Abstract
OBJECTIVE
To evaluate programmed death ligand 1 (PD-L1) expression in urothelial carcinoma of the bladder in relationship with tumor-infiltrating CD8+ T cells.
MATERIALS AND METHODS
Tissue microarrays were prepared from 56 cystectomy specimens performed at our hospital (1994–2002). PD-L1 immunoexpression was assessed using the murine antihuman PD-L1 monoclonal antibody 5H1. Extent of membranous PD-L1 expression was assigned in each spot. Spots showing ≥5% expression were considered positive. Average PD-L1 expression per tumor was also calculated (5% positivity cutoff). “High CD8 density” was defined as the presence of ≥60 CD8+ intraepithelial lymphocytes per high power field in a given spot. A tumor was considered high density if ≥50% of its spots were of high density.
RESULTS
PD-L1 expression was positive in approximately 20% of tumors. None of the benign urothelium spots expressed PD-L1. High CD8 density was observed in approximately 20% of cases. CD8 density did not correlate with PD-L1 expression. Overall survival (OS) and disease-specific survival (DSS) rates were 14% and 28%, respectively (median follow-up, 31.5 months). PD-L1 expression was associated with age at cystectomy (P = .01). Remaining clinicopathologic parameters were not associated with PD-L1 expression or CD8 density. High CD8 density was associated with favorable OS (P = .02) and DSS (P = .02). The same was true when CD8 density was adjusted for demographic and clinicopathologic parameters. There was no correlation between PD-L1 expression and outcome.
CONCLUSION
High intratumoral CD8+ T cell density is associated with better OS and DSS in invasive urothelial carcinoma of the bladder. We found no correlation between PD-L1 expression and outcome.
Bladder cancer is the fifth most commonly diagnosed malignant neoplasm in the United States.1 The vast majority of newly diagnosed bladder tumors are superficial non–muscle invasive that are prone to recur and ultimately lead to progression.2,3 The majority of disease-related mortality is because of muscle-invasive bladder cancer, with development of metastasis in approximately half of these patients. Radical cystectomy is the most recommended treatment for muscle-invasive bladder cancer, however approximately half of the patients develop metastasis after surgery.4 Identifying molecular biomarkers that can predict prognosis and response to targeted therapy in bladder urothelial carcinoma is needed.
Bladder cancer is known to show an acquired immune dysfunction, particularly affecting lymphocytes.5 Intravesical instillation of bacille Calmette-Guérin (BCG) is an established treatment modality for high-risk non– muscle invasive bladder carcinoma that has been shown to decrease their likelihood of recurrence and progression.6 One-third of patients initially fail to respond to BCG, and up to 74% of initial responders will relapse.7
B7H1 or programmed death ligand 1 (PD-L1) is a cell surface glycoprotein that functions as an inhibitor of T cells and plays a crucial role in suppression of cellular immune response. It is implicated in tumor immune escape by inducing apoptosis in activated antigen-specific CD8+ T cells, impairing cytokine production and diminishing the cytotoxicity of activated T cells.8,9 PD-L1 expression has been demonstrated in several malignancies, such as melanoma and renal cell carcinoma, and was found to be associated with worse prognosis.10 Furthermore, PD-L1 expression is described to be inversely correlated with the density of intratumoral CD8+ T cells.11 Only few studies have addressed PD-L1 expression in bladder cancer.5,12,13 PD-L1 appears as a promising biomarker as new data in immunotherapies targeting the PD-L1 pathway emerge.
In the present study, we evaluate PD-L1 expression by immunohistochemistry in urothelial carcinoma (UC) of the bladder in patients undergoing cystectomy. The relationship between PD-L1 expression, tumor-infiltrating CD8+ cells, and outcome is addressed.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Johns Hopkins University.
Patient Cohort and Tissue Microarray Construction
Fifty-six consecutive formalin-fixed paraffin-embedded (FFPE) cystectomy specimens performed between 1994 and 2002 were retrieved from our archival material. All sections were reviewed for confirmation of the original diagnosis by a urologic pathologist on the study (G.J.N.) and staged according to the 2010 American Joint Committee on Cancer-TNM Classification.14 The 2 tissue microarrays (TMAs) used here were part of a larger set of cystectomy TMAs that we constructed at Johns Hopkins Hospital following a previously described protocol.15 A triplicate tumor sample and paired benign urothelium were spotted from each specimen using 1.5-mm cores.
