensates for the CD59 deficiency on PNH erythrocytes, but not the CD55 deficiency. Thus, PNH patients on eculizumab accumulate C3 fragments on their CD55-deficient red cells, leading to extravascular hemolysis through the accumulation of opsonins that are recognized by the reticulo-endothelial system (see Fig. 2).76 Laboratory evidence of extravascular hemolysis in eculizumab-containing patients includes increased reticulocytes, persistent anemia, and often direct antiglobulin testing that is positive for C3 deposition. These patients may remain asymptomatic, but others have symptomatic anemia and remain dependent on transfusions.39 Thus, there is need for additional work toward a complement inhibitor that reduces C3 accumulation on PNH erythrocytes.
The evidence that C3 activation represents a potential target, unique from C5 blockade, for complement modulation is an ongoing area of research both in vitro and in vivo. However, there have been concerns that C3 inhibitor might be associated with increased infectious toxicity.77 Nonetheless, active research is ongoing to study this therapeutic possibility.
There is an antibody-based anti-C3 strategy that targets activated C3 (C3b/iC3b). This anti-C3b/iC3b murine monoclonal antibody 3E7 and its chimeric-deimmunized derivative H17 were shown to selectively inhibit the activity of C3 and C5 convertases of the CAP only, providing the opportunity for a selective inhibition of different complement pathway.78 These antibodies were tested in vitro on PNH erythrocytes, and were shown to be effective in preventing complement-mediated hemolysis of CD55/CD59 deficient erythrocytes.78 This approach has yet to be translated into the clinic.
Targeted C3 complement inhibition has also included strategies based on small peptide inhibitors, which may be closer to clinical translation. The best example of this is compstatin, which selectively binds to C3 and its active fragment C3b.79,80 Compstatin prevents the conversion of C3 to C3b and thus it impairs all initiation, amplification, and terminal pathways of the complement cascade.81 Preliminary data show that compstatin analogs inhibit complement activation on PNH erythrocytes, preventing both hemolysis and C3 deposition.82 There are also ongoing investigations of peptidic C3 inhibitor, compstatin Cp40, and its long-acting form (polyethylene glycol–Cp40) in PNH in vitro models. Thus, peptide inhibitors of C3 activation effectively prevent hemolysis and C3 opsonization of PNH erythrocytes83 and are another potential therapeutic in this disease.
A similar but distinct path that could also inhibit complement in these PNH patients involves C1 esterase inhibitor (C1INH). This is an endogenous human plasma protein in the family of serine protease inhibitors (SERPINs) and it has broad inhibitory activity in the complement and coagulation pathways. C1INH inhibits the classical pathway of complement by binding C1r and C1s and inhibits the mannose-binding, lectin-associated serine proteases in the lectin pathway.84,85 It has already been shown in humans that the commercially available plasma derived C1INH (Cinryze) prevents PNH erythrocyte lysis induced by the AP.86 Importantly, C1INH was able to block the accumulation of C3 degradation products on CD55-deficient erythrocytes from PNH patients on therapy with eculizumab in vitro.86 This is significant clinically in patients treated with eculizumab who fail to achieve transfusion independence.39,66,87 Patients who do not respond to eculizumab therapy could theoretically respond to a C1INH, either alone or in combination with C5 blockade. A clinical trial is anticipated to explore this hypothesis in vivo.
Last, strategies of complement inhibition that deliver a selective inhibition of early phases (C3 activation) of the AP of the complement cascade are being developed. These strategies retain intact functioning of the other 2 complement pathways. This strategy uses FH, a complement inhibitor that modulates the initial AP activation in the fluid phase by preventing C3 convertase activity and by promoting C3b inactivation into iC3b.88 FH modulates the AP amplification loop and it has been demonstrated to inhibit lysis in vitro.89 There are 2 FH-derived agents currently studied. The first is TT30, which is a recombinant fusion protein between complement FH and another complement-related protein, complement receptor 2, which delivers FH activity locally at the site of complement activation. This was investigated in an in vitro model, which showed that TT30 completely inhibited complement-mediated hemolysis of PNH erythrocytes and effectively prevents initial C3 activation and further C3 deposition on PNH erythrocytes.90 There was a phase I clinical trial for PNH patients to study the use of TT30 (clinicaltrials.gov) that was closed owing to inability to enroll. Mini-FH is the second analogous agent that results in selective inhibition of activation and amplification of the AP, without affecting the other 2 pathways. Mini-FH was found to be more effective than TT30, with full inhibition achieved at concentrations about 1 log lower than TT30.91 This has yet to be translated into the clinic to date.
