Abstract
The past several years have witnessed a resurgence of interest in cancer immunotherapy. The development of blocking antibodies against the inhibitory programmed death-1 (PD-1) pathway represents a clinical break-through in the treatment of solid tumors such as melanoma, and these agents show great promise in renal cell carcinoma (RCC). The early data have been surprising in that they demonstrate that blockade of a single immune checkpoint can elicit objective responses in patients with RCC, despite the recognized complexity of the immunosuppressive tumor microenvironment. Reinvigorating the patient’s own immune cells to reactivate and to target the tumor has the potential advantages of more selective killing and thus decreased toxicity. In addition, checkpoint blockade immunotherapy has the advantage of inducing a memory response that is unattainable with our current cytotoxic and targeted therapies. This Crossroads overview will highlight the emerging investigation of PD-1 blockade in RCC and how this T cell–targeted strategy may thwart the tumor’s escape mechanisms and shift the immune system/tumor balance back to a state of equilibrium and even to tumor elimination.
Introduction
Renal cell carcinoma (RCC), like many other tumor types, is characterized by complex interactions between the host immune response and a variety of immunosuppressive pathways operative in the tumor microenvironment (TME; refs. 1–5). An array of effector cells, such as CD8+ and CD4+ T cells, as well as suppressive cell populations, including regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), are present in the tumor infiltrate, but the precise role of these cells and their impact on prognosis remains elusive (1–5). This immune cell infiltrate may signify an active immune response, or it might be the consequence of cytokine secretion by the tumor that recruits T cells to the microenvironment (3). Despite reaching the tumor site, these effector lymphocytes may encounter a variety of factors in the TME that thwart their effects. These impediments include defects in the tumor-cell antigen-processing machinery, recruitment of suppressive cell populations, and increased expression of inhibitory molecules, such as PD-L1, on tumor cells (1, 6, 7).
PD-L1 is one of two major ligands for programmed death-1 (PD-1), a receptor expressed on both activated and then exhausted T cells (Fig. 1; refs. 6, 8). PD-L1 binding to PD-1 negatively regulates the immune response—inhibiting cytokine production, proliferation, and cytotoxic activity of antitumor T cells (9–11). Indeed, PD-L1 expression on tumor cells and tumor-infiltrating lymphocytes (TIL) has been associated with more aggressive tumor behavior and poorer survival (8–10, 12, 13). Most RCCs express PD-L1, and across multiple series, PD-L1 expression has been observed in approximately 16% to 66% of RCC samples tested (8, 9, 12–18). These variable results may be attributed to the differences in the antibodies used for immunohistochemical (IHC) analysis, the definitions of what constitutes PD-L1 “positivity” (e.g., >1%, >5%, and >10%), as well as the age of the specimens and the processing techniques used (19).
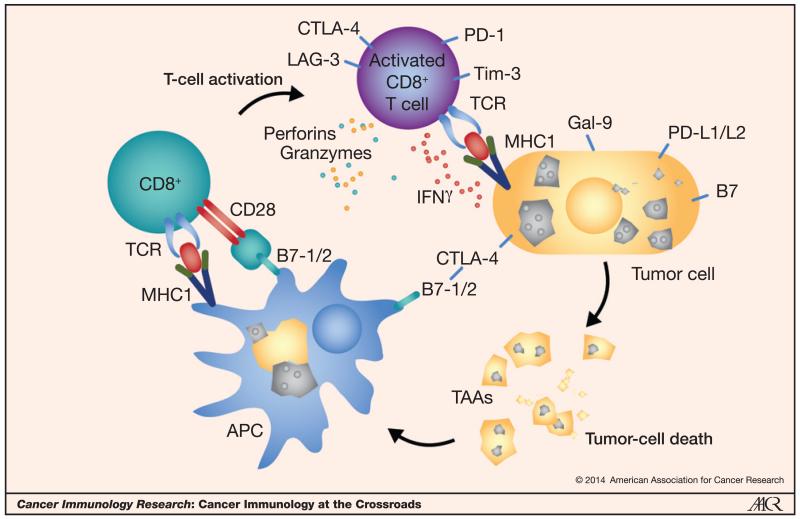
Figure 1.
Complex interplay between the host immune cells and the tumor and its microenvironment. Multiple inhibitory and stimulatory interactions are integrated to block or drive the host immune response, respectively. Antigen-presenting cells (APC) such as dendritic cells present tumor antigens (TAA) to a naïve CD8+ T cell. When the TCR-MHC1-peptide binding along with a stimulatory second signal, such as from CD28 binding to B7-1, is not overcome by the higher affinity and avidity binding of the inhibitory CTLA-4 receptor, the T cell is activated. The tumor-specific activated CD8+ T cell hones in on the malignant cell and induces killing. A variety of mediators such as perforins, granzymes, and IFNγ facilitate the cytolytic function. This host immune response can be thwarted by the tumor’s upregulation of inhibitory ligands on its cell surface, such as PD-L1 or galectin-9. Not depicted here, but when PD-L1 on the tumor cell binds to PD-1 on the T cell, it effectively turns off the T cell, thus evading the host immune response.
Significant improvement in clinical outcomes of patients with metastatic RCC (mRCC) has been realized in the past 10 years, and was triggered by the introduction of the targeted antiangiogenesis therapies (20). However, the inability of these agents to achieve deep or sustained therapeutic responses that translate into cure underscores the need to develop more potent therapies based on new mechanistic insights. RCC is clearly sensitive to immunomodulation as evidenced by the ability of high-dose interleukin-2 (IL2) to elicit complete and durable responses in a small percentage of patients with metastatic disease. However, the majority of patients do not derive benefit from IL2 administration, and the associated toxicity is substantial. Although the identification of biomarkers that predict who might respond to IL2 would be advantageous, the path to higher cure rates and better overall outcomes requires the development of more broadly effective strategies to harness the host immune system. To this end, cytokine injections, immune-stimulatory growth factors (e.g., GM-CSF), and various allogeneic and autologous tumor cell, dendritic cell, or peptide vaccine approaches have been attempted in the treatment of RCC (21, 22). Unfortunately, these maneuvers rarely elicit objective or durable responses despite clear evidence of immune system activation at the cellular level.
