Abstract
Background
The purpose of this study was to elucidate factors associated with pharyngoesophageal strictures after treatment for head and neck squamous cell carcinoma (SCC).
Methods
We conducted a retrospective review of patients receiving cisplatin and 5-fluorouracil chemotherapy combined with concurrent hyperfractionated radiation therapy for oropharyngeal squamous cell carcinoma.
Results
Strictures developed in 13 of 67 patients (19%). Strictures were associated with tumor location (tonsil vs base of tongue; p = .03), neck dissection after completion of therapy (p = .03), and the duration of treatment-induced mucositis (weeks with mucositis grade ≥2; National Cancer Institute (NCI) Common Toxicity Criteria; p < .001). Age, sex, race, tumor stage, nodal stage, American Joint Committee on Cancer (AJCC) stage, human papillomavirus (HPV) status, smoking, radiation dose, maximum severity of mucositis, amifostine use, and pretreatment swallow dysfunction were not significantly associated with stricture. In multivariate analysis, only duration of mucositis, after controlling for age, sex, and tumor location, remained highly significant (p < .01).
Conclusion
The duration of treatment-related mucositis is an independent risk factor for stricture formation in patients with oropharyngeal SCC treated with concurrent chemotherapy and radiation therapy.
Keywords: stricture, pharyngoesophageal, oropharyngeal, squamous cell carcinoma, chemoradiation
Definitive concurrent chemotherapy and radiation therapy for treatment of oropharyngeal squamous cell carcinoma (SCC) is a well-established treatment modality with excellent long-term local control and disease-free survival.1,2 However, significant swallowing dysfunction can impact the long-term quality of life for survivors long after the acute toxicities of treatment have resolved.3
Pharyngoesophageal stricture is 1 of the known complications of organ-sparing treatment protocols for head and neck cancer. Previous studies have examined treatment and patient risk factors for stricture formation, but have included patients undergoing diverse treatment modalities and with tumors in a range of locations. Consequently, these studies have primarily identified differences in treatment protocols between patients groups that are associated with stricture: twice-daily versus daily radiation,4 volumetric radiation dose to superior or middle pharyngeal constrictors,5,6 or high-risk tumor locations such as the hypopharynx.4,6 The only patient-specific risk profiles thus far identified are female patients4 and non- or ex-smokers6 who have been reported to have a higher rate of stricture formation. An improved ability to predict patients at risk for pharyngoesophageal stricture could potentially lead to preventive strategies and targeted therapies, including early intervention for stricture formation.
The epidemiological increase in human papillomavirus (HPV)-associated oropharyngeal cancers7 provides additional impetus to clearly define those at risk for long-term swallowing complications. Outcomes for these patients are excellent, with 2-year survival upward of 95%.8 Although treatment de-intensification may be possible in this cohort without sacrifice of oncologic outcomes, these patients are typically treated with the same regimen as those who are HPV negative. Avoiding long-term swallowing morbidity and stricture formation is of paramount importance in those patients who are anticipated to have excellent oncologic results.
In the current study, we sought to identify risk factors for stricture formation in a well-defined patient cohort with advanced-stage oropharyngeal SCC undergoing a uniform, concurrent chemotherapy and radiation therapy protocol. We hypothesized that by examining stricture formation in a population with a uniform disease site and treatment protocol, we would be able to define patient-specific risk factors associated with stricture formation.
Patients and Methods
Study Subjects
An institutional review board-approved retrospective medical chart review was performed on all patients with oropharyngeal SCC who underwent a structured concurrent chemotherapy and radiation therapy treatment protocol at the Milton J. Dance, Jr. Head and Neck Center at the Greater Baltimore Medical Center from 2000 through 2007. Seventy-four patients were identified, of whom 67 had follow-up greater than 1 year and were, therefore, eligible for further analysis.
