Abstract
BACKGROUND
We sought to define trends in the use of epidural analgesia (EA) for hepatopancreatic procedures, as well as to characterize inpatient outcomes relative to the use of EA.
METHODS
The Nationwide Inpatient Sample database was queried to identify all elective hepatopancreatic surgeries between 2000 and 2012. In-hospital outcomes were compared among patients receiving EA vs conventional analgesia using propensity matching.
RESULTS
EA utilization was 7.4% (n = 3,961). The use of EA among minimally invasive procedures increased from 3.8% in 2000 to 9.1% in 2012. The odds of sepsis (odds ratio [OR] .72, 95% confidence interval [CI] .56 to .93), respiratory failure (OR .79, 95% CI .69 to .91), and postoperative pneumonia (OR .77, 95% CI .61 to .98), as well as overall in-hospital mortality (OR .72, 95% CI .56 to .93) were lower in the EA cohort (all P < .05). In contrast, no association was noted between EA and postoperative hemorrhage (OR .81, 95% CI .65 to 1.01, P = .06).
CONCLUSIONS
EA use among patients undergoing hepatopancreatic procedures remains low. After controlling for confounding factors, EA remained associated with a reduction in specific pulmonary-related complications, as well as in-hospital mortality.
Keywords: Epidural analgesia, Liver resection, Pancreatic resection, Outcome
Epidural analgesia (EA) has been used to manage peri- and postoperative pain among patients undergoing major abdominal surgery over the last several decades.1,2 More recently, there has been increasing interest in locoregional analgesia as EA has become an important component of enhanced recovery after major surgery (ERAS) programs.3 EA has been proposed as a mechanism to help improve the perioperative surgical and anesthetic management of patients undergoing surgical procedures in general. Several studies have suggested that EA may not only improve pain control after surgery,4,5 but is also associated with better surgical outcomes compared with conventional analgesia.6–8 Improved outcomes with EA may be related to the ability of locoregional anesthesia to suppress physiologic surgical stress through blockade of nociceptive afferent nerve signaling while preserving motor function.9,10 Furthermore, less narcotic use with EA may also be associated with better bowel function, preservation of pulmonary function, and earlier ambulation after surgery.1,11
Compared with other general surgical procedures, hepatic and pancreatic surgery has historically been associated with poor pain control and more prolonged hospital stays.12,13 The use of EA may therefore be particularly relevant among this patient population. Witzigmann et al14 reported that patients who had better pain relief following hepatopancreatic surgery had less psychological distress, fewer surgical complications, and faster mobilization. In a small randomized clinical trial, Basu et al15 demonstrated the efficacy of EA among patients undergoing liver resection.
EA, however, has not been universally adopted for patients undergoing hepatopancreatic surgery. While some studies have reported that EA was associated with decreased postoperative complications, shortened length of hospital stay, and less hospital costs,2,16 other reports have suggested that EA may be associated with increased chance of rapid fluid shifts, more intraoperative hypotension, and perhaps higher blood loss.6,12,17 Moreover, epidural hematoma, a potential complication, may dissuade some surgeons from using EA especially in the setting of a major hepatectomy when postoperative coagulopathy may be a concern.18,19
Currently, data on the utilization of EA among patients undergoing hepatopancreatic surgery are limited. Most previous reports on the use of EA among patients undergoing hepatopancreatic surgery have been limited to single institutions, which may not reflect population-based outcomes.7,12,15,20 As such, we sought to evaluate the relative use of EA among patients undergoing hepatopancreatic surgery in a nationally representative dataset. In particular, the objective of the current study was to define trends in the use of EA for hepatopancreatic procedures, as well as to characterize the perioperative outcomes of patients relative to the use of EA vs non-EA analgesia.
Patients and Methods
Data sources and samples
An analysis of the National Inpatient Sample (NIS) database between January 1, 2001 and December 31, 2012 was performed. The NIS database is the largest publicly available all-payer inpatient care database in the United States that is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project. NIS contains deidentified data on patients enrolled in Medicare, Medicaid, private insurances, and the uninsured. The NIS contains nationally representative data on approximately 8 million hospital discharges gathered from about 1,000 hospitals sampled annually, which represents an approximate 20% stratified sample of all the community hospitals in the United States. To increase the information of the total sample of discharge and improve the estimates representing the entire hospitals, NIS was redesigned in 2012 drawing a sample of discharges with sample size of 20% from all hospitals. The NIS collects data on patient demographics, diagnosis codes, procedure codes, and hospital features. Information regarding laparoscopic procedures was available for all time periods; however, data for robotic information was only available since October 2008.21
All patients with an International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) procedure code for liver resection (50.12, 50.22, 50.3, and 50.4) and pancreas resection (52.5, 52.51, 52.52, 52.53, 52.59, 52.6, and 52.7) who underwent elective surgery were included in the analysis. Urgent and emergent cases were excluded. Minimally invasive surgery (MIS) was defined as a composite of laparoscopic and robotic procedures using ICD-9-CM coding (laparoscopy: 54.21, robotic: 17.4, 17.41, 17.42, 17.43, and 17.49). Patients who underwent EA placement for perioperative pain control were identified using ICD-9-CM procedure codes 03.90 and 03.91. All other cases were included in the “conventional analgesia” group. For all patients, demographic-specific data on age, sex, race, payer type, hospital location, hospital teaching status, household income, hospital region, and admission type were collected. The Charlson comorbidity index22,23 was used to assess comorbidities. In-hospital perioperative complications were defined using the corresponding ICD-9-CM diagnostic codes and categorized as sepsis, wound infection, wound complication, bleeding complication, pneumonia, respiratory failure, ileus, thromboembolic events, urinary tract infection, liver failure, cerebrovascular accident, cardiac complication, and postoperative shock. In-hospital mortality and length of stay (LOS) were extracted directly from the database. The composite endpoint of postoperative complications defined as 1 or more perioperative complications or death was used as a primary endpoint for analysis.