There has been a recent resurgence of interest in manipulating the immune system to treat cancer, with considerable excitement over the results of the initial testing of PD-1–PD-L1 pathway blockade in RCC. Immunologically, the concept of immunoediting provides a logical context in which to think about the forces at play between the host immune system and the tumor. Rather than the more dichotomous analogy of tipping the scales to favor immunosurveillance (23), the immunoediting hypothesis incorporates three phases that describe the varying degrees of balance between the tumor and the immune system, including elimination, equilibrium, and escape (Fig. 2; refs. 24–26). In the elimination stage, immune cells, such as natural killer (NK) cells or CD8+ effector cells, recognize and eliminate tumors that are small or highly immunogenic before they are even detectable radiographically. However, some tumors elude the initial host defense mechanisms and progress to a state in which they coexist with the immune system, in an ongoing battle called equilibrium. In this state, it is postulated that the tumors attempt to proliferate but are generally restrained by the immune system and are maintained in a state of functional dormancy. Under the constant immune system pressures, tumor cell variants that can resist immune cell recognition evolve through such mechanisms as antigen loss, defects in antigen presentation (i.e., MHC class I loss), or by upregulation of components of immunoinhibitory pathways such as PD-L1/PD-1, class II MHC/LAG-3, Galectin-9/Tim-3, and VEGF (16, 27–30). These are the tumors that eventually escape the immune system’s defense mechanisms, and that we ultimately must contend with in the clinic. This concept of “escape of immune control” has recently been recognized as one of the “hallmarks of cancer” (31). This Crossroads overview highlights the emerging investigation of PD-1 blockade in RCC and how this strategy may functionally transit an “escaping” tumor back to equilibrium and occasionally to elimination.
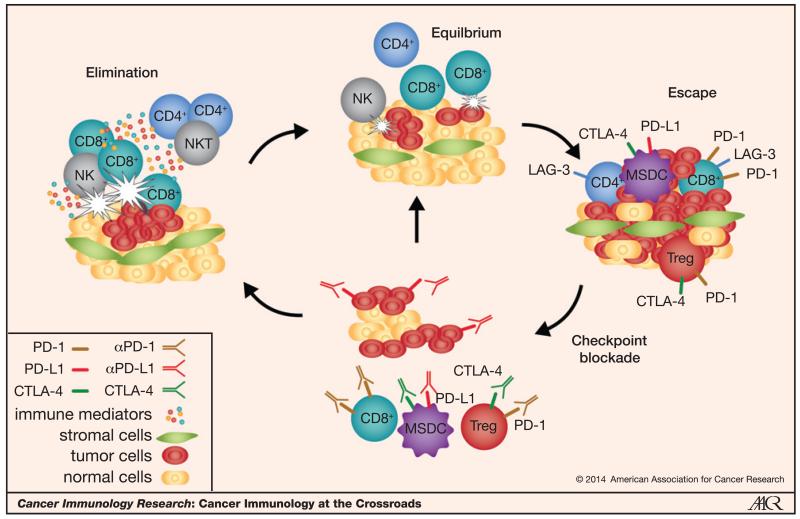
Figure 2.
The concept of immunoediting at work and the potential effects of immune checkpoint blockade on the equilibrium between the tumor and the host immune system. The relationship between a tumor and the host immune system may be best conceptualized by the immunoediting hypothesis, which encompasses three phases: tumor elimination, equilibrium, and tumor escape. In the elimination stage, immune cells such NK cells or CD8+ effector cells recognize and eliminate small or highly immunogenic tumors before they are even detectable radiographically. However, some tumors elude the initial host defense and transition to a state of equilibrium and coexist with the immune system; tumors try to grow but are generally functionally restrained by the immune system. Tumor-cell variants can evolve that can resist immune cell recognition by highjacking host mechanisms including upregulation of components of immunoinhibitory pathways such as PD-L1–PD-1, class II MHC/LAG-3, Galectin-9/Tim-3, and VEGF. Having escaped the immune system’s defense mechanisms, these tumors proliferate and are the ones we face in the clinic. Novel blocking antibodies targeting immune checkpoints, such as PD-1 and CTLA-4, are being evaluated in RCC and have shown encouraging preliminary efficacy. These therapies increase the host antitumor T-cell response and may induce disease stabilization (“equilibrium”) and in some cases, force the balance back to the tumor elimination stage, which may be reflected in the objective responses including CRs that have been observed in patients.
PD-1 Blockade in the Clinic: Initial Monotherapy Studies
Two phase I clinical trials investigating nivolumab (Bristol-Myers Squibb), an IgG4 antibody against PD-1, offered the first signal of efficacy in RCC (Table 1; refs. 6, 32). Nivolumab was administered at varying doses to more than 300 patients, 35 of whom had advanced RCC. An expansion cohort of 34 patients with RCC was treated with doses of either 1 mg/kg or 10 mg/kg (6). These patients were fairly heavily pretreated, with 45% having received three or more prior therapies (33). Median progression-free survival (PFS) was 7.3 months (33). Objective responses were observed in 29% of patients and were generally rapid with a median time to response of 8 weeks. Median duration of response was 56.1 weeks. At least 5 patients had an objective response after discontinuation of therapy. Of the 7 patients who discontinued treatment for reasons other than progressive disease, 40% had persistent responses off therapy for ≥16 weeks. The overall survival (OS) results were encouraging with 70% of these heavily pretreated patients alive at 1 year and 50% at 2 years with a median OS of close to 2 years (22 months; ref. 33). These early data were surprising in that they demonstrated that blockade of a single immune checkpoint could mediate objective responses in patients with RCC, despite the complex immunosuppressive milieu as discussed above.
