Abstract
Radiation (RT), temozolomide (TMZ), and dexamethasone in newly diagnosed high grade gliomas (HGG) produces severe treatment-related lymphopenia (TRL) that is associated with early cancer-related deaths. This TRL may result from inadvertent radiation to circulating lymphocytes. This study reinfused lymphocytes, harvested before chemo-radiation, and assessed safety, feasibility, and trends in lymphocyte counts. Patients with newly diagnosed HGG and total lymphocyte counts (TLC) ≥ 1000 cells/mm3 underwent apheresis. Cryopreserved autologous lymphocytes were reinfused once radiation was completed. Safety, feasibility, and trends in TLC, T cell subsets and cytokines were studied. Serial TLC were also compared with an unreinfused matched control group. Ten patients were harvested (median values: age 56 years, dexamethasone 3 mg/day, TLC/CD4 1980/772 cells/mm3). After 6 weeks of RT/TMZ, TLC fell 69 % (p < 0.0001) with similar reductions in CD4, CD8 and NK cells but not Tregs. Eight patients received lymphocyte reinfusions (median = 7.0 × 107 lymphocytes/kg) without adverse events. A post-reinfusion TLC rise of ≥300 cells/mm3 was noted in 3/8 patients at 4 weeks and 7/8 at 14 weeks which was similar to 23 matched controls. The reduced CD4/CD8 ratio was not restored by lymphocyte reinfusion. Severe lymphopenia was not accompanied by elevated serum interleukin-7 (IL-7) levels. This study confirms that severe TRL is common in HGG and is not associated with high plasma IL-7 levels. Although lymphocyte harvesting/rein-fusion is feasible and safe, serial lymphocyte counts are similar to unreinfused matched controls. Studies administering higher lymphocyte doses and/or IL-7 should be considered to restore severe treatment-related lymphopenia in HGG.
Keywords: High grade glioma, Lymphopenia, Radiation, Lymphocyte reinfusion, IL-7
Introduction
Current standard therapy for patients with newly diagnosed with high grade gliomas (HGG) included radiation, temozolomide and glucocorticoids. All of these are associated with significant lymphopenia [1–4]. An NCI funded prospective study that followed serial CD4 counts in 96 patients with newly diagnosed HGG documented severe CD4 lymphopenia (CD4 counts < 200 cells/mm3) in 40 % of patients 2 months after the initiation of concurrent radiation (RT) and temozolomide (TMZ) [5]. Multivariate analysis revealed that this lymphopenia was independently associated with shorter overall survival and that death resulted from tumor progression rather than opportunistic infections. Similar observations have now been reported in patients with treated with chemoradiation for resected or unresectable pancreatic cancers and stage III non-small cell lung cancers [6–9]. Nearly half of these patients developed severe lymphopenia total lymphocyte counts (TLC) < 500 cells/mm3) and those with the lowest lymphocyte counts died early of progressive cancer. In addition, HPV negative head and neck cancer patients with TLC < 500 cells/mm3 2-month after beginning chemoradiation had significantly worse progression free survival than those with higher total lymphocyte counts [10]. Each of these studies suggests that treatment-related lymphopenia (TRL) is common, severe and long-lasting. Of note, the only common modality among the treatment regimens studied is radiation.
The immune system and lymphocytes are important in the prevention and control of cancers [11]. Patients with severe immune deficiencies have a higher incidence of cancer and poorer survival than those with normal immune systems [12, 13]. Even pretreatment lymphopenia has been documented to be a poor prognostic factor in patients with solid tumors and lymphomas [14]. The function of the immune system depends in a large part on interleukins. Interleukin-7 (IL-7) is required for human T cell development and for maintaining and restoring homeostasis of mature T cells. IL-7 is the main homeostatic driver of T cell numbers. IL-7 blood levels are inversely correlated with peripheral T CD4 cell counts, similar to erythropoietin blood levels and their inverse correlation with red blood cells counts. Although IL-7 is limited in normal conditions, it accumulates during lymphopenic conditions [15]. It has been indicated that TGF-β is a significant negative regulator for IL-7 by down-regulating stromal IL-7 secretion [16]. Numerous vaccines and oncolytic virus have been studied in early phase clinical trials in patients with glioblastoma [17]. Reservation of the immune function could be critical in the current stage of cancer immunotherapy.