Statistical analysis
Descriptive statistics of the study population were reported as frequencies with percentages for categorical variables or median values with interquartile ranges (IQRs) for continuous variables. Standard demographic and clinicopathologic data including age, sex, race, comorbidities, household income, payer type, surgical procedures, hospital size, hospital location, hospital teaching status, and hospital region were analyzed in the study. Chi-square test and Wilcoxon rank-sum test were used for univariable analysis. Multivariable logistic regression and propensity score matching subgroup analysis were used to compare outcomes among patients receiving EA vs conventional analgesia. Propensity score methodology with a nearest neighbor algorithm was used to account for patient and hospital differences among patients receiving EA vs conventional analgesia. The propensity score was calculated using a logistic regression model that included age, sex, hospital bed size, hospital location and teaching status, household income, type of operation, surgical approach (open vs MIS), patient location by county, primary payer, and Charlson comorbidity index. The difference in the composite outcome of greater than or equal to 1 complication or inpatient mortality was estimated before and after propensity matching. We performed sensitivity analyses comparing outcomes among patients who received epidural catheters on the day of surgery vs patients getting the catheter on or after postoperative day 1. All analyses were carried out with STATA version 12.0 (StataCorp, College Station, TX). All tests were 2 sided and a P value less than .05 was considered statistically significant.
Results
Patient and hospital characteristics of cohort from 2000 to 2012
The characteristics of the 53,712 patients (pancreas, n = 28,706, 53.5%; liver, n = 24,349, 45.3%; combined pancreas and liver, n = 657, 1.2%) who underwent elective surgery during the 13-year study period stratified by type of analgesia are shown in Table 1. Among the entire cohort, the median age was 61 years (IQR 51 to 71 years) and 25,602 patients (47.8%) were male. The majority of patients were white (n = 33,558, 76.5%). About one third of patients had a Charlson comorbidity index greater than 3 (n = 17,413, 32.4%). The majority of cases were performed in large (n = 42,529, 79.5%), urban-teaching (n = 44,381, 83.0%) hospitals with most cases being done in the South region (n = 17,939, 33.4%). Most patients had either private (n = 26,143, 48.9%) or Medicare (n = 21,327, 39.9%) insurance. The majority of hepatopancreatic procedures were performed as an open procedure (n = 50,893, 94.7%), whereas the remaining 2,819 cases (5.3%) were MIS procedures. The most common pancreatic procedure was pancreaticoduodenectomy (n = 15,688, 29.2%), while the most common liver procedure was partial hepatectomy (n = 15,930, 29.7%).
Table 1.