The concurrent chemotherapy and radiation therapy protocol consisted of conventional external beam radiation of 125 cGy delivered twice daily for a total dose of 70 Gy to the primary site.1 Involved lymph nodes in the neck were treated to a total dose of 60 Gy, and clinically and radiologically negative lymph node basins in the neck were treated to a total dose of 50 Gy. Bolus IV cisplatin (12 mg/m2) over 1 hour and 5-fluorouracil (600 mg/m2) as a continuous infusion was delivered concurrently at the start of radiation therapy and with the completion of radiotherapy. Neck dissection was offered to all patients with N2 or higher disease upon presentation and to those patients who did not have a complete response in the neck at the conclusion of concurrent chemotherapy and radiation therapy. One patient died of a myocardial infarction after 1 cycle of chemotherapy, but no other patient experienced toxicity-related treatment breaks.
Demographic and clinicopathologic data were obtained from patient medical records and previously maintained databases. Patient age, sex, tumor site, tumor stage, nodal stage, performance of a neck dissection, American Joint Committee on Cancer (AJCC) stage, and current disease status was recorded for each patient according to available clinical, radiologic, and operative documentation. Smoking history was scored as a binary variable with a positive history indicating any past or current tobacco use. HPV status was determined by in situ hybridization catalyzed signal amplification method in formalin-fixed and paraffin-embedded tissues using probes against HPV-16 DNA. Interpretation was performed by a head and neck pathologist.
All radiation was delivered via conventional external beam and any variation from the standard dose of 70 Gy to the primary site was noted and recorded as equal to, greater than, or less than 70 Gy to analyze any potential dosing effects. As radiation dose to the neck varied with nodal disease, it was recorded as a 4-part variable: a maximum dose of 50 Gy to each side of the neck, 1 side of the neck treated up to 60 Gy, both sides of the neck treated to 60 Gy, or at least 1 side of the neck receiving a boost higher than 60 Gy. Radiation fields remained largely uniform to the primary site and nodal disease as no intensity-modulated radiotherapy (IMRT) was used during the course of this study. If amifostine was administered as a preventative agent for xerostomia throughout the entirety of, or during a portion of therapy, its use was recorded.
The majority of patients (40 of 67; 60%) in the study received a pretreatment modified barium swallow, and a Dysphagia and Outcomes Severity Score (DOSS, scale 1–7) was calculated for these patients. The score was reduced to a binary variable: those with functionally normal or minimally impaired swallow function (DOSS score 6 or 7), and those with impaired swallow (DOSS score <6).
Some patients treated with the uniform concurrent chemotherapy and radiation therapy protocol were independently enrolled in a prospective randomized controlled institutional review board–approved swallowing intervention trial (36 of 67 patients; 54%). Their radiation schedule, chemotherapy, or adjuvant surgical therapy did not change as a result of their enrollment in this study. Patients were randomized to swallow interventions consisting of a full range of oral motor exercises to improve strength and range of motion of the oropharyngeal musculature. The control group was not instructed on these exercises. As part of the data collection for the study, both control and treatment groups underwent frequent, comprehensive dietary and prospective nursing assessments, which included grading of oral mucositis induced by treatment according to the National Cancer Institute (NCI) Common Toxicity Criteria Version 2.0. For the purposes of the current study, the number of weeks that each patient experienced mucositis greater or equal to grade 2 was retrospectively collected from patient medical records and recorded as a continuous variable. Univariate analysis indicated that enrollment in the study as a control or experimental subject was not statistically associated with the primary outcome of stricture (p = .13).
All patients had a percutaneous gastrostomy tube (PEG) placed before beginning treatment and the duration of PEG-dependence was calculated from the first day of therapy to the date of PEG decannulation.
The primary outcome of pharyngoesophageal stricture was determined by modified barium swallow (MBS) and was defined as a narrowed area which either partially or completely obstructed bolus transport from the pharynx into the proximal esophagus. Posttreatment MBS studies were performed for any patient with persistent dysphagia after conclusion of treatment, determined by a patient's subjective swallowing complaint, reduced ability to maintain adequate nutrition by mouth, or an inability for the patient to advance diet level. Routine MBS studies were not performed on any subset of patients, including patients enrolled in a swallowing study, and were, therefore, limited to patients with subjective complaints. The MBS was performed conjointly by the speech pathologist and radiologist within the range of 3 months to 3 years after the conclusion of treatment. Overall, 30 of 67 (45%) of all patients underwent formal MBS swallow evaluations, with 12 of 30 (40%) of these patients receiving a diagnosis of pharyngoesophageal stricture based on the MBS results.