Table 1.
Reported studies of PD-1 pathway blocking antibodies in RCC
| Trial (ref.) | Phase | N a | Patient population |
Primary endpoint |
RR | Median PFS |
Median OS |
|---|---|---|---|---|---|---|---|
| Nivolumab monotherapy | |||||||
|
| |||||||
| Drake et al. (33) NCT00730639 |
I | 34/35 RCC | Advanced solid tumors |
Safety Tolerability |
29% | 7.3 mo | 22 mo |
| Choueiri et al. (36) NCT01358721 |
I | 91 | Metastatic ccRCC with prior antiangiogenic therapy or treatment naïve |
Immunomodulatory activity |
17% | 36% at 24 wks |
NR |
| Motzer et al. (34, 35) NCT01354431 |
II Dose ranging |
168 | Metastatic ccRCC with prior antiangiogenic therapy |
PFS | 20%–22% | 2.7–4.2 mo | 18.2–25.5 mo |
|
| |||||||
| Nivolumab plus sunitinib, pazopanib, or ipilimumab | |||||||
|
| |||||||
| Hammers et al. (46); Amin et al. (42) NCT01472081 |
I | 44 Ipi 43 S/P |
mRCC: prior antiangiogenic therapy or treatment naive |
Safety Tolerability |
|||
| N3/I1 | 43% | 37 wks | NR | ||||
| N1/I3 | 48% | 38 wks | NR | ||||
| N + S | 52% | 49 wks | |||||
| N + P | 45% | 31 wks | |||||
|
| |||||||
| MPDL3280A | |||||||
|
| |||||||
| Cho et al. (15) | I | 69 RCC | Advanced solid tumors |
DLT | 15% | 24 wks | NR |
| McDermott et al. (37) NCT01375842 |
aAllowed non–clear cell RCC |
||||||
|
| |||||||
| MPDL3280A plus bevacizumab | |||||||
|
| |||||||
| McDermott et al. (37) | I | 10 RCC | Treatment naïve | Safety | 40% | NR | NR |
| BMS-936559 Brahmer et al. (11) NCT00729664 |
I | 17 RCC | Advanced solid tumors | Safety, MTD, DLT | 12% | 53% at 24 wks |
NR |
Abbreviations: DLT, dose-limited toxicity; I, ipilimumab; N, nivolumab; NR, not reached; P, pazopanib; S, sunitinib.
N, number evaluable over total enrolled when presented in ratio.
The tolerability of PD-1 blockade is another attractive aspect of this strategy especially when contrasted to that of high-dose IL2. In the phase IB nivolumab expansion cohort, no maximum-tolerated dose (MTD) was defined (33). Fatigue, rash, diarrhea, and pruritus were the most frequently cited adverse events (AE). Predefined treatment-related “select” AEs that were potentially autoimmune in etiology occurred in 85% of patients, but only 18% of the AEs were grade 3/4. These events tended to be dermatologic (any grade/grade 3/4: 35%/3%), endocrinologic (18%/0%), gastrointestinal (18%/0%), hepatic (12%/3%), infusional (6%/0%), or pulmonary (pneumonitis, 6%/3%) in nature.
On the basis of the initial phase I studies in solid tumors showing a lack of a dose–toxicity relationship at the 0.3 and 10 mg/kg doses, Motzer and colleagues (34) undertook a phase II study to assess whether dose affected clinical efficacy in terms of response rate and PFS. In that study, 168 patients with advanced, tyrosine kinase inhibitor (TKI)-refractory metastatic clear cell RCC were randomized to one of three dose levels of nivolumab given every 3 weeks: 0.3, 2, or 10 mg/kg. By the Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY) prognostic risk group criteria, the majority of patients were favorable or intermediate risk whereas a quarter were poor risk. The majority (62%) had received at least one and a third (33%) had received two prior antiangiogenic agents. Interestingly, no dose–response relationship was observed. Median PFS was not significantly different among the three cohorts, ranging from 2.7 [80% confidence interval (CI), 1.9–30] to 4 (80% CI, 2.8–4.2) to 4.2 (80% CI, 2.8–5.5) months in the 0.3, 2, and 10 mg/kg cohorts, respectively (P = 0.9). Objective responses were observed in 20%, 22%, and 20% of patients, respectively. The two complete responses (CR) occurred with the lower doses. Stable disease was attained in 37% to 44% while 32% to 40% of patients exhibited primary treatment-refractory disease. Responses tend to be durable with a median duration of response of 22.3 months (4.8, NR) in the 10mg/kg arm and had not been reached in the lower two doses (35). The median number of doses administered ranged from 6 to 8. Reasons for treatment discontinuation were progressive disease in 75% of patients and drug-related toxicity in 6% of patients. In the entire cohort, 22% of patients were treated beyond progression.
The median OS results for this phase II study were encouraging: 18.2 (80% CI, 16–24), 25.5 (80% CI, 20–29), and 24.7 (80% CI, 15–26) months in the three cohorts. When broken down by MSKCC risk groups, median OS in the favorable group had not been reached while it was 20.3 and 12.5 months in the intermediate and poor risk groups. Similarly, patients with one prior treatment had a median OS that had not been reached compared with 18.7 months (80% CI, 13.4–26) in those with two or more prior therapies. The toxicity profile observed here mirrored that of the phase I studies with fatigue (22%–35%, all grade 1 or 2), nausea (10%–13%), pruritus (9%–11%), rash (7%–13%), and diarrhea (3%–15%, no grade >2) being the most frequently observed. Grade 3/4 events occurred in 5% of patients receiving the 0.3-mg/kg dose compared with 17% of patients receiving the 2-mg/kg dose, and 13% of patients receiving the 10-mg/kg dose. There was no grade 3 or 4 pneumonitis. Overall, these data confirmed the activity and tolerability of nivolumab in RCC, but as above, failed to demonstrate a clear dose–response relationship at the dose levels studied.