Distribution of clinical and demographic data of patients undergoing hepatopancreatic surgery stratified by anesthesia type
| All patients (n = 53,712) | Conventional (n = 49,751) | Epidural (n = 3,961) | P value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 61 (51–71) | 61 (51–71) | 61 (50–70) | .57 |
| Male sex (n = 53,606) | 25,602 (47.8) | 23,676 (47.7) | 1,926 (48.7) | .20 |
| Ethnicity (n = 43,860) | <.001 | |||
| White | 33,558 (76.5) | 30,871 (76.0) | 2,687 (83.4) | |
| Black | 3,739 (8.5) | 3,548 (8.7) | 191 (5.9) | |
| Hispanic | 3,389 (7.7) | 3,226 (7.9) | 163 (5.1) | |
| Other | 3,174 (7.3) | 2,994 (7.4) | 180 (5.6) | |
| Comorbidity index ≥3 | 17,413 (32.4) | 16,099 (32.4) | 1,314 (33.2) | .29 |
| Household income (n = 52,411) | <.001 | |||
| Low | 9,601 (18.3) | 8,940 (18.4) | 661 (17.0) | |
| Medium | 12,198 (23.3) | 11,255 (23.2) | 943 (24.3) | |
| High | 13,566 (25.9) | 12,398 (25.6) | 1,168 (30.1) | |
| Highest | 17,046 (32.5) | 15,939 (32.8) | 1,107 (28.5) | |
| Primary payer (n = 53,425) | .11 | |||
| Private | 26,143 (48.9) | 24,204 (48.9) | 1,939 (49.1) | |
| Medicare | 21,327 (39.9) | 19,736 (39.9) | 1,591 (40.3) | |
| Medicaid | 3,223 (6.1) | 3,018 (6.1) | 205 (5.2) | |
| Self/other | 2,732 (5.1) | 2,517 (5.1) | 215 (5.4) | |
| Surgical approach | .14 | |||
| MIS | 2,819 (5.3) | 2,631 (5.3) | 188 (4.8) | |
| Open | 50,893 (94.7) | 47,120 (94.7) | 3,773 (95.2) | |
| Operation type | ||||
| Pancreas | <.001 | |||
| Proximal pancreatectomy* | 415 (.8) | 382 (.8) | 33 (.8) | |
| Distal pancreatectomy | 9,287 (17.3) | 8,632 (17.4) | 655 (16.6) | |
| Subtotal pancreatectomy | 260 (.5) | 231 (.5) | 29 (.7) | |
| Total pancreatectomy | 1,341 (2.5) | 1,242 (2.5) | 99 (2.5) | |
| Whipple | 15,688 (29.2) | 14,212 (28.5) | 1,476 (37.3) | |
| Others | 1,715 (3.19) | 1,576 (3.2) | 139 (3.5) | |
| Liver | ||||
| Partial hepatectomy | 15,930 (29.7) | 14,984 (30.1) | 946 (23.9) | |
| Lobectomy | 7,570 (14.1) | 7,085 (14.2) | 485 (12.2) | |
| Total hepatectomy | 849 (1.6) | 804 (1.6) | 45 (1.1) | |
| Combined pancreas/liver | 657 (1.2) | 603 (1.2) | 54 (1.4) | |
| Hospital size (n = 53,500) | <.001 | |||
| Small | 3,395 (6.3) | 3,069 (6.2) | 326 (8.2) | |
| Medium | 7,576 (14.2) | 7,038 (14.2) | 538 (13.6) | |
| Large | 42,529 (79.5) | 39,433 (79.6) | 3,096 (78.2) | |
| Hospital location/teaching (n = 53,500) | .006 | |||
| Rural | 1,215 (2.3) | 1,097 (2.2) | 118 (3.0) | |
| Urban nonteaching | 7,904 (14.7) | 7,308 (14.8) | 596 (15.0) | |
| Urban teaching | 44,381 (83.0) | 41,135 (83.0) | 3,246 (82.0) | |
| Hospital region (n = 53,712) | <.001 | |||
| Northeast | 12,034 (22.4) | 11,421 (23.0) | 613 (15.4) | |
| Midwest | 12,179 (22.7) | 10,524 (21.1) | 1,655 (41.8) | |
| South | 17,939 (33.4) | 17,017 (34.2) | 922 (23.3) | |
| West | 11,560 (21.5) | 10,789 (21.7) | 771 (19.5) |
IQR = interquartile range; MIS = minimally invasive surgery.
Duodenum-preserving resection of the head of the pancreas such as Beger’s or Frey’s procedure.
Overall utilization and factors associated with the use of epidural analgesia
Overall utilization was low, as only 7.4% (n = 3,961) of patients undergoing a hepatopancreatic procedure had EA. Certain characteristics such as median age, sex, and Charleston comorbidity index were comparable among patients who did and did not have EA. Other factors were noted to be different. For example, patients who had EA were more likely to be white (odds ratio [OR] 1.59, 95% confidence interval [CI] 1.45 to 1.75), have a higher household income (OR 1.10, 95% CI 1.01 to 1.20), as well as have their procedure performed at a rural hospital (OR 1.37, 95% CI 1.13 to 1.67) (all P < .05). Differences were also seen with respect to payer type, as EA was more likely to be used among patients with private insurance when compared with Medicaid patients (OR 1.18, 95% CI 1.02 to 1.37, P = .03).