Statistical Analysis
Patient demographics and clinical variables were summarized using descriptive statistics. Associations between categorical variables and the presence of a stricture were statistically assessed with the Fisher exact test. For each continuous variable, a 2-sample Wilcoxon rank-sum test tested the hypothesis that the values for patients with and without stricture are from populations with the same distribution. For all models reported, the Hosmer– Lemeshow test results supported the null hypothesis that the models fit the data acceptably, and link tests fail to detect misspecification. All analyses were conducted using Stata 11.0 software (StataCorp, College Station, TX).
Results
Seventy-four patients began the concurrent chemotherapy and radiation therapy for biopsy-proven oropharyngeal SCC. Three of these patients were lost to follow-up, 2 died of intercurrent illnesses, 1 died of treatment-related toxicity, and 1 died of disease, all within the first year after therapy. The data for the 67 patients with at least 1 year of follow-up is summarized in Table 1.
Table 1.
Patient demographics.
| Covariate | Patients without stricture (%) | Patients with stricture (%) | p value |
|---|---|---|---|
| Age | 1 | ||
| ≤55 | 23 (42.6) | 6 (46.2) | |
| >55 | 31 (57.4) | 7 (53.8) | |
| Sex | .39 | ||
| Male | 47 (87) | 10 (76.9) | |
| Female | 7 (13) | 3 (23.1) | |
| Race | 1 | ||
| White | 47 (87) | 12 (92.3) | |
| African American | 6 (11.1) | 1 (7.7) | |
| Other | 1 (1.9) | 0 (0) | |
| Tumor location | .03 | ||
| Tonsil | 12 (24.1) | 8 (61.5) | |
| Base of tongue | 39 (72.2) | 5 (38.5) | |
| Other oropharynx | 2 (3.7) | 0 (0) | |
| Tumor classification | .79 | ||
| 1 | 2 (3.7) | 0 (0) | |
| 2 | 21 (38.9) | 5 (38.5) | |
| 3 | 14 (25.9) | 5 (38.5) | |
| 4 | 17 (31.5) | 3 (23.1) | |
| Nodal classification | .24 | ||
| 0 | 4 (7.4) | 0 (0) | |
| 1 | 15 (27.8) | 1 (7.7) | |
| 2 | 29 (53.7) | 11 (84.6) | |
| 3 | 6 (11.1) | 1 (7.7) | |
| AJCC stage | .27 | ||
| 3 | 13 (24.1) | 1 (7.7) | |
| 4 | 41 (75.9) | 12 (92.3) | |
| HPV | 1 | ||
| Negative | 13 (39.4) | 3 (42.9) | |
| Positive | 20 (60.6) | 5 (57.1) | |
| Tobacco history | .74 | ||
| No | 17 (31.5) | 3 (23.1) | |
| Yes | 37 (68.5) | 10 (76.9) | |
| Neck dissection | .03 | ||
| No | 22 (40.7) | 1 (7.7) | |
| Yes | 32 (59.3) | 12 (92.3) | |
| Primary site radiation | .66 | ||
| <7000 Gy | 1 (1.9) | 0 (0) | |
| 7000 Gy | 49 (90.7) | 13 (100) | |
| >7000 Gy | 4 (7.4) | 0 (0) | |
| Neck radiation | .59 | ||
| 5000 Gy 5000 Gy | 4 (7.4) | 0 (0) | |
| 6000 Gy 5000 Gy | 34 (63.0) | 10 (76.9) | |
| 6000 Gy 6000 Gy | 10 (18.5) | 3 (23.1) | |
| >6000 Gy Any Gy | 6 (11.1) | 0 (0) | |
| Amifostine use | .42 | ||
| None | 34 (63.0) | 6 (46.2) | |
| Partial course | 7 (12.9) | 3 (23.1) | |
| Complete course | 6 (11.1) | 4 (30.8) | |
| Maximal mucositis (CTC 3.0) | .09 | ||
| 1 | 3 (11.5) | 0 (0) | |
| 2 | 10 (38.5) | 1 (10) | |
| 3 | 13 (50.0) | 9 (90) | |
| 4 | 0 (0) | 0 (0) | |
| Duration of mucositis (weeks) | < .001 | ||
| Mean (SD) | 4.5 (3.8) | 20.5 (15.6) | |
| Median (IQR) | 4 (3.8) | 19.5 (15.3) | |
| Pretreatment dysphagia | 1 | ||
| No (DOSS ≥ 6) | 23 (82.1) | 10 (83.3) | |
| Yes (DOSS <6) | 5 (17.9) | 2 (16.7) | |
| Posttreatment dysphagia | .06 | ||
| No (DOSS ≥ 6) | 12 (66.7) | 3 (25.0) | |
| Yes (DOSS <6) | 6 (33.3) | 9 (75.0) | |
| Participation in swallow study | .13 | ||
| Control | 13 (52) | 2 (20) | |