To explore biomarkers predictive of response or resistance, a parallel biomarker-centered study, involving multiple biopsies, was conducted (NCT01358721; ref. 36). This study used the three dose levels discussed above in treatment-refractory cohorts (n = 67), but it also included a cohort of treatment-naïve patients treated at 10 mg/kg (n = 24). The results echoed the phase II efficacy and toxicity findings and provided additional evidence of the immunomodulatory effects of nivolumab. The objective response rate (ORR) in the previously treated cohorts ranged from 9% to 23% for the 0.3, 2, and 10 mg/kg doses and was 13% in the treatment-naïve cohort. The ORR by RECIST v1.1 was 17%. Stable disease was achieved in another 32% of patients with a PFS rate at 24 weeks of 36%. The toxicity profile was similar to that seen in the phase II study (35) with no new, concerning safety signals.
A significant increase in effector T cells and their transcripts in tumor biopsies provided evidence of nivolumab’s immunomodulatory activity. In the 33 matched pairs available, investigators observed an overall trend in proliferation of CD3+ and CD8+ cells with median increases of 73% and 88%, respectively. These findings were corroborated by a similar degree of increase in the mRNA transcripts of the two cell populations. Posttreatment increases in certain cytokines such as IFNγ in the TME and serum chemokines such as CXCL9 and CXCL10, which are both inducible by IFNγ, suggested that nivolumab may promote T-cell migration and expansion. Increased migration of effector T cells to the tumor was suggestive of clinical response to nivolumab with a trend to higher median increases in CD3+ and CD8+ cells in responders compared with nonresponders.
Finally, investigators hypothesized that baseline measures of T-cell exhaustion would be indicative of response to nivolumab. For example, higher baseline levels of PD-L1 expression on tumors should correlate with responses. Of the 56 evaluable fresh treatment biopsies (minimum of 100 tumor cells required), 32% of the tumor samples (n = 18/56) were PD-L1+ as defined by ≥5% of plasma membrane staining. The ORR in the PD-L1–positive patients was 22% (4 of 18) compared with 8% in the negative patients (3 of 38). Whether PD-L1 expression on the tumor increases in response to anti-PD-1 is an area of intense investigation. In the 27 available matched specimens, there was a less than 5% increase in tumor membrane PD-L1 expression from baseline to on treatment (cycle 2) by IHC. In contrast, gene expression profiling of PD-L1 expression demonstrated evidence of significant pharmacodynamic change. This study was especially notable for its success in tissue acquisition; 85% of patients had specimens for IHC at baseline and on treatment biopsies were procured from 72% of patients (36). Successful tissue procurement is very important for the field as standardization of assays will help address the variability in treatment results. Analysis of tumor tissues is critical to improve our understanding of the mechanisms of action of these agents as well as the modes of tumor and host resistance. Standardized assays on tumor tissues will facilitate the identification of predictive biomarkers that will enhance patient selection. We assert that success of tissue acquisition should be considered as an outcome measure in future studies.
Putting the Early Results into Perspective
These initial phase I and II studies demonstrated the clinical activity of nivolumab across multiple dose levels in more than 200 patients with RCC, with an acceptable safety and tolerability profile and encouraging OS results (6, 32, 34, 36). When compared with findings from the phase I study, the phase II results were somewhat discouraging in terms of traditional response and PFS criteria. However, these parameters may not be the best markers of success for immunotherapy. Indeed, upward of 60% of patients achieved clinical benefit as defined by stable disease or objective response.
The notion that OS and disease control rates may better reflect the clinical activity of PD-1 blockade in RCC than ORR is perhaps best understood in the context of the immunoediting hypothesis (25). Clearly, there are some tumors that “escape” and, exhibit upfront resistance. In the phase II study, the incidence of primary refractory disease was approximately 40% (34). On the opposite end of the spectrum, some tumors are “responders” by traditional RECIST criteria, and likely traverse from escape to equilibrium and eventually back to elimination (Fig. 2). For some tumors, this process takes a considerable amount of time, as multiple resistance mechanisms must be overcome. Thus, it is not surprising that at least by imaging, more patients achieve disease stabilization consistent with equilibrium than achieve a significant degree of tumor eradication (i.e., elimination). The durability of responses, which are notable with the anti-PD-1 strategy, is also consistent with reaching this equipoise (the equilibrium stage). In fact, this long-lasting equilibrium, potentially coupled with the induction of T cell–mediated memory responses may be what translates into improvements in OS. A more definitive answer to the impact of nivolumab on OS in RCC is forthcoming from an accrued phase III study. In this study, nivolumab is being compared with standard-of-care everolimus in more than 800 patients with RCC who had received one or two prior antiangiogenic agents (NCT01668784).
PD-L1 Blockade
An additional approach to blocking the PD-L1–PD-1 axis is via monoclonal antibodies (mAb) directed against the ligand PD-L1; these agents have the theoretical advantage of sparing the interaction between PD-L2 and PD-1. This strategy could be clinically relevant, because the PD-L2-PD-1 interaction is also inhibitory and the more selective anti-PD-L1 antibodies potentially could mediate a lower rate of autoimmune toxicity. The first anti-PD-L1 antibody tested, BMS-936559 (Bristol-Myers Squibb), was evaluated in a large phase I solid tumor study, which included 17 patients with treatment-refractory mRCC who were treated at various doses every 2 weeks (11). Although this treatment was tolerable, with no cases of pneumonitis reported, only 12% of the treated mRCC patients experienced an objective response. Disease stabilization was observed in 41% of patients, and 53% of patients were progression free at 24 weeks. Given the parallel phase I study of nivolumab with its more impressive ORR, this antibody is not being developed further at this time.