Of the 56 cases, 50 were invasive cases (46 usual UC, 4 UC with divergent differentiation) and 6 were noninvasive (1 low grade, 4 high grade, and 1 carcinoma in situ). In 52 cases, paired benign urothelium was available for evaluation.
Clinicopathologic Data
All pertinent clinicopathologic data were retrieved from electronic medical records at Johns Hopkins Hospital. These included patient demographics and preoperative information such as treatment modality and clinical stage. Follow-up data on disease-specific survival (DSS) and overall survival (OS) were also obtained.
Immunohistochemistry
Standard immunohistochemistry analysis was performed for PD-L1 using a murine antihuman B7-H1 monoclonal antibody (clone 5H1, isotype mouse IgG1)16 at a concentration of 2 μg/mL according to a standard protocol, the specificity of which has been previously validated.17
Sections were deparaffinized and antigen retrieval was performed using a Tris-EDTA buffer, pH 9.0 at 120°C for 10 minutes in a Decloaking Chamber (Biocare Medical). Endogenous peroxidase, biotin, and proteins were blocked (CSA system K1500, DAKO; avidin/biotin blocking kit SP-2001, Vector Laboratories; Serotec Block ACE, AbD Serotec, Raleigh, NC). The primary antibody was applied and allowed to incubate at 4°C for 20 hours. Secondary antibody (biotinylated antimouse IgG1; 553441 BD) at a concentration of 1 μg/mL was applied for 30 minutes at room temperature. The signal was then developed with amplification according to the manufacturer’s protocol (CSA system K1500; DAKO). Sections were counterstained with hematoxylin, dehydrated in graded ethanol, cleared in xylene, and a coverslip was applied. Tonsil tissue was used as positive control.
Immunohistochemistry for CD8 was conducted according to standard automated methods.18
TMA spots with artifactual folds or lacking tissue target representation were omitted from further analysis. The latter accounted for any variability in number of total evaluable spots among markers.
Scoring System
The extent of membranous PD-L1 expression in tumor cells and benign urothelium was assigned in each spot (0%–100%), guided by the corresponding hematoxylin and eosin–stained TMA section. Spots showing at least 5% expression were considered positive. The average PD-L1 expression was calculated in each case and a 5% positivity cutoff was also used.
High CD8 density was defined as the presence of ≥60 CD8+ intraepithelial cells per high power field in a given spot.18 A tumor was defined as high density if at least half of its spots had high CD8 density.
Statistical Analysis
Findings were analyzed using Stata/SE 11.0 (Stata Corp LP, College Station, TX). Association between CD8 density and PD-L1 expression was assessed using the exact McNemar significance probability test. Proportions were compared using the Fisher exact test. Logistic regression was used for outcome analysis.
RESULTS
Clinicopathologic Characteristics
Demographics and clinicopathologic characteristics are summarized in Table 1. Median follow-up time was 31.5 months (range, 1.37–215 months). Tumor OS and DSS rates were 14% and 28%, respectively.
Table 1.
Demographic and clinicopathologic characteristics of 56 cystectomy patients
| Demographic and Pathologic Features | |
|---|---|
| Age (y) | |
| Median (range) | 67.4 (34.8–89.2) |
| Mean (SD) | 65.8 (11.9) |
| Gender, n (%) | |
| Male | 45/56 (80.4) |
| Female | 11/56 (19.6) |
| Ethnicity, n (%) | |
| Caucasian | 51/56 (91) |
| African American | 5/56 (9) |
| pT category, n (%) | |
| pTa | 2/56 (3.6) |
| pTis | 4/56 (7.1) |
| pT1 | 5/56 (8.9) |
| pT2 | 17/56 (30.4) |
| pT3 | 22/56 (39.3) |
| pT4 | 6/56 (10.7) |
| Lymph node metastasis, n (%) | |
| No | 39/49 (79.6) |
| Yes | 10/49 (20.4) |
| Lymphovascular invasion, n (%) | |
| No | 38 (67.9) |
| Yes | 18 (32.1) |
| Treatment | |
| Neoadjuvant radiotherapy, n (%) | |
| No | 53/55 (96.4) |
| Yes | 2/55 (3.6) |
| Neoadjuvant chemotherapy, n (%) | |
| No | 50/55 (90.9) |
| Yes | 5/55 (9.1) |
| Intravesical therapy, n (%) | |
| No | 26/52 (50) |
| Yes | 26/52 (50) |
| Adjuvant radiotherapy, n (%) | |
| No | 50/55 (90.9) |
| Yes | 5/55 (9.1) |
| Adjuvant chemotherapy, n (%) | |
| No | 32/55 (58.2) |
| Yes | 23/55 (41.8) |
SD, standard deviation.