SUMMARY
PNH is caused by a somatic mutation in PIGA that leads to a marked deficiency or absence of the complement regulatory proteins CD55 and CD59. The disease manifests with intravascular hemolysis, bone marrow failure, and thrombosis. Complement inhibition through the C5 monoclonal antibody eculizumab has led to dramatic clinical improvement in PNH. Although this therapeutic approach is safe and effective, there is residual complement activity resulting from upstream complement components that account for suboptimal responses in patients. A novel era for complement regulation in PNH is upon us and the goal is to find targeted and specific treatments for PNH and other complement-mediated diseases.
KEY POINTS.
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, clonal, hematopoietic stem cell disorder with 3 clinical features: hemolytic anemia from uncontrolled complement activation, thrombosis, and bone marrow failure.
Eculizumab is a humanized monoclonal antibody that binds to C5 in complement system and decreases intravascular hemolysis, reduces thrombosis risk, and improves quality of life.
Persistent extravascular hemolysis in PNH while on eculizumab remains a relevant clinical issue and multiple therapies are being examined to improve this.
References
- 1.Rosse WF. Paroxysmal nocturnal hemoglobinuria as a molecular disease. Medicine. 1997;76(2):63–93. doi: 10.1097/00005792-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(12):3699–709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148(8):587–95. doi: 10.7326/0003-4819-148-8-200804150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–55. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 5.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144(9):3478–83. [PubMed] [Google Scholar]
- 6.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 7.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344(15):1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 8.Holt DS, Botto M, Bygrave AE, et al. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 2001;98(2):442–9. doi: 10.1182/blood.v98.2.442. [DOI] [PubMed] [Google Scholar]
- 9.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983;80(17):5430–4. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pangburn MK, Muller-Eberhard HJ. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann N Y Acad Sci. 1983;421:291–8. doi: 10.1111/j.1749-6632.1983.tb18116.x. [DOI] [PubMed] [Google Scholar]
- 11.Medof ME, Gottlieb A, Kinoshita T, et al. Relationship between decay accelerating factor deficiency, diminished acetylcholinesterase activity, and defective terminal complement pathway restriction in paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1987;80(1):165–74. doi: 10.1172/JCI113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776–87. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–78. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson-Weller A, March JP, Rosenfeld SI, et al. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983;80(16):5066–70. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. C3b deposition during activation of the alternative complement pathway and the effect of deposition on the activating surface. J Immunol. 1983;131(4):1930–5. [PubMed] [Google Scholar]
- 16.Holguin MH, Fredrick LR, Bernshaw NJ, et al. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meri S, Morgan BP, Davies A, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox LA, Ezzell JL, Bernshaw NJ, et al. Molecular basis of the enhanced susceptibility of the erythrocytes of paroxysmal nocturnal hemoglobinuria to hemolysis in acidified serum. Blood. 1991;78(3):820–9. [PubMed] [Google Scholar]
- 19.Telen MJ, Green AM. The Inab phenotype: characterization of the membrane protein and complement regulatory defect. Blood. 1989;74(1):437–41. [PubMed] [Google Scholar]
- 20.Ham TH. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria. A study of the mechanism of hemolysisin relation to acid-base equilibrium. N Engl J Med. 1937;217:915–7. [Google Scholar]
- 21.Ham TH, Dingle JH. Studies on destruction of red blood cells. II. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria: certain immunological aspects of the hemolytic mechanism with special reference to serum complement. J Clin Invest. 1939;18(6):657–72. doi: 10.1172/JCI101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosse WF, Dacie JV. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966;45(5):736–48. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosse WF. The life-span of complement-sensitive and -insensitive red cells in paroxysmal nocturnal hemoglobinuria. Blood. 1971;37(5):556–62. [PubMed] [Google Scholar]