A second anti-PD-L1 antibody (MPDL3280A; Genentech) is of the IgG1 isotype, and thus has limited or no antibody-dependent cellular toxicity (ADCC) capacity. An expansion arm in the large phase I solid tumor study of this agent (NCT01375842) has enrolled 69 patients with clear cell and non–clear cell RCC (15, 37). This trial was notable for including patients with non–clear cell disease (10%), poor-risk patients by MSKCC criteria (26%), and patients with more aggressive histologic disease, such as those with Fuhrman grade 4 or sarcomatoid histology (29%). Of the evaluable patients, 15% experienced an objective response (1 CR), and an additional 32% achieved disease stabilization. Although exploratory, subset analysis revealed interesting findings of higher ORRs for patients with MSKCC poor-risk disease (27% in all patients, n = 15; 57% in high PD-L1 expressers, n = 7), and for Fuhrman grade 4 or sarcomatoid disease (22%, n = 18). In all patients, median PFS was 24 weeks (5–98+) and 51% were progression-free at 24 weeks.
Similar to nivolumab, MPDL3280A was well tolerated, with no MTD reached at the doses studied. Median duration of treatment was long at 239 days (21–834 days). Only 16% of patients (n = 11) experienced grade 3 treatment-related toxicities, which included anemia (4%), dehydration (3%), fatigue (3%), and hypophosphatemia (3%). No grade 4 toxicities, grade >2 pneumonitis or colitis, or other immune-related AEs were noted.
Given these promising results, this agent is being developed further in RCC. An ongoing randomized study will compare the efficacy of MPDL3280A monotherapy with a combination arm of MPDL3280A + bevacizumab to a third control arm of standard-of-care sunitinib in 300 previously untreated patients with mRCC (NCT01984242). The primary endpoint of this study is PFS. Patients on the sunitinib arm are allowed to cross over to the combination of MPDL3280A plus bevicizumab upon progression. Initial results from the phase I component in 10 patients with RCC demonstrated that the combination was safe with no grade 4 AEs or deaths due to MPDL3280A (37). Of these 10 patients, 90% experienced some degree of clinical benefit with an ORR of 40% and stable disease in 50% of patients.
Combination Studies Based on PD-1 Blockade
Combining PD-1 blockade with other agents such as VEGF-targeted therapies, such as the bevacizumab trial described above, has already proven effective in RCC and is a rational approach to gain additive efficacy or synergy by targeting two different mechanisms of action and potential resistance pathways. In addition to the antiangiogenic and antiproliferative effects of suppressing VEGF with the VEGF-targeted therapies, inhibition of VEGF may induce a more hospitable immune environment. For example, VEGF has been shown to impede dendritic cell maturation and function thereby hindering their antigen-presenting functions in the host (27, 38, 39). VEGF-targeted therapies may also reverse the phenotype of some immunosuppressive cell populations such as Tregs and MDSCs to create a less hostile TME for the effector T cells induced by PD-1 blockade (40, 41).
Amin and colleagues recently presented the initial results of a trial combining nivolumab with VEGF blockade using the TKIs sunitinib or pazopanib. This phase I study was designed to test the safety and tolerability of the combined regimen in addition to determining the MTD of the combination for further phase II testing (42). Initially only pretreated patients were evaluated and nivolumab was tested at a 2-mg/kg dose with both TKIs. Four dose-limiting toxicities (DLT) were observed in the first 20 pazopanib patients, and thus, an expansion cohort of that combination was not pursued. The sunitinib/nivolumab combination appeared to be more tolerable, and after 7 patients, the nivolumab dose was escalated to 5 mg/kg (n = 7). Once tolerability was assured, a dose expansion cohort enrolled 19 patients with the difference that they were treatment naïve. In the whole trial cohort, these patients were generally of favorable or intermediate risk by MSKCC criteria (90% or greater in each arm). Clinical activity was observed with both TKI/nivolumab combinations. In the sunitinib arms, 52% of patients (n = 33) had a confirmed objective response with one CR while another 30% had disease stabilization. Only 1 patient had primary refractory disease, and 4 patients were not evaluable. In the pazopanib arm, 45% had a confirmed objective response (no CRs) and another 35% had disease stabilization. Four patients (20%) had primary refractory disease. Median durations of response with either TKI ranged from 30 to 37 weeks, and the majority of responses (59%) on the sunitinib arm were ongoing at the time of reporting. Keeping in mind that 58% of the sunitinib patients were treatment naïve, median PFS was encouraging at approximately 49 and 31 weeks, respectively, for the sunitinib and pazopanib cohorts. In patients who discontinued treatment for reasons other than disease progression, 24% and 11% continued to respond in the sunitinib and pazopanib arms.
Although intriguing, these data beg the question as to whether the potentially enhanced activity of a TKI/nivolumab combination merits the increased toxicity. Undeniably, these early studies demonstrate that the activity comes at a cost, with a high rate of grade 3/4 toxicities with either TKI/nivolumab combination: 71%–85% in the sunitinib arms and 70% in the pazopanib arm. As described above, the pazopanib arm was terminated because of DLTs. However, the majority of the DLTs observed were consistent with those known to occur with TKIs but at a higher rate than expected. For example, grade 3/4 diarrhea occurred in 12% of the dose-escalation sunitinib cohort and 20% of the pazopanib patients, as opposed to 5% and 3% on the pivotal trials of sunitinib and pazopanib, respectively (43, 44). No treatment-related deaths occurred and there were two cases of pneumonitis. More liver and renal toxicity occurred than was anticipated: 15% to 18% transaminitis with both TKIs and in the sunitinib arm, 9% had acute renal failure (3% grade 3/4). Approximately 36% of patients on the sunitinib arm discontinued the combination for a drug-related AE (42).