| Treatment | 12 (48) | 8 (80) | |
| Duration of PEG-dependence, days | .19 | ||
| Mean (SD) | 710 (2108) | 1153 (2800) | |
| Median (IQR) | 193 (168) | 231 (336) | |
| Disease status | .64 | ||
| Alive | 42 (77.8) | 12 (92.3) | |
| Dead of disease | 10 (18.5) | 1 (7.7) | |
| Death, other cause | 2 (3.7) | 0 (0) |
Abbreviations: AJCC, American Joint Committee on Cancer; HPV, human papillomavirus; CTC, common toxicity criteria; IQR, interquartile range; DOSS, dysphagia outcomes and severity score.
Thirteen patients (19%) had pharyngoesophageal strictures diagnosed by MBS studies. All patients with strictures underwent therapeutic dilations, with a mean number of 3.6 dilations (range, 1–19).
Using univariate analysis, more patients with strictures had primary tumors located in the tonsil versus base of tongue (8 [62%] vs 5 [24%]; p = .03) and were more likely to have undergone a neck dissection (12 [92%] vs 1 [60%]; p = .03) compared with those who did not have stricture formation. Although neck dissections were most commonly performed for patients with a nodal status of N2 or N3, nodal status did not significantly correlate with stricture formation (p = .24).
Among the 36 patients who had detailed mucositis toxicity data available, the duration of treatment-induced mucositis was strongly correlated with stricture formation. Those patients who formed strictures had a significantly longer median duration of mucositis compared with those who did not form strictures (20 weeks vs 4 weeks; p < .001). The maximal severity of mucositis was not significantly associated with stricture formation (p = .09).
All other clinical or pathologic variables were not significantly associated with stricture formation, including age, sex, race, tumor stage, nodal stage, AJCC stage, HPV-16 positivity, radiation dose to the primary site, radiation dose to the neck, amifostine use, preoperative dysphagia, disease status, or enrollment in the swallowing trial.
Multivariate logistic regression models were created to control for clinical variables that could be confounding the correlations identified in univariate analyses (Table 2). Due to the low numbers of index stricture cases, these models can best be regarded as hypothesis generating, however inference for tumor location or duration of mucositis did not qualitatively change even when omitting age, sex, or smoking history. Multiple models, both adjusted and unadjusted, were created to confirm the stability of the findings, including models that controlled for the 2 patient-specific risk factors for strictures identified in previous studies: female sex4 and smoking.6 When controlling for age, sex, and duration of mucositis, tumor location was no longer significant. Duration of mucositis, however, remained significantly and independently associated with stricture formation (odds ratio = 1.32 for each additional week of mucositis, p < .01). Each week of additional treatment-induced mucositis is, therefore, estimated to be associated with a 32% increase in the risk of stricture formation. Although neck dissection was a significant factor on univariate analysis, no stable multivariate analyses could be constructed with this variable, as only 1 patient with a stricture did not have a neck dissection. We can therefore conclude that neck dissection may correlate with stricture formation but cannot confirm its independent association.
Table 2.