- 24.Vanderschoot CE, Huizinga TW, van ‘t Veer-Korthof ET, et al. Deficiency of glycosyl-phosphatidylinositol-linked membrane glycoproteins of leukocytes in paroxysmal nocturnal hemoglobinuria, description of a new diagnostic cytoflourometric assay. Blood. 1990;76(9):1853–9. [PubMed] [Google Scholar]
- 25.Rotoli B, Robledo R, Scarpato N, et al. Two populations of erythroid cell progenitors in paroxysmal nocturnal hemoglobinuria. Blood. 1984;64(4):847–51. [PubMed] [Google Scholar]
- 26.Maciejewski JP, Sloand EM, Sato T, et al. Impaired hematopoiesis in paroxysmal nocturnal hemoglobinuria/aplastic anemia is not associated with a selective proliferative defect in the glycosylphosphatidylinositol-anchored protein-deficient clone. Blood. 1997;89(4):1173–81. [PubMed] [Google Scholar]
- 27.Dameshek W. Riddle: what do aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH) and “hypoplastic” leukemia have in common? (Editorial) Blood. 1967;30(2):251–4. [PubMed] [Google Scholar]
- 28.Nakakuma H, Kawaguchi T. Pathogenesis of selective expansion of PNH clones. Int J Hematol. 2003;77(2):121–4. doi: 10.1007/BF02983210. [DOI] [PubMed] [Google Scholar]
- 29.Inoue N, Izui-Sarumaru T, Murakami Y, et al. Molecular basis of clonal expansion of hematopoiesis in 2 patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2006;108(13):4232–6. doi: 10.1182/blood-2006-05-025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwanaga M, Furukawa K, Amenomori T, et al. Paroxysmal nocturnal haemoglobinuria clones in patients with myelodysplastic syndromes. Br J Haematol. 1998;102(2):465–74. doi: 10.1046/j.1365-2141.1998.00794.x. [DOI] [PubMed] [Google Scholar]
- 31.Griscelli-Bennaceur A, Gluckman E, Scrobohaci ML, et al. Aplastic anemia and paroxysmal nocturnal hemoglobinuria: search for a pathogenetic link. Blood. 1995;85:1354–63. [PubMed] [Google Scholar]
- 32.Bessler M, Mason P, Hillmen P, et al. Somatic mutations and cellular selection in paroxysmal nocturnal haemoglobinuria. Lancet. 1994;343(8903):951–3. doi: 10.1016/s0140-6736(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 33.Nagakura S, Ishihara S, Dunn DE, et al. Decreased susceptibility of leukemic cells with PIG-A mutation to natural killer cells in vitro. Blood. 2002;100(3):1031–7. doi: 10.1182/blood.v100.3.1031. [DOI] [PubMed] [Google Scholar]
- 34.Hanaoka N, Kawaguchi T, Horikawa K, et al. Immunoselection by natural killer cells of PIGA mutant cells missing stress-inducible ULBP. Blood. 2006;107(3):1184–91. doi: 10.1182/blood-2005-03-1337. [DOI] [PubMed] [Google Scholar]
- 35.Lewis SM, Dacie JV. The aplastic anaemia–paroxysmal nocturnal haemoglobinuria syndrome. Br J Haematol. 1967;13(2):236–51. doi: 10.1111/j.1365-2141.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 36.Gargiulo L, Papaioannou M, Sica M, et al. Glycosylphosphatidylinositol-specific, CD1d-restricted T cells in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(14):2753–61. doi: 10.1182/blood-2012-11-469353. [DOI] [PubMed] [Google Scholar]
- 37.Dezern AE, Symons HJ, Resar LS, et al. Detection of paroxysmal nocturnal hemoglobinuria clones to exclude inherited bone marrow failure syndromes. Eur J Haematol. 2014;92:467–70. doi: 10.1111/ejh.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheinberg P, Marte M, Nunez O, et al. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95(7):1075–80. doi: 10.3324/haematol.2009.017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeZern AE, Dorr D, Brodsky RA. Predictors of hemoglobin response to eculizumab therapy in paroxysmal nocturnal hemoglobinuria. Eur J Haematol. 2013;90(1):16–24. doi: 10.1111/ejh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107(4):1308–14. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- 41.Nakao S, Sugimori C, Yamazaki H. Clinical significance of a small population of paroxysmal nocturnal hemoglobinuria-type cells in the management of bone marrow failure. Int J Hematol. 2006;84(2):118–22. doi: 10.1532/IJH97.06077. [DOI] [PubMed] [Google Scholar]
- 42.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–96. doi: 10.1182/blood-2012-09-311381. quiz: 5105. [DOI] [PubMed] [Google Scholar]
- 43.Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2003;102(10):3587–91. doi: 10.1182/blood-2003-01-0009. [DOI] [PubMed] [Google Scholar]