PD-L1 Expression and Intraepithelial CD8+ Cells
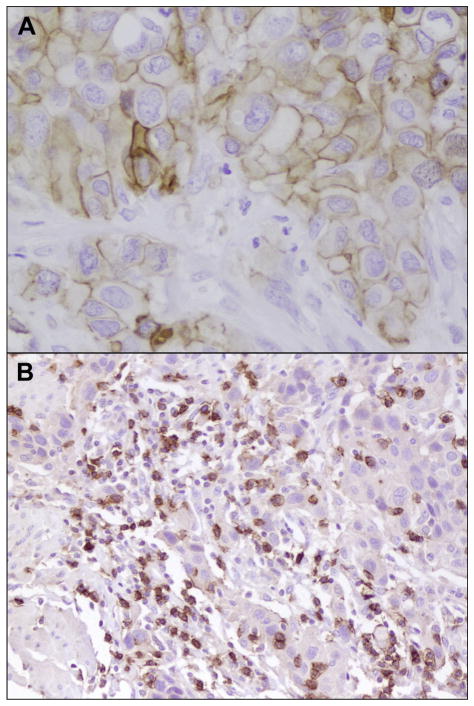
Immunohistochemical PD-L1 expression and CD8 density is depicted in Figure 1.
Figure 1.
(A) Membranous programmed death ligand 1 expression in tumor cells (×400). (B) Intratumoral CD8+ T cells (×400).
PD-L1 expression was positive in 28 of 166 (17%) all examined tumor spots. No benign urothelium or noninvasive tumor spots expressed PD-L1 (0 of 101 and 0 of 15, respectively). We found strong correlation in PD-L1 expression among TMA spots within the same tumor (correlation coefficient >0.6; P < .001). High CD8 density was observed in 29 of 161 (18%) tumor spots, whereas 8 of 97 (8.3%) benign spots had high CD8 density.
There was no correlation between CD8 density and PD-L1 expression (P = .86). High CD8 density was seen in 11 of 27 (41%) PD-L1–positive and in 18 of 134 (13%) PD-L1–negative tumor spots.
Overall, PD-L1 was positive in 10 of 56 (18%) tumors. High CD8 density was observed in 11/56 (19.6%) tumors. There was no difference in high CD8 density among tumors with positive PD-L1 and negative PD-L1 expression (50% vs 13%, respectively; P = .27). The same was true when only the invasive cases were analyzed (40% and 15%, respectively; P = 1). Among the invasive tumors, 10 of 50 (20%) presented positive PD-L1 expression and 10 of 50 (20%) had high CD8 density.
Association of PD-L1 Expression and CD8 Density With Clinicopathologic Characteristics
PD-L1 expression was more frequently seen in tumors from younger patients (P = .01) as shown in Table 2. There was no association between either PD-L1 expression or CD8 density and any remaining clinicopathologic characteristic, such as gender (P = .1 and P = 1, respectively), race (P = .57 and P = .57, respectively), pT category (P = .78 and P = .58, respectively), lymph node metastasis (P = 1 and P = .36, respectively), lympho-vascular invasion (P = .71 and P = 1, respectively), neoadjuvant radiotherapy (P = .3 and P = 1, respectively), neoadjuvant chemotherapy (P = 1 and P = 1, respectively), intravesical chemotherapy (P = .14 and P = .73, respectively), adjuvant radiotherapy (P = .58 and P = .57, respectively), and adjuvant chemotherapy (P = .47 and P = .49, respectively).
Table 2.
Association of clinicopathologic characteristics with PD-L1 expression and CD8 density in 56 cystectomy patients
| Clinicopathologic Parameter | PD-L1 Positive | P Value | CD8 High | P Value |
|---|---|---|---|---|
| Age (y), median (range) | 54.8 (34.8–73.9) | .01* | 58.6 (49–78) | .26 |
| pT category, n (%) | .78 | .58 | ||
| pTa | 0/2 (0) | 0/2 (0) | ||
| pTis | 0/4 (0) | 0/4 (0) | ||
| pT1 | 0/5 (0) | 0/5 (0) | ||
| pT2 | 5/17 (29.4) | 4/17 (23.5) | ||
| pT3 | 4/22 (18.2) | 6/22 (27.3) | ||
| pT4 | 1/6 (16.7) | 0/6 (0) | ||
| Lymph node metastasis, n (%) | 1 | .36 | ||
| No | 7/39 (18) | 6/39 (15.4) | ||
| Yes | 2/10 (20) | 3/10 (30) | ||
| Lymphovascular invasion, n (%) | .71 | 1 | ||
| No | 6/38 (15.8) | 7/38 (18.4) | ||
| Yes | 4/18 (22.2) | 3/18 (16.7) | ||
| Intravesical chemotherapy, n (%) | .14 | .73 | ||
| No | 7/26 (26.9) | 6/26 (23.1) | ||
| Yes | 2/26 (7.7) | 4/26 (15.4) |
PD-L1, programmed death ligand 1.