Logistic regression models.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Tumor location | 0.20* (0.05–0.76) | 0.76 (0.07–8.37) | |
| Age | 1.03 (0.95–1.10) | 1.07 (0.94–1.23) | 1.07 (0.94–1.23) |
| Sex | 0.60 (0.12–2.98) | 0.20 (0.01–3.19) | 0.21 (0.01–3.45) |
| Smoking history | 1.14 (0.25–5.21) | ||
| Duration of mucositis | 1.34* (1.09–1.63) | 1.32* (1.08–1.63) | |
| Observations | 65 | 36 | 36 |
| Homer–Lemeshow test p value | 0.32 | 0.64 | 0.66 |
| Link test fitted value squared p value | 0.18 | 0.59 | 0.65 |
Odds ratios; 95% confidence intervals in parentheses.
p < .05.
Patients are sorted by their individual duration of mucositis in Figure 1. Patients with stricture formation have a significantly higher mean and median duration of mucositis, and there also seems to be a critical duration of mucositis above which patients are at very high risk for stricture formation. Of the 26 patients without strictures, none had a duration of mucositis longer than 14 weeks. In contrast, 7 of the 10 patients with stricture (70%) had a duration of mucositis lasting longer than 19 weeks. The simple criterion of 15 weeks of mucositis, therefore, has excellent predictive power in identifying those patients ultimately at risk for stricture formation (Specificity = 100%, Sensitivity = 70%) should report confidence intervals for those estimates: The exact 95% CI for sensitivity is .35 to .93, and for specificity it is .81 to 1.
Figure 1.
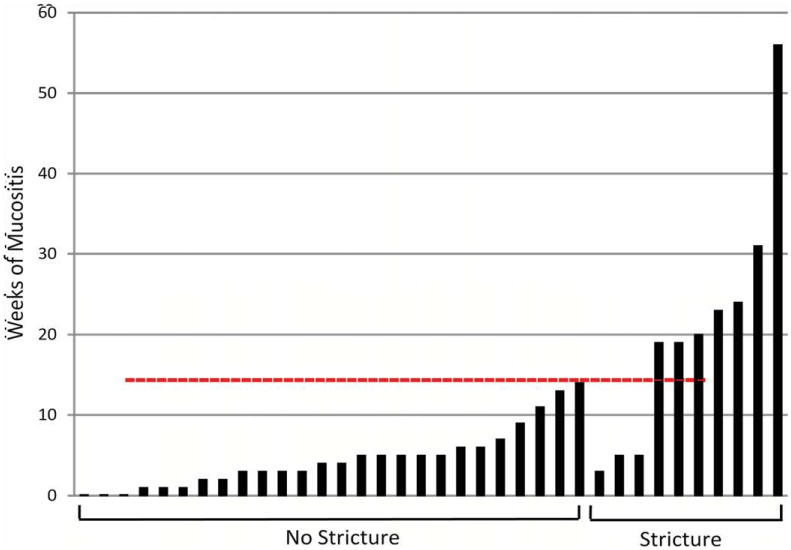
Duration in weeks of severe mucositis after definitive concurrent chemotherapy and radiation therapy for oropharyngeal squamous cell carcinoma. Individual patients sorted by stricture outcome. Severe mucositis defined as grade ≥2, National Cancer Institute Common Toxicity Criteria. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
As 100% of patients in our cohort had prophylactic PEG tubes placed before beginning concurrent chemotherapy and radiation therapy, we examined if the presence of a stricture influenced the short-term or long-term rate of PEG-dependence. Figure 2 plots the rate of PEG-dependence for patients with or without strictures, whereas the estimates for patients with stricture exceed those for patients without stricture at all time points, the curves are not significantly different (p = .14). The 1-year and 2-year PEG-independence rate for patients without strictures was 83% and 93%, respectively, and for those with strictures it was 69% and 77%, respectively. All disease-free patients eventually achieved PEG independence, with a range of PEG dependence from 66 to 1036 days. Four patients with local/regional recurrence died with their PEG in place (indicated by tick marks in Figure 2).
Figure 2.