- 44.Moyo VM, Mukhina GL, Garrett ES, et al. Natural history of paroxysmal nocturnal hemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126:133–8. doi: 10.1111/j.1365-2141.2004.04992.x. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura J, Kanakura Y, Ware RE, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83(3):193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 46.Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 47.Louwes H, Vellenga E, de Wolf JT. Abnormal platelet adhesion on abdominal vessels in asymptomatic patients with paroxysmal nocturnal hemoglobinuria. Ann Hematol. 2001;80(10):573–6. doi: 10.1007/s002770100350. [DOI] [PubMed] [Google Scholar]
- 48.Wiedmer T, Hall SE, Ortel TL, et al. Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood. 1993;82(4):1192–6. [PubMed] [Google Scholar]
- 49.Ninomiya H, Hasegawa Y, Nagasawa T, et al. Excess soluble urokinase-type plasminogen activator receptor in the plasma of patients with paroxysmal nocturnal hemoglobinuria inhibits cell-associated fibrinolytic activity. Int J Hematol. 1997;65(3):285–91. doi: 10.1016/s0925-5710(96)00559-2. [DOI] [PubMed] [Google Scholar]
- 50.Sloand EM, Pfannes L, Scheinberg P, et al. Increased soluble urokinase plasminogen activator receptor (suPAR) is associated with thrombosis and inhibition of plasmin generation in paroxysmal nocturnal hemoglobinuria (PNH) patients. Exp Hematol. 2008;36(12):1616–24. doi: 10.1016/j.exphem.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritis K, Doumas M, Mastellos D, et al. A novel C5a receptor-tissue factor crosstalk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177(7):4794–802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 52.Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–8. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 53.Weitz IC, Razavi P, Rochanda L, et al. Eculizumab therapy results in rapid and sustained decreases in markers of thrombin generation and inflammation in patients with PNH independent of its effects on hemolysis and microparticle formation. Thromb Res. 2012;130(3):361–8. doi: 10.1016/j.thromres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Emadi A, Brodsky RA. Successful discontinuation of anticoagulation following eculizumab administration in paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2009;84(10):699–701. doi: 10.1002/ajh.21506. [DOI] [PubMed] [Google Scholar]
- 55.Carmichael FJ. Recent developments in hemoglobin-based oxygen carriers–an update on clinical trials. Transfus Apher Sci. 2001;24(1):17–21. doi: 10.1016/s0955-3886(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 56.Azizi E, Dror Y, Wallis K. Arginase activity in erythrocytes of healthy and ill children. Clin Chim Acta. 1970;28(3):391–6. doi: 10.1016/0009-8981(70)90063-x. [DOI] [PubMed] [Google Scholar]
- 57.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heitlinger E. Learnings from over 25 years of PNH experience: the era of targeted complement inhibition. Blood Rev. 2013;27(Suppl 1):S1–6. doi: 10.1016/S0268-960X(13)00080-5. [DOI] [PubMed] [Google Scholar]
- 59.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113(26):6522–7. doi: 10.1182/blood-2009-03-195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brodsky RA, Mukhina GL, Li S, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114(3):459–66. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borowitz MJ, Craig FE, Digiuseppe JA, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78(4):211–30. doi: 10.1002/cyto.b.20525. [DOI] [PubMed] [Google Scholar]
- 62.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–7. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 63.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256–64. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 64.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350(6):552–9. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 65.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–43. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 66.Hillmen P, Muus P, Röth A, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162:62–73. doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–92. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 68.Nishimura J, Yamamoto M, Hayashi S, et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370(7):632–9. doi: 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]
- 69.Rondelli T, Risitano AM, Peffault de Latour R, et al. Polymorphism of the complement receptor 1 gene correlates with the hematologic response to eculizumab in patients with paroxysmal nocturnal hemoglobinuria. Haematologica. 2014;99(2):262–6. doi: 10.3324/haematol.2013.090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly R, Arnold L, Richards S, et al. The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. Br J Haematol. 2010;149(3):446–50. doi: 10.1111/j.1365-2141.2010.08099.x. [DOI] [PubMed] [Google Scholar]