Significant P value.
Outcome Analyses
Table 3 shows the association between CD8 density and outcome in 50 invasive UCs.
Table 3.
Association between CD8 density and outcome in the subset of 50 cystectomies with invasive (≥pT1) urothelial carcinoma
| CD8 Density | Overall Survival
|
Disease-specific Survival
|
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Unadjusted | 0.12 (0.02–0.68) | .02 | 0.14 (0.03–0.78) | .02 |
| Adjusted for demographic parameters* | 0.1 (0.02–0.69) | .02 | 0.06 (0.01–0.53) | .01 |
| Adjusted for pathologic parameters† | 0.1 (0.01–0.69) | .02 | 0.05 (0.01–0.62) | .02 |
| Adjusted for neoadjuvant therapy‡ | 0.04 (0.004–0.46) | .01 | 0.1 (0.02–0.6) | .01 |
| Adjusted for intravesical therapy | 0.09 (0.01–0.58) | .01 | 0.11 (0.02–0.7) | .02 |
CI, confidence interval; OR, odds ratio.
Age and gender.
pT category, lymph node metastasis and lymphovascular invasion.
Neoadjuvant chemotherapy and neoadjuvant radiotherapy.
DSS was associated with gender (P = .02) and adjuvant chemotherapy (P = .04) in invasive UC. There was no association between any other clinicopathologic feature including age, pT category, presence of lymph node metastasis, lymphovascular invasion, neoadjuvant treatment, and intravesical chemotherapy and outcome.
High CD8 density was associated with improved OS (P = .02) and DSS (P = .02) in invasive UC. It remained as such after adjustment for demographic parameters, pathologic characteristics, neoadjuvant therapy, and intravesical chemotherapy.
There was no correlation between PD-L1 expression and outcome.
COMMENT
Improvement in understanding of tumor-host immune relationship has allowed the identification of signaling pathways that regulate anticancer immune response. Programmed death 1 (PD-1), a member of B7 family, plays a key role in mediating tumor-induced immune suppression19 and is expressed in tumor-infiltrating CD8+ T cells. PD-L1 is a ligand of PD-1 that inhibits immune responses and tumor cell apoptosis induced by antigen-specific CD8+ T cells. It has been shown that reverse signaling through PD-L1 in T cells regulates cytokine production and inhibits survival of activated T cells.8,9 Several clinical trials evaluating the therapeutic role of anti-PD-1 and anti-PD-L1 monoclonal antibodies are currently underway in melanoma, renal cell carcinoma, and non–small cell lung carcinoma.20 Agents blocking PD-1 or PD-L1 are thought to mediate tumor regression by enhancing endogenous antitumor immune responses. An association between membranous PD-L1 expression and tumor response to anti-PD-1 therapy has also been suggested.21
In the present study, PD-L1 expression was demonstrated in 18% of bladder UCs. Our findings are in contrast with those of Nakanishi et al12 who reported membranous and cytoplasmic PD-L1 expression in all 65 UCs analyzed, including 50 bladder cancer, 7 renal pelvic carcinoma, and 8 ureteral carcinoma; with a median percentage of PD-L1–positive cells of 21.1 (range, 2.1%–47.1%). In the latter study, however, frozen tissue rather than FFPE was used and a monoclonal antibody against human B7-H1 (MIH1, mouse IgG1) as opposed to clone 5H1 used in the present study. Inman et al13 found PD-L1 membranous expression in 28% of FFPE bladder cancer using the same clone as our study but a lower cutoff of 1% (ie, any positive cell considered a case as positive). Notably, Boorjian et al,5 like our study, demonstrated a relatively lower PD-L1 membranous expression of 12.4% of UC in a large cystectomy cohort, using the same antibody clone (5H1) applied to FFPE tissue and using a similar 5% cutoff like in the present study. The discrepant results among some of the aforementioned studies could be in part because of the lack of specificity of previously used commercial antibodies, given the documented substantial difficulty in developing reagents and methods for detection of PD-L1 in archival tissue.9 Other factor at play is the difference in scoring strategies used to evaluate PD-L1 expression among the various studies. Like most previous studies on PD-L1 expression,5,10,17 we chose a 5% membranous staining cutoff for PD-L1–positive expression.