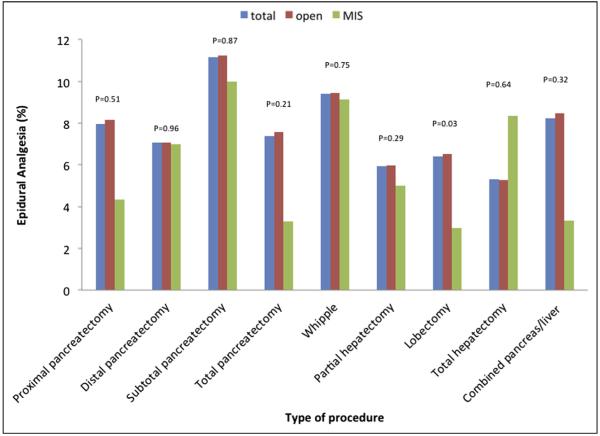
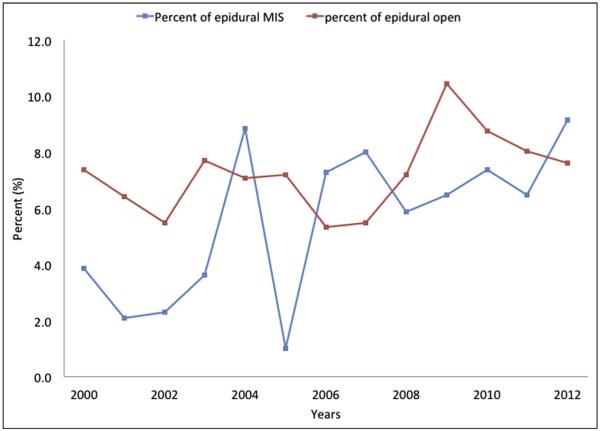
Surgical procedure type was also associated with the odds of EA receipt. Patients undergoing a pancreatic procedure were more likely to have EA vs patients who underwent a hepatic operation (OR 1.43, 95% CI 1.34 to 1.53, P < .001). In addition, there were trends in EA utilization among the different types of pancreatic and hepatic procedures (Fig. 1). For example, patients who underwent a pancreaticoduodenectomy (9.4%) were more likely to have EA compared with patients having a distal pancreatectomy (7.1%) (P < .001). In contrast, among hepatectomy procedures, there was no difference in EA use in patients undergoing a partial hepatectomy (5.9%) vs a major hepatectomy (5.3%) (P = .44). Regarding surgical approach (ie, open vs MIS), EA was preferentially utilized in patients undergoing an open (6.5%) vs an MIS (3.0%) procedure (P = .03). Over the 13 years of the study period, there was no overall increase in EA utilization. Of note, there was, however, an increase in the use of EA among patients who underwent MIS hepatopancreatic procedures (2000: 3.8% vs 2012: 9.1%, P = .004) (Fig. 2).
Figure 1.
Relative use rate of EA over time stratified by receipt of MIS vs open surgery.
Figure 2.
Relative use rate of EA stratified by procedure type.
Perioperative outcomes: impact of epidural analgesia vs conventional anesthesia
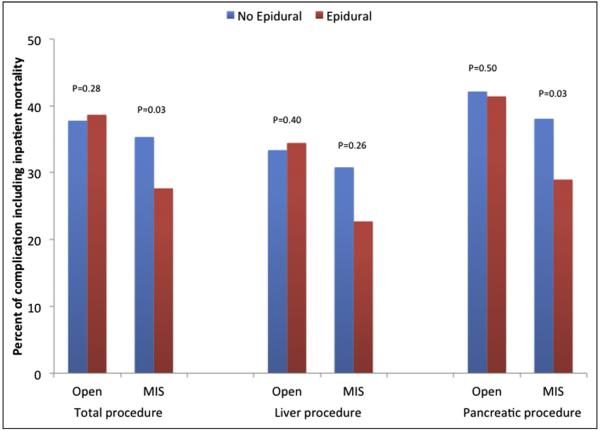
A total of 18,854 patients had at least one complication for a morbidity rate of 35.1%. While there was no difference in the overall incidence of complications in the EA (36.2%) vs non-EA (35.0%) cohorts (P = .12), several complication-specific differences were noted (Table 2). In particular, patients who received EA were less likely to develop sepsis (OR .75, 95% CI .61 to .94), postoperative hemorrhage (OR .79, 95% CI .66 to .94), and liver failure (OR .69, 95% CI .49 to .98) (all P < .05). In addition, EA use was associated with a lower risk of postoperative pneumonia (OR .73, 95% CI .60 to .90) and respiratory failure (OR .89, 95% CI .79 to .99) (both P < .05). Overall, inhospital mortality was 3.2% (pancreatic: 3.2%; hepatic: 2.9%). Receipt of EA was associated with overall lower in-hospital mortality (OR .66, 95% CI .53 to .83). While EA was associated with lower mortality among patients undergoing a pancreatic operation (EA: 2.1% vs non-EA: 3.1%, P < .001), no such association was noted among patients who underwent a hepatic procedure (EA: 2.3% vs non-EA: 3.0%, P = .14). In a subset analysis of only patients who underwent a Whipple procedure, EA had lower in-hospital mortality (EA: 2.9% vs non-EA: 4.2%), respiratory failure (EA: 10.8% vs non-EA: 13.6%), sepsis (EA: 3.0% vs non-EA: 4.8%), and postoperative hemorrhage (EA: 3.5% vs non-EA: 5.2%) compared with the conventional analgesia group (all P < .05). In addition, among patients undergoing distal pancreatectomy, the receipt of EA was associated with lower in-hospital mortality (EA: .5% vs non-EA: 1.8%) and respiratory failure (EA: 5.7% vs non-EA: 8.4%) (both P < .05). Among the subgroup of patients who underwent an MIS operation, the incidence of complications and in-hospital mortality was lower in the EA (27.7%) vs the conventional analgesia (35.3%) cohort (P = .03) (Fig. 3).