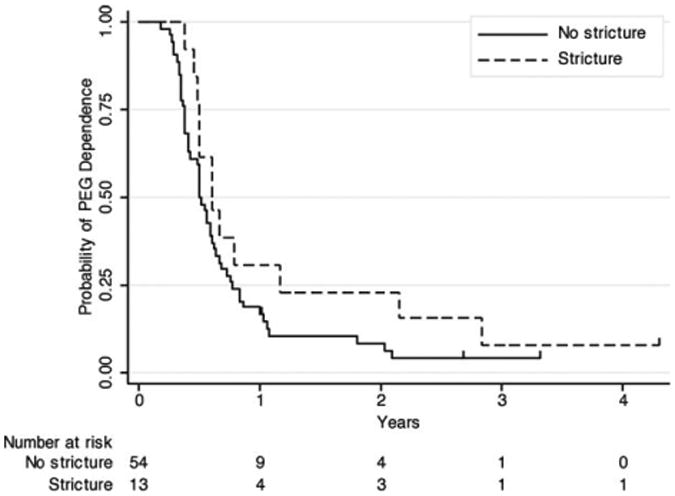
Probability of percutaneous gastrostomy (PEG) dependence after definitive concurrent chemotherapy and radiation therapy for oropharyngeal squamous cell carcinoma. Patients sorted by stricture outcome, censored events (patient died with PEG in place) indicated by tick marks.
Discussion
Comparison with Previous Studies
In the current study, we have studied a homogenously treated patient population with a single diagnosis — SCC of the oropharynx — to identify causative factors that are associated with a subset of these patient developing pharyngoesophageal strictures. We report the novel finding that the duration of treatment-induced mucositis is strongly and independently associated with stricture formation, and that a critical duration of 15 weeks of mucositis strongly predicts stricture formation.
The rate of pharyngoesophageal stricture reported in this study (19%) is similar to other previously reported contemporary series. Although those studies included patients with SCCs located in a variety of subsites, the overall rate of symptomatic strictures lies within a narrow range (21%,4 20.5%,6 and 32%5). Similar to other series, the rate of pharyngoesophageal stricture was determined by diagnostic workup in a subset of patients with persistent dysphagia weeks or months posttreatment and not by routinely performing MBS studies. Therefore, the clinically silent, radiographic rate of stricture may be higher than commonly reported, as some patients likely have functional reserve to overcome the resistance to swallowing imposed by the stricture and do not symptomatically present to healthcare providers.
Our restricted patient cohort has advantages and disadvantages in determining the factors most strongly associated with stricture formation. As every patient in our cohort received twice daily hyperfractionated external beam radiation, we are unable to evaluate previously reported associations with stricture such as twice daily versus daily radiation4 or IMRT dose to superior or middle pharyngeal constrictors.6 The possible effect of the exact radiation fields accounted for in studies using IMRT can only be indirectly accounted for by analyzing the tumor location and nodal disease in this study, as these clinical factors determine the radiation fields. However, the uniformity of radiation dosing and similarity of radiation fields used in planning does allow this study to focus on other radiation-induced factors such as mucositis.
Similarly, because our patient population was limited to oropharyngeal cancer, we cannot evaluate the association between stricture formation and treatment for hypopharyngeal or laryngeal cancers.4,6 However, our cohort did allow for detailed analysis of patient factors associated with stricture formation rather than treatment factors. In the patient population described here, female patients and smoking status did not seem to have an association with stricture formation, as previously reported.4,6
Factors Associated with Stricture Formation
We have identified a novel risk factor for stricture formation—the duration of treatment-induced mucositis during concurrent chemotherapy and radiation therapy in patients with advanced-stage oropharyngeal SCC. This is the strongest association with strictures in our cohort and it seems to be an independent risk factor on multivariate analysis, although due to the small numbers of strictures in our series, these models could not adjust for all possible contributory variables. This finding provides a clear pathophysiologic mechanism for stricture formation— long-term opposing ulcerated mucosal surfaces leading to circumferential scarring—a mechanism that has been proposed to be the cause of stricture formation but without direct evidence. As can be seen in Table 3, the NCI Common Toxicity Criteria that was used to score mucositis in this study specifically addresses ulceration, and the duration of mucositis was calculated by the number of weeks with recorded mucosal ulceration. Further supporting the theory of long-term opposing mucosal ulceration is the finding that although the duration of mucositis is the most important risk factor for stricture formation, the maximum severity of mucositis is not associated with strictures. In addition, we report here a strong duration response with 15 weeks of treatment-induced mucositis as a highly sensitive and specific predictor of ultimate stricture formation.