- 71.Marasca R, Coluccio V, Santachiara R, et al. Pregnancy in PNH: another eculizumab baby. Br J Haematol. 2010;150(6):707–8. doi: 10.1111/j.1365-2141.2010.08258.x. [DOI] [PubMed] [Google Scholar]
- 72.Danilov AV, Brodsky RA, Craigo S, et al. Managing a pregnant patient with paroxysmal nocturnal hemoglobinuria in the era of eculizumab. Leuk Res. 2010;34(5):566–71. doi: 10.1016/j.leukres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 73.Townsley DM, Young NS. Blood consult: paroxysmal nocturnal hemoglobinuria and its complications. Blood. 2013;122(16):2795–8. doi: 10.1182/blood-2013-07-360081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brodsky RA, Luznik L, Bolaños-Meade J, et al. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42(8):523–7. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suenaga K, Kanda Y, Niiya H, et al. Successful application of nonmyeloablative transplantation for paroxysmal nocturnal hemoglobinuria. Exp Hematol. 2001;29(5):639–42. doi: 10.1016/s0301-472x(01)00632-4. [DOI] [PubMed] [Google Scholar]
- 76.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113:4094–100. doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 77.Botto M, Walport MJ. Hereditary deficiency of C3 in animals and humans. Int Rev Immunol. 1993;10(1):37–50. doi: 10.3109/08830189309051170. [DOI] [PubMed] [Google Scholar]
- 78.Lindorfer MA, Pawluczkowycz AW, Peek EM, et al. A novel approach to preventing the hemolysis of paroxysmal nocturnal hemoglobinuria: both complement-mediated cytolysis and C3 deposition are blocked by a monoclonal antibody specific for the alternative pathway of complement. Blood. 2010;115(11):2283–91. doi: 10.1182/blood-2009-09-244285. [DOI] [PubMed] [Google Scholar]
- 79.Sahu A, Soulika AM, Morikis D, et al. Binding kinetics, structure-activity relationship, and biotransformation of the complement inhibitor compstatin. J Immunol. 2000;165(5):2491–9. doi: 10.4049/jimmunol.165.5.2491. [DOI] [PubMed] [Google Scholar]
- 80.Sahu A, Lambris JD. Complement inhibitors: a resurgent concept in anti-inflammatory therapeutics. Immunopharmacology. 2000;49(1–2):133–48. doi: 10.1016/s0162-3109(00)80299-4. [DOI] [PubMed] [Google Scholar]
- 81.Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–92. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Risitano AM, Patrizia R, Caterina P, et al. Novel complement modulators for paroxysmal nocturnal hemoglobinuria: peptide and protein inhibitors of C3 convertase prevent both surface C3 deposition and subsequent hemolysis of affected erythrocytes in vitro. Blood (ASH Annual Meeting Abstracts) 2012;120:370. [Google Scholar]
- 83.Risitano AM, Ricklin D, Huang Y, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123(13):2094–101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cai S, Dole VS, Bergmeier W, et al. A direct role for C1 inhibitor in regulation of leukocyte adhesion. J Immunol. 2005;174(10):6462–6. doi: 10.4049/jimmunol.174.10.6462. [DOI] [PubMed] [Google Scholar]
- 85.Beinrohr L, Harmat V, Dobó J, et al. C1 inhibitor serpin domain structure reveals the likely mechanism of heparin potentiation and conformational disease. J Biol Chem. 2007;282(29):21100–9. doi: 10.1074/jbc.M700841200. [DOI] [PubMed] [Google Scholar]
- 86.DeZern AE, Uknis M, Yuan X, et al. Complement Blockade with a C1 Esterase Inhibitor in Paroxysmal Nocturnal Hemoglobinuria. Exp Hematol. 2014;42:857–61. e1. doi: 10.1016/j.exphem.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hillmen P, Elebute M, Kelly R, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85(8):553–9. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- 88.Ferreira VP, Pangburn MK. Factor H mediated cell surface protection from complement is critical for the survival of PNH erythrocytes. Blood. 2007;110(6):2190–2. doi: 10.1182/blood-2007-04-083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whaley K, Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976;144(5):1147–63. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Risitano AM, Notaro R, Pascariello C, et al. The complement receptor 2/factor H fusion protein TT30 protects paroxysmal nocturnal hemoglobinuria erythrocytes from complement-mediated hemolysis and C3 fragment. Blood. 2012;119(26):6307–16. doi: 10.1182/blood-2011-12-398792. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt CQ, Bai H, Lin Z, et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J Immunol. 2013;190(11):5712–21. doi: 10.4049/jimmunol.1203548. [DOI] [PMC free article] [PubMed] [Google Scholar]