Table 2.
Unadjusted perioperative outcomes of epidural analgesia
| OR (95% CI) | P value | |
|---|---|---|
| Any complication | 1.05 (.99–1.13) | .12 |
| Sepsis | .75 (.61–.94) | .01* |
| Wound infection | 1.12 (.98–1.28) | .1 |
| Wound complication | 1.04 (.82–1.31) | .74 |
| Postoperative hemorrhage | .79 (.66–.94) | .01* |
| Respiratory failure | .89 (.79–.99) | .03* |
| Postoperative pneumonia | .73 (.60–.90) | .003* |
| Cardiac complication | .94 (.79–1.11) | .47 |
| Ileus | 1.22 (1.11–1.35) | <.001* |
| Liver failure | .69 (.49–.98) | .04* |
| Thromboembolic event | .95 (.73–1.22) | .66 |
| Postoperative shock | .80 (.52–1.22) | .3 |
| Cerebrovascular accident | .69 (.25–1.88) | .47 |
| Urinary tract infection | 1.08 (.93–1.25) | .31 |
| Transfusion | 1.06 (.98–1.15) | .14 |
| Prolonged length of hospital stay |
1.11 (1.04–1.19) | .003* |
| Nonroutine discharge (n = 51,958) |
1.14 (1.06–1.22) | <.001* |
| In hospital mortality (n = 53,682) |
.66 (.53–.82) | <.001* |
CI = confidence interval; OR = odds ratio.
Prolonged length of hospital stay is defined by greater than or equal to 10 days.
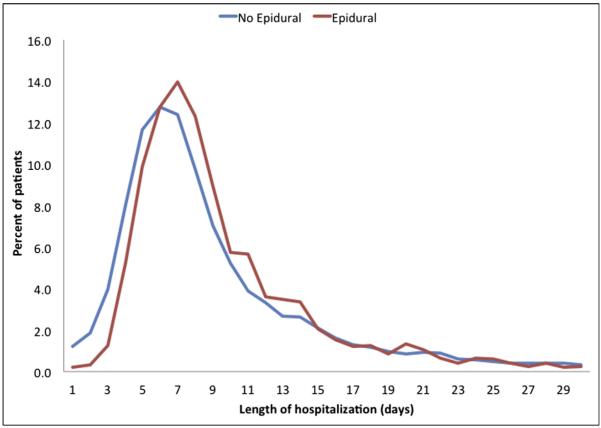
Figure 3.
Length of hospital stay according to anesthesiology type.
Median LOS was 8 days (IQR = to 12 days) (pancreatic: 9 days [IQR 7 to 14 days]; hepatic: 6 days [IQR = to 8 days]). The use of EA was associated with a slightly longer overall median LOS (EA: 8 days vs non-EA: 7 days, P < .001) (Fig. 4). This difference persisted in hepatic procedures (EA: 7 days vs non-EA: 6 days, P < .001) when LOS was stratified by procedure type, while a marginal difference was noted in pancreatic procedures (EA: 9 days [mean 11.7 days] vs non-EA: 9 days [mean 12.4 days], P < .001). Of note, median hospital stay was longer among patients who underwent an MIS procedure (EA: 8 days vs non-EA: 7 days, P < .001). Sensitivity analyses showed no remarkable differences in the likelihood of adverse outcomes or LOS between patients who received epidural catheters on the day of surgery or 1 day prior vs patients who had them placed in the postoperative period.
Figure 4.
Percent of patients experiencing a complication including inpatient mortality according to anesthesia type among MIS and open surgery.
On propensity matched analysis, after matching for patient age, sex, race, payer type, household income, hospital characteristics, comorbidities, and procedure type, patients in the EA group stayed in the hospital about an average .12 days longer than patients in the conventional group (median LOS; EA: 8 [IQR 6 to 12 days; mean 10.49 days] vs non-EA: 8 days [IQR = to 12 days; mean 10.37 days], P < .05). There was no difference in the odds of a blood transfusion among patients treated with EA vs conventional analgesia in the propensity score-matched analysis (OR .98, 95% CI .89 to 1.08, P = .67). Other differences in perioperative outcomes persisted, with patients in the EA cohort having a lower risk of sepsis (OR .72, 95% CI .56 to .93), postoperative pneumonia (OR .77, 95% CI .61 to .98), respiratory failure (OR .79, 95% CI .69 to .91), as well as in-hospital mortality (OR .72, 95% CI .56 to .93) (Table 3). This mortality benefit was noted for pancreas (OR .69, 95% CI .50 to .96, P = .03), while no remarkable benefit was noted for liver operations (OR .77, 95% CI .51 to 1.16, P = .21). On propensity matched analysis, EA use was not associated with an increase in hospital charges (EA: $92,160.9 vs non-EA: $94,374.5, P = .32).