If a TKI/nivolumab combination is to be pursued further, the optimal timing of these agents should be considered. For example, should immunotherapy be given upfront during the time when the patient is most likely to be healthiest and most likely to have a potentially longer progression-free interval during which an immune response could develop? Conversely, should PD-1 blockade be initiated after or during concurrent VEGF blockade, in keeping with the notion that VEGF-targeted agents could induce a more hospitable immune environment. An additional consideration involves the concept that VEGF inhibition may modulate tumor PD-L1 expression. Conflicting results from preclinical and clinical work suggest that VEGF blockade may increase PD-L1 expression potentially leading to immune tolerance and that this upregulation may be a mechanism of acquired resistance to the VEGF-targeted agents. Preclinical work by Drake suggests that TKI administration induces PD-L1 expression (C. Drake; unpublished data). In counterpoint, the only prospective study in humans observed decreases in the levels of PD-L1 expression (34% decrease; P < 0.05) and of FOXP3 Tregs (P < 0.05) in nephrectomy specimens after neoadjuvant TKI administration (45). Several randomized combination VEGF inhibitor/PD-1 blockade studies are accruing or in the planning stages; these studies will provide further insight into whether the potential increased efficacy of combination treatment is sufficient to counterbalance the expected added toxicity.
Perhaps more intriguing is the concept of blocking multiple immune checkpoint pathways to control RCC. The same phase I study that explored the VEGF TKI/nivolumab combinations also included cohorts in which both CTLA-4 and PD-1 were blocked simultaneously, using the mAbs ipilimumab and nivolumab (46). Ipilimumab is a fully human anti-CTLA-4 antibody that is FDA approved for the treatment of metastatic melanoma (47). In a small phase II study of patients with advanced RCC, ipilimumab treatment resulted in a modest response rate of 13% but with 33% grade 3/4 immune-related toxicities (48). In metastatic melanoma, the combination of ipilimumab and nivolumab resulted in a 40% ORR, with a manageable albeit high rate of grade 3/4 toxicity (55%; ref. 49). These results drove the testing of the combination forward in RCC. In the phase I study, the two ipilimumab arms included 44 patients with previously treated or treatment-naïve mRCC. These patients were randomized to receive either nivolumab 3 mg/kg i.v. plus ipilimumab 1 mg/kg i.v. (N3/I1, n = 21), or nivolumab 1 mg/kg i.v. plus ipilimumab 3 mg/kg i.v., every 3 weeks for four cycles (N1/I3, n = 23; ref. 46). After four induction cycles of combined therapy, patients who had not progressed were continued on nivolumab, 3 mg/kg every 2 weeks. The primary endpoint was safety. By MSKCC prognostic criteria, all patients were favorable and intermediate risk. Although 20% were treatment naïve, 80% had had prior systemic treatment including antiangiogenic agents (78%–81%), cytokines (26%–57%), or mTOR inhibitors (24%–30%). Approximately 30% of patients had had two or more lines of therapy.
In general, the lower dose of ipilimumab (N3/I1) was more tolerable; 29% of the patients in the lower-dose cohort had grade 3/4 events compared with 61% in the N1/I3 group. Most notable were diarrhea (5% vs. 13%), elevations in transaminases (0% vs. 26%), and elevations in lipase (14% vs. 26%).
No high-grade pneumonitis or deaths related to treatment transpired. Low-grade autoimmune events such as endocrinopathies (14% vs. 35%), renal insults (10% vs. 10%), and skin disorders (38% vs. 39%) were observed. In all, 26% of patients on the N1/I3 arm discontinued treatment due to toxicity compared with 10% on the N3/I1 arm. These discontinuations were generally due to increases in liver (n = 2) or pancreatic (n 4) enzymes, diarrhea (n = 1) or pneumonitis (n = 1).
ORRs were statistically significant in both arms: 43% to 48%, with a median duration of response of 31 weeks in the N3/I1 cohort, and had not been reached in the N1/I3 patients (median follow-up, 36–40 weeks). Approximately 80% of patients continued to have an ongoing response at the time the data were presented. One patient in the N1/I3 arm had a CR while 24% to 35% had stable disease. Primary progressive disease occurred in 13% to 24% of patients. The 24-week PFS was similar in both arms at 64% to 65%. The median PFS was similar in the two cohorts (38 vs. 37 weeks), suggesting that the increased toxicity with the higher dose is not warranted. Similar to what was observed in the TKI/nivolumab combination arms, a fairly high percentage of patients who discontinued treatment before progression had continued responses off drug (33%–46%), albeit with a relatively short follow-up time.
Overall, combined checkpoint blockade with nivolumab plus ipilimumab showed an acceptable and manageable safety profile and evidence of clinical activity in mRCC (46). The ORR was greater than that observed previously with nivolumab or ipilimumab monotherapies, and responses tended to be durable even after treatment had discontinued. The clinical benefit was especially impressive, given that 80% of enrolled patients were treatment refractory. However, this combination can be quite toxic. Practitioners will need to be well informed as to the expected common and uncommon immune-related events and will need to be proficient in their management, which may require multidisciplinary input. A phase III study testing the combination against a sunitinib control arm is planned and will further delineate the efficacy and toxicity profile of the combined regimen (NCT02231749).
Patient Selection and Predictive Biomarkers for PD-1 Pathway Blockade
The significant percentage of RCC cases that are refractory to monotherapy with nivolumab (40%) underscores the need to elucidate resistance mechanisms, which may inform combination strategies, and to identify patient selection criteria that will better predict outcomes with this approach. Almost all of the early-phase clinical studies described above strove to identify potential predictive biomarkers. Expression of PD-L1 on tumor cells, was shown to be a potential predictive biomarker in the phase IB nivolumab study, and thus has been the top candidate, but results have been variable in RCC. Across the monotherapy trials, it appears that patients with PD-L1–positive tumors have a higher chance of responding to PD-1 blockade, but between 10% and 20% of patients with PD-L1–negative tumors still responded. In the phase II dose-ranging nivolumab study, there was a trend toward improved response (31% vs. 18%), PFS (2.9 vs. 4.9 months), and OS (NR vs. 18.2 months) for patients with high PD-L1 tumor expression (i.e., ≥5%; ref. 35). Conclusions were limited by missing expression data in 61 patients and by the lack of a comparator arm.