Recent modeling studies suggest that a standard 60-Gy course of brain radiation administered in thirty fractions will provide potentially lethal doses of radiation to over 95 % of circulating lymphocytes [18]. Recently published studies in pancreatic cancer and lung cancer confirm the associations between radiation volumes, TRL, and survival [8, 9]. These data suggest that inadvertent radiation to circulating lymphocytes passing through a treatment field could be an important factor in the development of TRL. One approach to reducing lymphocyte exposure to radiation could involve removing circulating lymphocytes before radiation and reinfusing them once radiation is complete. The reconstitution of immune status following anti-neoplastic therapy has been investigated in several hematologic malignancies and solid tumors [19–21].
This study was designed to prospectively explore the feasibility and safety of harvesting lymphocytes using a single apheresis and a peripheral line in this patient population. It also sought to determine if reinfusion would provide an increase in lymphocyte counts after lymphocyte reinfusion. A secondary objective was to monitor serial changes in lymphocyte subtypes and serum cytokine levels for a period of 20 weeks after the initiation of treatment. Our study is part of a larger effort that attempts to preserve immune system from the effects of radiation and chemotherapy.
Patient and methods
Patient population
This study was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions (protocol # J11162). Eligible patients were required to have newly diagnosed anaplastic astrocytomas (WHO grade III) or glioblastomas (WHO grade IV) who were scheduled to receive standard concomitant RT/TMZ followed by six cycles of standard adjuvant TMZ. Other eligibility criteria include age >18 years, normal bone marrow function, TLC ≥ 1000 cells/mm3, and Karnofsky performance status ≥60 %. Patients were not eligible if they had received prior radiation or chemotherapy, required anticoagulation, had a new central nervous system bleed, or received antineoplastic agents other than standard radiation and temozolomide.
Lymphocyte harvesting and freezing
Autologous lymphocyte collection was performed 1–10 days prior to initiating standard RT/TMZ. Lymphocytes were collected via peripheral intravenous access with a planned harvest of 2 × 108 lymphocytes with one apheresis procedure. The cells were transferred to conical tubes and centrifuged for 10 min at 2–8 °C at 1200 rpm. The plasma was removed and the cell pellets were re-suspended in cryoprotectant [6 % Hetastarch in 0.9 % sodium chloride injection supplemented with 2 % human serum albumin (HSA) and 5 % DMSO]. The cells were cryopreserved in multiple cryopreservation bags at maximum nucleated cell concentration of 2 × 108 cells/mL. The lymphocytes products were frozen in a controlled-rate freezer at −80 °C and stored in the vapor phase of a liquid nitrogen freezer at less than −135 °C.
Re-infusion of the autologous lymphocytes
Re-infusion of autologous lymphocytes was scheduled to occur within 5 days of completion of concomitant RT/ TMZ. All patients were hydrated with 200 cc of D5 1/2NS over 1 h prior and were pre-medicated 30 min prior to the lymphocyte infusion with diphenhydramine 25 mg intravenously and acetaminophen 650 mg orally. The contents of the cryobags were thawed in a 37 °C water bath, and then within 1 min transferred into a 60 cc syringe and infused intravenously via syringe push through a 3-way stopcock and tubing primed with NS over a 5 min period. The infusion was interrupted for normal saline flushes if the patient experienced discomfort or coolness from the reinfusion. Patients received additional hydration 400 cc of D5 1/2NS over 2 h after lymphocyte reinfusion.
Hematologic monitoring
Heme-8 with differentials were collected prior to lymphocyte harvesting and then weekly for 20 weeks as is standard for this patient population. Lymphocyte subsets and CD4 count were monitored by flow cytometry at the time of lymphocyte collection, prior lymphocyte reinfusion, and biweekly thereafter for 14 weeks. At the same time, serum was collected for cytokine analysis and stored at −80 °C.
Flow cytometry
Flow cytometry was performed using standard whole blood lysis clinical laboratory procedures using IVD reagents. Aliquots of the samples were labeled with anti CD3-FITC, anti CD4 PerCp, and anti CD8 APC (Beckton Dickinson, Mountain View, CA, USA). Analysis was performed on a BD FACScalibur using Cell-Quest software (Beckton Dickinson, Mountain View, CA, USA). Reported values are for the CD3+CD4+, CD3+CD8+, CD56+CD16+ and CD3+CD4+CD25+FoxP3+ lymphoid populations.