Table 3.
Outcome of epidural analgesia among patients undergoing hepatopancreatic surgery in propensity score-matched cohort*
| Outcome | OR (95% CI) | P value |
|---|---|---|
| Any complication | .98 (.90–1.06) | .63 |
| Sepsis | .72 (.56–.93) | .01 |
| Wound infection | 1.12 (.95–1.32) | .18 |
| Wound complication | 1.07 (.82–1.40) | .62 |
| Postoperative hemorrhage | .81 (.65–1.01) | .06 |
| Respiratory failure | .79 (.69–.91) | .001 |
| Postoperative pneumonia | .77 (.61–.98) | .04 |
| Cardiac complication | 1.01 (.83–1.23) | .94 |
| Ileus | 1.00 (.89–1.13) | .99 |
| Liver failure | .69 (.46–1.03) | .07 |
| Thromboembolic event | .98 (.73–1.33) | .91 |
| Postoperative shock | .90 (.56–1.47) | .69 |
| Cerebrovascular accident | .91 (.26–3.19) | .88 |
| Urinary tract infection | .97 (.81–1.16) | .74 |
| Prolonged length of hospital stay‡ |
1.12 (1.03–1.22) | .01 |
| In-hospital mortality | .72 (.56–.93) | .01 |
CI = confidence interval; OR = odds ratio.
Age, race, sex, hospital bed size, hospital location/teaching status, household income, operation type, surgical approach (open vs MIS), patient location by county, primary payer, and Charlson comorbidity index (conventional analgesia is used as the reference group).
Prolonged length of hospital stay is defined by greater than or equal to 10 days.
Comments
Intravenous and external opioids are commonly employed pharmacologic strategies for pain management during and after major surgery. Unfortunately, opioid use can increase the risk of nausea and vomiting, mental status changes, prolonged ileus, and long-term opioid dependence.24 In contrast, EA may have an opioid-sparing effect in the perioperative period and has well-demonstrated effectiveness for postoperative pain relief and reduction in cardiopulmonary morbidity.1,25 Recently, there has been an increasing interest in EA for pain management during and after major abdominal surgery because of the short- as well as long-term benefits associated with its use.10,26,27 While the use of EA for patients undergoing some surgical procedures such as colectomy has been well documented, its use in hepatopancreatic surgery is much less defined.4,28 This study is important because it defined the overall utilization and outcome of EA among patients undergoing pancreatic or hepatic operative procedures in a nationally representative cohort. Of interest, we found that the overall use of EA among patients undergoing hepatopancreatic surgery was lowdless than 10%. In addition, while there was a modest increase in the use of EA among patients undergoing an MIS hepatopancreatic procedure over time, there was not a dramatic increase in overall EA utilization. Data from the anesthesia literature have similarly suggested no change in overall EA utilization among non-HPB surgical patients.8,28 While EA was associated with a slight prolongation of hospital stay, the increase was of marginal clinical significance (D0.12 days). In addition, EA use was associated with a decrease in certain specific complications such as respiratory failure, postoperative pneumonia, and overall in-hospital mortality.
Utilization of EA remains relatively low for abdominal surgical procedures, as it has not been established as the standard of care for perioperative pain management for these cases. For example, Halabi et al28 reported that EA was utilized in only about 2% of open colorectal surgery procedures between 2002 and 2010 without an obvious increasing trend over that time period. Similarly, in this study, we noted a relatively low use of EA for pancreatic and liver procedures as only 7.4% of patients received EA. The reason for the low use of EA among patients undergoing a pancreatic or liver procedure is likely multifactorial. Institutional practices, additional time, and personnel requirement for preoperative epidural catheter placement, invasiveness of EA, as well as the fear of catheter-related complications may factor into the decision to use EA or not. The concern for exacerbation of intraoperative hypotension may be particularly relevant to hepatopancreatic procedures, as these cases may be more prone to intraoperative blood loss and fluid shifts.7 In addition, there may be a perception that EA can potentially complicate management of venous thromboembolism prophylaxis. For example, Weinberg et al reported on postoperative changes in prothrombin time following hepatic resection that may have implications for perioperative analgesia.29 As such, the belief that a patient’s coagulation profile may be altered after major hepatic resection may contribute to EA’s limited use in hepatic surgery.27,29,30 To this point, data from this study demonstrated the relatively lower utilization of EA use among patients undergoing hepatic surgery (6.1%) compared with pancreatic resection (8.5%) (P < .001).