The Genentech study team has undertaken an alternative strategy of looking at the PD-L1 expression on the infiltrating immune cells and observed similar results with a predisposition to better outcome in the high expressers compared with low expressers when assessed by IHC. In the RCC cohort of the phase I study, patients with immune-infiltrating cells exhibiting high PD-L1 expression (IHC1: ≥1%, IHC2: ≥5%, or IHC3: ≥10%) had an ORR of 20% and a median PFS of 24 weeks compared with low expressers (IHC0 ≤1%), who experienced an ORR of 10% and median PFS of 20 weeks (37).
Conversely, the predictive power of PD-L1 expression may not hold true for combination studies; in the TKI/nivolumab studies PD-L1 expression actually appeared to be a negative predictor of response (42). However, the number of patients was small. These results require further investigation as to the possible effects of VEGF inhibition on PD-L1 expression (45) and the potential benefits of targeting these two different pathways.
Conclusions from the PD-L1 biomarker data are also limited by the retrospective nature of these studies, the disparate definitions of what constitutes a PD-L1–positive tumor (e.g., >1%, >5% expression), the unclear role of membrane versus cytoplasmic staining, and the use of different antibodies. The phenomenon of patients with PD-L1–negative tumors responding may be explained by intratumoral heterogeneity (50), discrepancies in tissue processing (19), and the inherent fact that the use of nephrectomy specimens or older biopsies may not accurately reflect the current PD-L1 status of the metastatic tumors (51, 52). Differences in expression levels between the primary tumor and metastases or in the same tumor when assessed serially over time may reflect adaptive upregulation of PD-L1 in response to different treatment or selective pressures as the disease advances (29, 53, 54). Why some patients with PD-L1–positive tumors do not respond to PD-1 pathway blockade is an additional unresolved question. Hypothetical explanations include the overwhelming expression of additional inhibitory immune checkpoint ligands on the tumor and their corresponding checkpoint molecules on tumor-infiltrating T cells (55), as well as other immunosuppressive molecules and cell populations in the TME that cannot be overcome by monotherapy.
Future Directions
Combination therapy is likely to be the path forward in RCC, as cancer is rarely cured or even controlled by one drug alone. Fortunately, the number of possible combinatorial approaches appears limitless with many more novel strategies yet to be identified. Vanneman and Dranoff (56) succinctly categorized several of the possibilities: (i) therapies that can augment antigen-presentation and enhance T-cell priming: that is, sunitinib, JAK2 inhibitors, anthracyclines, and radiation; (ii) drugs that can increase differentiation of memory T cells such as mTOR inhibitors; (iii) agents that improve antitumor T-cell function such as checkpoint blockade (e.g., anti-CTLA-4, TIM-3, or LAG-3), agonistic costimulatory antibodies (e.g., anti-OX40 anti-CD137), immunoinhibitors (CD244 and CD160), inhibitors of apoptosis (IAP); (iv) drugs that enhance CTL-mediated lysis of tumor cells such as HSP90 inhibitors, PI3K inhibitors, histone deacetylase (HDAC) inhibitors; (v) drugs that can reduce tumor-induced immunosuppression such as PI3K inhibitors; (vi) agents that decrease immunosuppressive cell populations such as Tregs and MSDCs (e.g., sunitinib, gemcitabine, and 5-fluorouracil); and (vii) novel vaccine approaches. The last approach is particularly intriguing because both vaccines and immune checkpoint inhibitors can simultaneously harness distinct components of the immune system with specificity and resultant low off-target toxicity. The development of memory cells with both of these approaches may induce long-term, persisting effects that are unattainable with traditional therapies (57, 58).
Conclusions and Current State of Affairs
The resulted studies evaluating PD-1 blockade in RCC support their clinical development especially given the encouraging durability of responses and initial OS results. The findings underscore several distinct features of PD-1 blockade that distinguish them from our currently available agents including the durability of responses and disease stabilization that can persist even off therapy. These enduring effects are consistent with induction of memory responses against the tumor, which are unachievable with the targeted agents. Remaining questions include how to optimally develop this line of therapies. Given the impact of multiple resistance mechanisms operative in the TME, combination therapies are likely key to overcoming resistance. The general tolerability of these agents makes them ideal candidates for use in combinations. We need to ascertain the best and simplest combinations and move them forward into clinical testing. The optimal timing for PD-1 pathway blockade, whether it be earlier in the adjuvant or treatmentnaïve setting or later in the advanced disease course, needs to be determined and is the focus of ongoing and planned trials. Perhaps utmost, we must identify and develop predictive biomarkers, which will direct selection of the optimal candidates for this strategy. This will be no easy task as even with the long-approved systemic agents in mRCC such as the cytokines, VEGF-targeted therapies, and mTOR inhibitors, we have yet to identify a predictive biomarker (59).
Footnotes
Disclosure of Potential Conflicts of Interest
L.C. Harshman is a consultant/advisory board member for Bristol-Myers Squib, Pfizer, Dendreon, and Aveo. C.G. Drake reports receiving a commercial research grant from Bristol-Myers Squib and Janssen; has ownership interest (including patents) in Compugen and NexImmune; and is a consultant/advisory board member for Bristol-Myers Squibb, Compugen, Dendreon, Roche/Genenetch, Novartis, and Merck. T.K. Choueiri is a consultant/advisory board member for Merck, Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Novartis, and Bayer.