Cytokine analysis
The Bioplex 200 platform was used to determine the concentration in pg/mL of multiple target proteins in previously banked serum samples. Luminex bead based immunoassays were performed following the manufacturers protocols and using the supplied cytokine standards the concentrations were determined using five parameter log curve fits using the supplied software. IL-7 was multiplexed using the Bioplex Pro Human Group 1 Panel and TGF-β was assessed using the the Bioplex Pro TGF panel (Biorad, Hercules CA, USA).
Statistical considerations
This study was designed to assess the feasibility of lymphocyte harvesting and reinfusion in patients with newly diagnosed HGG. Feasibility was defined as having at least 5 of the 10 patients (50 %) achieve an absolute increase of ≥300 lymphocytes/mm3 4 weeks after lymphocyte reinfusion. The study was designed to have 83 % power for detecting a minimum of 40 % difference from a null of 10 % using a one-sided type I error rate of 5 %. Patient baseline characteristics were summarized using descriptive statistics. The proportion of patients who achieved a TLC increase ≥300 cell/mm3 at 4 weeks and at 14 weeks after lymphocyte reinfusion were estimated using binomial distribution along with 90 % confidence intervals. Student’s t test and paired t test were used for continuous data between and within group comparison, respectively. Laboratory data was analyzed using Graphpad Prism (GraphPad Software). p values <0.05 were considered statistically significant. All clinical data were analyzed using SAS software (version 9.2).
Results
Patient demographics, baseline, and treatment characteristics
Ten patients were enrolled on this study between July, 2012 and May 2013. Eight patients (80 %) were male, 9 (90 %) had undergone a debulking surgical procedure, 7 (70 %) had a pathologic diagnosis of glioblastoma (WHO grade IV) and 3 (30 %) had anaplastic astrocytoma (WHO grade III). The median age of the enrolled patients was 55.5 years (range 40–67) with a median Karnofsky Performance Status Score (KPS) of 90 (range 80–100). Baseline total lymphocyte counts in these patients ranged from 1060 to 2550 cells/mm3 (median 1980 cells/mm3) and baseline CD4+ counts ranged from 296 to 1369 cells/ mm3 (median 772 cells/mm3).
Lymphocyte harvest
All ten patients underwent lymphocyte collection with a single apheresis using a peripheral intravenous line. One patient (Patient ID #1) had an abbreviated (67 min) collection due to poor IV access. The remaining patients all completed the 2 h apheresis without incident. Seventy percent of the participants (7/10 patients) were taking dexamethasone at the time of lymphocyte harvesting. The median dose was 3 mg/day (range 0–4 mg/day) (Table 1). A median of 8.85 × 107 lymphocytes/kg were collected (range 3.44 × 107–11.13 × 107 lymphocytes/kg) [22].
Table 1.