A recent meta-analysis of randomized controlled trials comparing EA with conventional analgesia after major, open abdominal surgeries in the setting of an enhanced recovery protocol showed no significant difference in morbidity or LOS.31 Moreover, in a previous randomized clinical trial of patients undergoing an open liver procedure, the investigators noted no difference in time to first mobilization or overall morbidity among patients treated with EA vs conventional analgesia.32 In contrast, Nishimori et al5 reported in a recent systematic review that EA did indeed influence the risk of specific perioperative complications. Particularly, the incidence of respiratory failure, gastrointestinal complications, myocardial infarction, and renal complications were reduced among patients who received EA. Concordant with these previous studies, national data on EA use from this study showed no difference in the overall incidence of complications and only a modest increase in LOS of 1 day among patients undergoing a liver operation who were treated with EA. Similar to the data reported by Nishimori et al, we found that EA was associated with a lower incidence of postoperative respiratory failure, postoperative pneumonia, as well as in-hospital mortality compared with the conventional analgesia group. While the lower mortality associated with EA use may have been related to selection bias, we tried to account for many potential confounders by using propensity matching. Of note, when we did propensity matching on age, sex, race, payer type, household income, hospital characteristics, comorbidities, and procedure type, the relative benefit of EA with regard to pulmonary-specific complications and overall mortality persisted. These data strongly suggest that, while EA may not decrease the risk of complications overall, EA may reduce a patient’s chance of pulmonary complications in the perioperative period.
While the use of EA was higher among patients undergoing a pancreatic procedure, overall utilization was still low. Perhaps not surprisingly, EA utilization was somewhat higher among patients who underwent a pancreaticoduodenectomy (9.4%) compared with patients having a distal pancreatectomy (7.1%) (P < .001). The impact of EA on outcomes following pancreatic surgery remains controversial. Pratt et al7 noted increased fluid shifts and excessive intraoperative hemorrhage associated with EA in a retrospective study of patients undergoing pancreatoduodenectomy. In contrast, Amini et al2 reported an overall morbidity incidence of complications that was lower among patients receiving EA compared with conventional analgesia. Based on national data reported herein, we demonstrated that EA use for patients undergoing pancreatectomy was safe and associated with no difference in perioperative outcomes. Specifically, there was no significant difference in the overall incidence of complications among pancreatectomy patients who had EA vs conventional analgesia. While LOS was slightly different with EA use, the difference is likely to be of no clinical significance. Perhaps more importantly, similar to EA use for hepatectomy patients, EA use was associated with decreased postoperative pulmonary complications, as well as improved in-hospital mortality even after propensity matching.
This study had several limitations. As with all studies using administrative data, the accuracy of information was dependent on appropriate coding for procedures and complications.33,34 NIS data are, however, a well-recognized source of accurate in-hospital registry data.21 The NIS do lack data regarding pain levels, patient satisfaction, possible procedure-related complications such as epidural hematoma and hypotension, and timing of discontinuation of EA. As such, none of these important and potentially relevant factors could be assessed relative to EA use. The purpose of this study was not, however, to assess EA efficacy, but rather to define the utilization and in-hospital outcomes of EA vs conventional analgesia.
Conclusions
In conclusion, EA use among patients undergoing liver and pancreatic procedures remains low with fewer than 1 in 10 patients receiving EA. While the use of EA among patients undergoing MIS hepatopancreatic procedures seems to be increasing, its use among open procedures did not change over the time periods examined. While EA use was associated with a slight increase in LOS, the increase is probably of marginal clinical significance. After controlling for several confounding factors using propensity matching, EA remained associated with a reduction in certain specific pulmonary-related complications, as well as overall in-hospital mortality. Future studies comparing EA with conventional analgesia among patients undergoing hepatopancreatic surgery are needed to better define which patients benefit the most from locoregional analgesic approaches in the perioperative setting.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359:1276–82. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 2.Amini A, Patanwala AE, Maegawa FB, et al. Effect of epidural analgesia on postoperative complications following pancreaticoduodenectomy. Am J Surg. 2012;204:1000–4. doi: 10.1016/j.amjsurg.2012.05.022. discussion, 1004–6. [DOI] [PubMed] [Google Scholar]
- 3.van Dam RM, Hendry PO, Coolsen MM, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–75. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 4.Carli F, Trudel JL, Belliveau P. The effect of intraoperative thoracic epidural anesthesia and postoperative analgesia on bowel function after colorectal surgery: a prospective, randomized trial. Dis Colon Rectum. 2001;44:1083–9. doi: 10.1007/BF02234626. [DOI] [PubMed] [Google Scholar]