Although several large retrospective studies suggested PD-L1 expression to be associated with more aggressive disease in solid tumors,10,22 including bladder cancer,5,12,13 other reports have failed to show such association.23,24 In fact, a more recent study seems to point to a correlation between PD-L1 expression and improved survival as well as influx of lymphocytes into the tumor micro-environment.17 Here, we found no association between PD-L1 expression and outcome. The conflicting findings emphasize the need for additional studies in larger cohorts.
Boorjian et al5 demonstrated decreased expression of PD-L1 and increased expression of PD-1 in patients that received BCG before cystectomy. In contrast, we found no association between PD-L1 expression or CD8 density and any treatment modality analyzed.
The presence of tumor-infiltrating lymphocytes (TILs) has been associated with favorable prognosis in several neoplasms25–27 including bladder cancer.28,29 In agreement, we found that high intratumoral CD8+ T cell density was a significant predictor of favorable OS and DSS.
Tumor cells evade recognition and destruction by the immune system through the PD-L1 pathway.10 Previous studies have demonstrated that PD-L1 expression inversely correlates with TILs.11,13 We found no difference in CD8 density between tumors with positive and negative PD-L1 expression. Our finding further supports that alternate regulators of T cell functions other than PD-1 and PD-L1 pathway, such as cytotoxic T lymphocyte-–associated protein 4, T cell immunoglobulin mucin 3, and lymphocyte activation gene 3, could be implicated.9
The limitations of the present study include the use of TMAs instead of whole tissue sections for evaluation of immunohistochemistry, given the heterogeneity of biomarker expression within areas of the same tumor. However, TMAs provide an efficient high-throughput way to evaluate large number of cases under the same immunohistochemistry conditions. In fact, several studies have supported the value of the TMA usage and the adequate representation of the overall expression levels using multiple TMA spots.30 Nevertheless, the prognostic significance of PD-L1 expression and its interaction with CD8+ T cell density in bladder cancer should be further evaluated using whole sections to further confirm our findings. Another limitation is the cohort size and the retrospective nature of the study. Prospective design will eliminate any potential bias inherent to retrospectively constructed cohort. A validation study of the present study, in 2 additional UC cohorts, is underway.
CONCLUSION
In summary, we found high intratumoral CD8+ T cell density to be associated with favorable OS and DSS in UC. There was no association between high intratumoral CD8+ T cell density and tumoral PD-L1 expression. Furthermore, we found no correlation between PD-L1 expression and outcome.
Acknowledgments
Funding Support: This study was partially supported by the Brady Urologic Institute Patana Fund for Research and the Clinical Innovator Award from Flight Attendant Medical Research Institute.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;2013:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto H, Brimo F, Schultz L, et al. Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Arch Pathol Lab Med. 2010;134:1160–1163. doi: 10.5858/2009-0403-OA.1. [DOI] [PubMed] [Google Scholar]
- 3.Chaux A, Karram S, Miller JS, et al. High-grade papillary urothelial carcinoma of the urinary tract: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Hum Pathol. 2012;43:115–120. doi: 10.1016/j.humpath.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Donat SM, Reuter VE. Management of low grade papillary bladder tumors. J Urol. 2007;178:1201–1205. doi: 10.1016/j.juro.2007.05.148. discussion: 1205. [DOI] [PubMed] [Google Scholar]
- 5.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–4808. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. EORTC Genito-Urinary Tract Cancer Group. The side effects of Bacillus Calmette-Guerin in the treatment of Ta T1 bladder cancer do not predict its efficacy: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol. 2003;44:423–428. doi: 10.1016/s0302-2838(03)00371-3. [DOI] [PubMed] [Google Scholar]
- 7.Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002;167:1573–1583. [PubMed] [Google Scholar]
- 8.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 11.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 14.Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Manual. 7. Official Publication of the American Joint Committee on Cancer; 2010. [DOI] [PubMed] [Google Scholar]
- 15.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2:662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapon M, Randriamampita C, Maubec E, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131:1300–1307. doi: 10.1038/jid.2011.30. [DOI] [PubMed] [Google Scholar]
- 23.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 24.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 25.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 26.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 27.Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liakou CI, Narayanan S, Ng Tang D, et al. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun. 2007;7:10–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol. 2008;26:5630–5637. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]