Baseline characteristics and dexamethasone dosing for all patients
| Patient | Age | Gender | KPS | Histology | Baseline TLC (cells/ mm3) | Baseline CD4 (cells/ mm3) | Total lymphocyte collected (107/kg) | DEX dose at lymphocyte harvest (mg/day) | DEX dose range during radiation (mg/day) | DEX dose at lymphocyte reinfusion (mg/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | F | 100 | Glioblastoma | 1570 | 499 | 3.4 | 0 | 0 | 0 |
| 2 | 40 | M | 100 | Anaplastic astrocytoma | 1060 | 296 | 11.1 | 1 | 0–8 | 8 |
| 3 | 63 | M | 80 | Anaplastic astrocytoma | 1320 | 626 | 6.2 | 0 | 0–4 | 4 |
| 4 | 42 | M | 80 | Glioblastoma | 2270 | 1006 | 9.3 | 0 | 0–4 | 4 |
| 5 | 52 | M | 80 | Glioblastoma | 2370 | 955 | 9.3 | 4 | 1–8 | 8 |
| 6 | 41 | M | 90 | Glioblastoma | 1100 | 450 | 8.1 | 4 | 1–4 | 1 |
| 7 | 60 | M | 90 | Glioblastoma | 1970 | 918 | 9.5 | 2 | 2–4 | 4 |
| 8 | 63 | F | 90 | Glioblastoma | 2290 | 1261 | 6.0 | 4 | 2–4 | 2a |
| 9 | 67 | M | 90 | Glioblastoma | 1990 | 674 | 8.4 | 4 | 2–4 | 2a |
| 10 | 43 | M | 100 | Anaplastic astrocytoma | 2550 | 1369 | 11.7 | 4 | 1–4 | 1 |
| Median | 56 | 80 % male | 90 | 70 % glioblastoma | 1980 | 772 | 8.85 | 3 | 1–4 | 4 |
DEX dexamethasone
Patients #8 and #9 did not receive lymphocyte reinfusion
Lymphocyte reinfusion
Eight of the ten harvested patients underwent lymphocyte reinfusions. Two patients elected to join a vaccine research study and thus did not receive their harvested lymphocytes. The first vaccine in this study was administered after completion of concurrent RT/TMZ. One patient required placement of a PICC line for lymphocyte reinfusion due to poor intravenous access. The remaining seven patients had their autologous lymphocytes infused via a 20 gauge peripheral intravenous line without complication. A median of 6.99 × 107 lymphocytes/kg (range 2.69 × 107–9.47 × 107 lymphocytes/kg) were infused over 20–30 min.
Safety and toxicity
No serious adverse events observed during the lymphocyte collection and reinfusion [22]. All the procedures were performed in the outpatient clinic in the Johns Hopkins Hospital. No infusion reactions, vital sign abnormalities, febrile reactions or pain were noted.
Effects of treatment and lymphocyte reinfusion on CD4+ and CD8+ cells
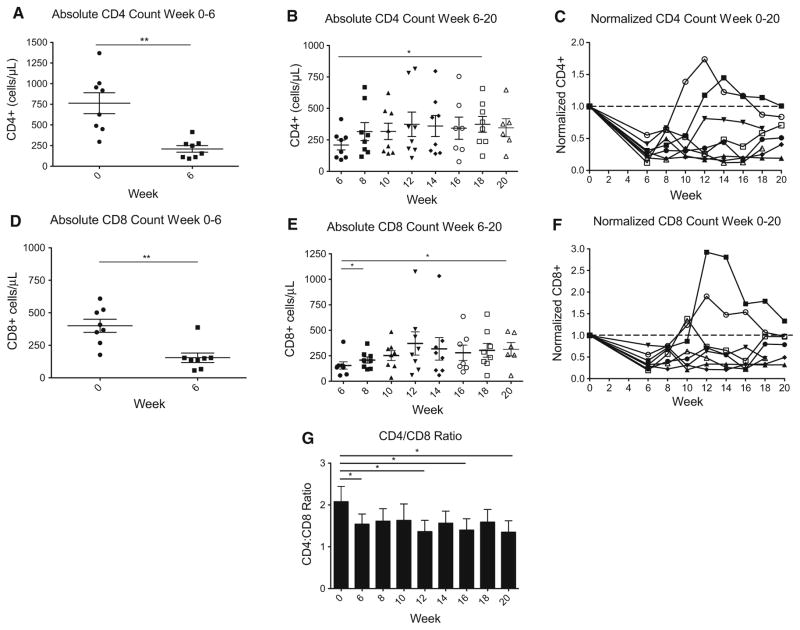
Baseline CD4+ counts in these patients ranged from 296 to 1369 cells/mm3 with a median of 772 cells/mm3. However, after 6 weeks of concurrent RT/TMZ the median CD4+ cell count fell to 202 cells/mm3 (range 92–415 cells/ mm3), representing a 74 % decrease (p = 0.0014, Fig. 1a). The median CD8+ cell count at baseline was 388 cells/ mm3 (range 177–609 cells/mm3). After 6 weeks of RT/ TMZ, the median CD8+ cell count dropped to 142 cells/ mm3 (range 57–388 cells/mm3) which is a 63 % reduction (p = 0.0011, Fig. 1d). After reinfusion, both CD4+ and CD8+ cell counts increased significantly. These post-infusion data are shown in Fig. 1b for CD4+ cells and in Fig. 1e for CD8+ cells. When normalized to baseline, CD8+ cell counts were less profoundly affected by concurrent RT/TMZ as compared to CD4+ cells (Fig. 1c, f). The ratio of CD4+/CD8+ cells was found to be significantly decreased after RT/TMZ at week 6 (p = 0.04), and was not restored by lymphocyte reinfusion (Fig. 1g).