- 5.Nishimori M, Low JH, Zheng H, et al. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. 2012;7:CD005059. doi: 10.1002/14651858.CD005059.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Sakowska M, Docherty E, Linscott D, et al. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg. 2009;33:1802–8. doi: 10.1007/s00268-009-0131-2. [DOI] [PubMed] [Google Scholar]
- 7.Pratt WB, Steinbrook RA, Maithel SK, et al. Epidural analgesia for pancreatoduodenectomy: a critical appraisal. J Gastrointest Surg. 2008;12:1207–20. doi: 10.1007/s11605-008-0467-1. [DOI] [PubMed] [Google Scholar]
- 8.Pöpping DM, Elia N, Marret E, et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg. 2008;143:990–9. doi: 10.1001/archsurg.143.10.990. discussion, 1000. [DOI] [PubMed] [Google Scholar]
- 9.Richman JM, Wu CL. Epidural analgesia for postoperative pain. Anesthesiol Clin North Am. 2005;23:125–40. doi: 10.1016/j.atc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia: their role in postoperative outcome. Anesthesiology. 1995;82:1474–506. doi: 10.1097/00000542-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Warner DO, Warner MA, Ritman EL. Human chest wall function during epidural anesthesia. Anesthesiology. 1996;85:761–73. doi: 10.1097/00000542-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Page A, Rostad B, Staley CA, et al. Epidural analgesia in hepatic resection. J Am Coll Surg. 2008;206:1184–92. doi: 10.1016/j.jamcollsurg.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang CL, Ye XZ, Zhang XD, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56:667–78. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- 14.Witzigmann H, Max D, Uhlmann D, et al. Quality of life in chronic pancreatitis: a prospective trial comparing classical whipple procedure and duodenum-preserving pancreatic head resection. J Gastrointest Surg. 2002;6:173–80. doi: 10.1016/s1091-255x(01)00023-3. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Tamijmarane A, Bulters D, et al. An alternative method of wound pain control following hepatic resection: a preliminary study. HPB (Oxford) 2004;6:186–9. doi: 10.1080/13651820410030844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Pietri L, Siniscalchi A, Reggiani A, et al. The use of intrathecal morphine for postoperative pain relief after liver resection: a comparison with epidural analgesia. Anesth Analg. 2006;102:1157–63. doi: 10.1213/01.ane.0000198567.85040.ce. [DOI] [PubMed] [Google Scholar]
- 17.Revie EJ, Massie LJ, McNally SJ, et al. Effectiveness of epidural analgesia following open liver resection. HPB (Oxford) 2011;13:206–11. doi: 10.1111/j.1477-2574.2010.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oguro A, Taniguchi H, Daidoh T, et al. Factors relating to coagulation, fibrinolysis and hepatic damage after liver resection. HPB Surg. 1993;7:43–9. doi: 10.1155/1993/91843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzimas P, Prout J, Papadopoulos G, et al. Epidural anaesthesia and analgesia for liver resection. Anaesthesia. 2013;68:628–35. doi: 10.1111/anae.12191. [DOI] [PubMed] [Google Scholar]
- 20.Choi DX, Schoeniger LO. For patients undergoing pancreatoduodenectomy, epidural anesthesia and analgesia improves pain but increases rates of intensive care unit admissions and alterations in analgesics. Pancreas. 2010;39:492–7. doi: 10.1097/MPA.0b013e3181bdfc76. [DOI] [PubMed] [Google Scholar]
- 21.HCUP National Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality R; MD: 2012. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed December 20, 2014. [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40:675–85. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- 25.Yeager MP, Glass DD, Neff RK, et al. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66:729–36. doi: 10.1097/00000542-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg. 2003;238:663–73. doi: 10.1097/01.sla.0000094300.36689.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halabi WJ, Jafari MD, Nguyen VQ, et al. A nationwide analysis of the use and outcomes of epidural analgesia in open colorectal surgery. J Gastrointest Surg. 2013;17:1130–7. doi: 10.1007/s11605-013-2195-4. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg L, Scurrah N, Gunning K, et al. Postoperative changes in prothrombin time following hepatic resection: implications for perioperative analgesia. Anaesth Intensive Care. 2006;34:438–43. doi: 10.1177/0310057X0603400405. [DOI] [PubMed] [Google Scholar]
- 30.Matot I, Scheinin O, Eid A, et al. Epidural anesthesia and analgesia in liver resection. Anesth Analg. 2002;95:1179–81. doi: 10.1097/00000539-200211000-00009. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Hughes MJ, Ventham NT, McNally S, et al. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg. 2014;149:1224–30. doi: 10.1001/jamasurg.2014.210. [DOI] [PubMed] [Google Scholar]
- 32.Revie EJ, McKeown DW, Wilson JA, et al. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB (Oxford) 2012;14:611–8. doi: 10.1111/j.1477-2574.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano PS, Mark DH. Bias in the coding of hospital discharge data and its implications for quality assessment. Med Care. 1994;32:81–90. doi: 10.1097/00005650-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–23. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]