Table 3.
National Cancer Institute Common Toxicity Criteria.
| Grade | Description |
|---|---|
| 1 | Erythema of the mucosa |
| 2 | Patchy ulcerations or pseudomembranes |
| 3 | Confluent ulcerations or pseudomembranes; bleeding with minor trauma |
| 4 | Tissue necrosis; significant spontaneous bleeding; life-threatening consequences |
Mucositis is a well-described treatment complication of radiation protocols9 and is worsened by the addition of chemotherapy.1,10,11 Its effects range from oral pain and need for increased analgesics to hospitalizations and unscheduled treatment interruptions.12 Significant effort has gone into development of agents to treat or prevent mucositis, including amifostine,13,14 which in the current study was not shown to be associated with a decreased risk of strictures. A Cochrane review of treatments to prevent oral mucositis revealed that few therapies had strong evidence supporting their use, but ice chips and Chinese medicine did show statistically significant treatment effect.15 Novel therapies such as recombinant human growth factors are also being investigated.16,17 To the list of significant side effects of mucositis we can now add the risk of pharyngoesophageal stricture, and this only adds impetus to the efforts to target this complication of concurrent chemotherapy and radiation therapy protocols.
It has been posited by other authors that patients with PEG tubes may be at higher risk of stricture formation because of the decreased need to use pharyngeal musculature to maintain nutrition and hydration.18 Because every patient in our study had a PEG placed, we could not evaluate this association, but in our cohort there was not an association between pretreatment swallow dysfunction and stricture (ie, patients who were dependent on PEGs rather than prophylactic PEGs), nor did patients with strictures have a statistically longer duration of PEG-dependence.
Interestingly, in the current study, we also report an association between the development of pharyngoesophageal stricture and the performance of a neck dissection. Our models did not allow us to confirm this as an independent risk factor, but there is literature to support the association between neck dissection and swallowing complications after treatment for head and neck cancer. A retrospective study of patients treated with primary concurrent chemotherapy and radiation therapy reported a higher incidence of PEG dependence in patients who underwent a posttreatment neck dissection.19 Although soft tissue edema and disruption of cervical rootlets provides an explanation for postoperative dysphagia, it is less clear what role these factors would have in explaining the development of late strictures. Larger patient samples and detailed information about the exact timing of stricture formation are needed to clarify this association.
Oropharyngeal Cancer and Future Directions
HPV-positive oropharyngeal cancer seems to be a distinct clinical entity. Studies show an increasing incidence of HPV-positive oropharyngeal carcinomas despite a decrease in the incidence of SCC in other subsites that mirrors the overall decline in tobacco use.7 It is a disease that has distinct clinical risk factors,20 and evidence from epidemiological studies,21 retrospective studies,22 and prospective trials8,23 indicate that patients who are HPV positive have a better clinical prognosis than patients with tobacco-associated SCC.
As the current study and other large series indicate, the toxicity of concurrent chemotherapy and radiation therapy is nontrivial and experienced by a substantial proportion of patients treated with these protocols. Late complications of treatment including PEG dependence, laryngeal dysfunction, and pharyngoesophageal dysphagia are experienced by up to 43% of patients24 with stricture formation in approximately 20% of patients.
If treatment modalities can be altered to decrease the toxicities in those patients with HPV disease, without sacrificing oncologic success, this would be a critical advancement. In addition to modified organ-sparing protocols, there may also be a role for surgical management, particularly using minimally invasive techniques such as transoral laser25 or robotic technology.26 Detailed information from clinical trials comparing complication rates are needed to guide clinicians in choosing treatment options.