References
- 1.Singer K, Kastenberger M, Gottfried E, Hammerschmied CG, Buttner M, Aigner M, et al. Warburg phenotype in renal cell carcinoma: high expression of glucose-transporter 1 (GLUT-1) correlates with low CD8(+) T-cell in filtration in the tumor. Int J Cancer. 2011;128:2085–95. doi: 10.1002/ijc.25543. [DOI] [PubMed] [Google Scholar]
- 2.Attig S, Hennenlotter J, Pawelec G, Klein G, Koch SD, Pircher H, et al. Simultaneous infiltration of polyfunctional effector and suppressor T cells into renal cell carcinomas. Cancer Res. 2009;69:8412–9. doi: 10.1158/0008-5472.CAN-09-0852. [DOI] [PubMed] [Google Scholar]
- 3.Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, et al. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer. 2003;89:1906–8. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–6. [PubMed] [Google Scholar]
- 5.Webster WS, Lohse CM, Thompson RH, Dong H, Frigola X, Dicks DL, et al. Mononuclear cell infiltration in clear-cell renal cell carcinoma independently predicts patient survival. Cancer. 2006;107:46–53. doi: 10.1002/cncr.21951. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–6. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749–56. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 14.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–7. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- 15.Cho DC, Sosman JA, Sznol M, Gordon MS, Hollebecque A, Hamid O, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2013;31(suppl) abstr 4505. [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 18.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquére K, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PloS ONE. 2011;6:e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Napoli A, Signoretti S. Tissue biomarkers in renal cell carcinoma: issues and solutions. Cancer. 2009;115:2290–7. doi: 10.1002/cncr.24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudes GR, Carducci MA, Choueiri TK, Esper P, Jonasch E, Kumar R, et al. NCCN Task Force report: optimizing treatment of advanced renal cell carcinoma with molecular targeted therapy. J Natl Compr Canc Netw. 2011;9:S1–29. doi: 10.6004/jnccn.2011.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal SK, Hu A, Figlin RA. A new age for vaccine therapy in renal cell carcinoma. Cancer J. 2013;19:365–70. doi: 10.1097/PPO.0b013e31829d74b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parish CR. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol. 2003;81:106–13. doi: 10.1046/j.0818-9641.2003.01151.x. [DOI] [PubMed] [Google Scholar]
- 24.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 26.Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. J Leuk Biol. 2008;84:988–93. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–10. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 29.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drake CG, McDermott DF, Sznol M, Choueiri TK, Kluger HM, Powderly JD, et al. Survival, safety, and response duration results of nivolumab (Anti-PD-1; BMS-936558; ONO-4538) in a phase I trial in patients with previously treated metastatic renal cell carcinoma (mRCC): long-term patient follow-up. J Clin Oncol. 2013;31(suppl) abstr 4514. [Google Scholar]
- 34.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel T, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): results of a randomized, dose-ranging phase II trial. J Clin Oncol. 2014;32(suppl):5s. doi: 10.1200/JCO.2014.59.0703. abstr 5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer RJ, Rini BI, McDermott DF, Redman B, Kuzel T, Harrison MR, et al. Randomized, dose-ranging phase II trial of nivolumab for metastatic renal cell carcinoma (mRCC); Proceedings of the European Society of Medical Oncology Meeting; Madrid, Spain. 2014. [Google Scholar]
- 36.Choueiri TK, Fishman MN, Escudier BJ, Kim JJ, Kluger HM, Stadler WM, et al. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): biomarker-based results from a randomized clinical trial. J Clin Oncol. 2014;32(suppl):5s. abstr 5012. [Google Scholar]
- 37.McDermott DF, Sznol M, Sosman JA, Soria JC, Gordon MS, Hamid O, et al. Immune correlates and long term follow up of a Phase Ia study of MPDL3280A, an engineered PD-L1 antibody, in patients with metastatic renal cell carcinoma (mRCC); Proceedings of the European Society of Medical Oncology Meeting; Madrid, Spain. 2014. [Google Scholar]
- 38.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 39.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. [PubMed] [Google Scholar]
- 40.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 41.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amin A, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2014;32(suppl):5s. abstr 5010. [Google Scholar]
- 43.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 44.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 45.Sharpe K, Stewart GD, Mackay A, Van Neste C, Rofe C, Berney D, et al. The effect of VEGF-targeted therapy on biomarker expression in sequential tissue from patients with metastatic clear cell renal cancer. Clin Cancer Res. 2013;19:6924–34. doi: 10.1158/1078-0432.CCR-13-1631. [DOI] [PubMed] [Google Scholar]
- 46.Hammers HJ, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2014;32(suppl):5s. doi: 10.1200/JCO.2016.72.1985. abstr 4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callea M, Genega EM, Gupta M, Cheng S, Fay AP, Song J, et al. PD-L1 expression in primary clear cell renal cell carcinomas (ccRCCs) and their metastases. J Clin Oncol. 2014;32(suppl):5s. abstr 4585. [Google Scholar]
- 52.Jilaveanu LB, Shuch B, Zito CR, Parisi F, Barr M, Kluger Y, et al. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer. 2014;5:166–72. doi: 10.7150/jca.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, et al. Interferon-gamma-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–93. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 55.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dranoff G. Tailor-made renal cell carcinoma vaccines. Cancer Cell. 2012;22:287–9. doi: 10.1016/j.ccr.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 58.Gupta R, Emens LA. GM-CSF-secreting vaccines for solid tumors: moving forward. Discov Med. 2010;10:52–60. [PMC free article] [PubMed] [Google Scholar]
- 59.Sonpavde G, Choueiri TK. Precision medicine for metastatic renal cell carcinoma. Urol Oncol. 2014;32:5–15. doi: 10.1016/j.urolonc.2013.07.010. [DOI] [PubMed] [Google Scholar]