Conclusion
The current study has shown that the duration of treatment-induced mucositis is independently associated with the late complication of pharyngoesophageal stricture. Fifteen weeks of mucositis is strongly associated with stricture formation and has excellent specificity and sensitivity in predicting those patients at risk for this complication. This finding suggests that treating physicians and speech language pathologists may be able to identify these patients before the stricture has formed. Early detection opens opportunities for early therapeutic intervention, and understanding the pathophysiology of stricture formation will hopefully be able to guide therapeutic strategies in the future for those patients treated with aggressive concurrent chemotherapy and radiation therapy protocols.
Acknowledgments
This work was presented at the Poster Session of the Combined Otolaryngology Spring Meeting (COSM), April 28–29, 2010, Las Vegas, Nevada.
References
- 1.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NP, Sallah S, Karlsson U, Antoine JE. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer. 2002;94:1131–1141. doi: 10.1002/cncr.10257. [DOI] [PubMed] [Google Scholar]
- 4.Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808–812. doi: 10.1002/hed.20427. [DOI] [PubMed] [Google Scholar]
- 5.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76:403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(10 Suppl):2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 8.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 9.Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106:329–336. doi: 10.1002/cncr.21622. [DOI] [PubMed] [Google Scholar]
- 10.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 12.Murphy BA, Beaumont JL, Isitt J, et al. Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manage. 2009;38:522–532. doi: 10.1016/j.jpainsymman.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Haddad R, Sonis S, Posner M, et al. Randomized phase 2 study of concomitant chemoradiotherapy using weekly carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancer. Cancer. 2009;115:4514–4523. doi: 10.1002/cncr.24525. [DOI] [PubMed] [Google Scholar]
- 14.Suntharalingam M, Jaboin J, Taylor R, et al. The evaluation of amifostine for mucosal protection in patients with advanced loco-regional squamous cell carcinomas of the head and neck (SCCHN) treated with concurrent weekly carboplatin, paclitaxel, and daily radiotherapy (RT) Semin Oncol. 2004;31(6 Suppl 18):2–7. doi: 10.1053/j.seminoncol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2007;4:CD000978. doi: 10.1002/14651858.CD000978.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Barasch A, Epstein J, Tilashalski K. Palifermin for management of treatment-induced oral mucositis in cancer patients. Biologics. 2009;3:111–116. doi: 10.2147/btt.2009.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson DE, Barker NP, Akhmadullina LI, et al. Phase II, randomized, double-blind, placebo-controlled study of recombi-nant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil-based chemotherapy. J Clin Oncol. 2009;27:4333–4338. doi: 10.1200/JCO.2008.21.2381. [DOI] [PubMed] [Google Scholar]
- 18.Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91:1785–1790. [PubMed] [Google Scholar]
- 19.Lango MN, Egleston B, Ende K, et al. Impact of neck dissection on long-term feeding tube dependence in patients with head and neck cancer treated with primary radiation or chemoradiation. Head Neck. 2010;32:341–347. doi: 10.1002/hed.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Kong W, Peng Y, Miao Q, Mackillop WJ. Temporal trends in the incidence and survival of cancers of the upper aerodigestive tract in Ontario and the United States. Int J Cancer. 2009;125:2159–2165. doi: 10.1002/ijc.24533. [DOI] [PubMed] [Google Scholar]
- 22.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 23.Jo S, Juhasz A, Zhang K, et al. Human papillomavirus infection as a prognostic factor in oropharyngeal squamous cell carcinomas treated in a prospective phase II clinical trial. Anticancer Res. 2009;29:1467–1474. [PMC free article] [PubMed] [Google Scholar]
- 24.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant DG, Hinni ML, Salassa JR, Perry WC, Hayden RE, Casler JD. Oropharyngeal cancer: a case for single modality treatment with transoral laser microsurgery. Arch Otolaryngol Head Neck Surg. 2009;135:1225–1230. doi: 10.1001/archoto.2009.185. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein GS, O'Malley BW, Jr, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133:1220–1226. doi: 10.1001/archotol.133.12.1220. [DOI] [PubMed] [Google Scholar]