Fig. 1.
Effects of treatment and lymphocyte reinfusion on CD4+ and CD8+ cells. a Absolute CD4+ cell counts from week 0 to 6 while patients were treated with concurrent RT/TMZ; b absolute CD4+ cell counts from week 6 to 20 after lymphocyte reinfusion; c normalized CD4+ cell counts from week 0 to 20 during the study period (normalized to baseline), plotted individually for each patient in the study; d absolute CD8+ cell counts from week 0 to 6 while patients were treated with concurrent RT/TMZ; e absolute CD8+ cell counts from week 6 to 20 after lymphocyte reinfusion; f normalized CD8+ cell counts from week 0 to 20 during the study period (normalized to baseline), plotted individually for each patient in the study; g ratio of CD4+/CD8+ overtime in the patients who underwent lymphocyte harvest and reinfusion asterisk denotes p value < 0.05. Double asterisks denotes p value < 0.01
Effects of treatment and lymphocyte reinfusion on NK and Treg cells
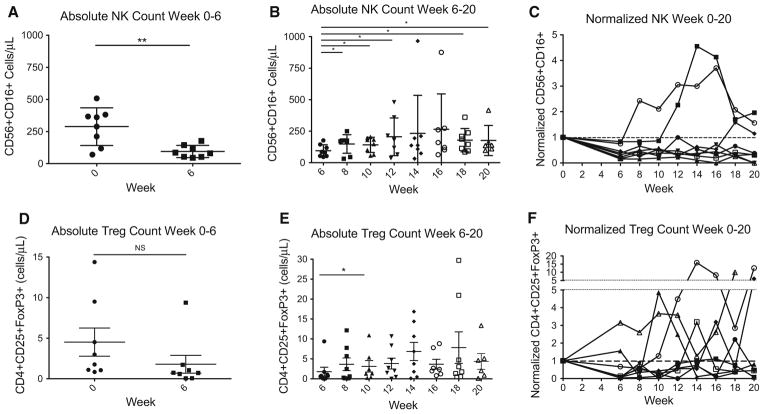
For these studies, Treg were defined as CD3+CD4+CD25HI T cells that co-express forkhead box P3 (FOXP3) via intra-cellular staining. NK cells were defined as CD56+CD16+ lymphocytes. As shown in Fig. 2a, and similar to CD4+ and CD8+ cells, absolute NK cell counts were significantly decreased at the conclusion of RT/TMZ at the 6 week time point (p = 0.0061). After lymphocyte reinfusion, median NK cell counts increased slightly (Fig. 2b). However, when normalized to baseline, the majority of patients did not recover NK cell counts to baseline levels by week 20 (Fig. 2c). Absolute Treg cell counts were not significantly decreased by the 6 weeks of concurrent RT/TMZ (Fig. 2d). In addition, lymphocyte reinfusion had only minor effects on Treg cell counts (Fig. 2e). When Treg cell counts were normalized to baseline, changes in Treg levels were quite variable (Fig. 2f).
Fig. 2.
Effects of treatment and lymphocyte reinfusion on NK and Treg cells. a Absolute NK cell counts from week 0 to 6 while patients were treated with concurrent RT/TMZ; b absolute NK cell counts from week 6 to 20 after lymphocyte reinfusion; c normalized NK cell counts from week 0 to 20 during the study period (normalized to baseline), plotted individually for each patient in the study; d absolute Treg cell counts from week 0 to 6 while patients were treated with concurrent RT/TMZ; e absolute Treg cell counts from week 6 to 20 after lymphocyte reinfusion; f normalized Treg cell counts from week 0 to 20 during the study period (normalized to baseline), plotted individually for each patient in the study asterisk denotes p value <0.05. Double asterisks denotes p value < 0.01
Effects of treatment and lymphocyte reinfusion on IL-7 and TGF-β levels
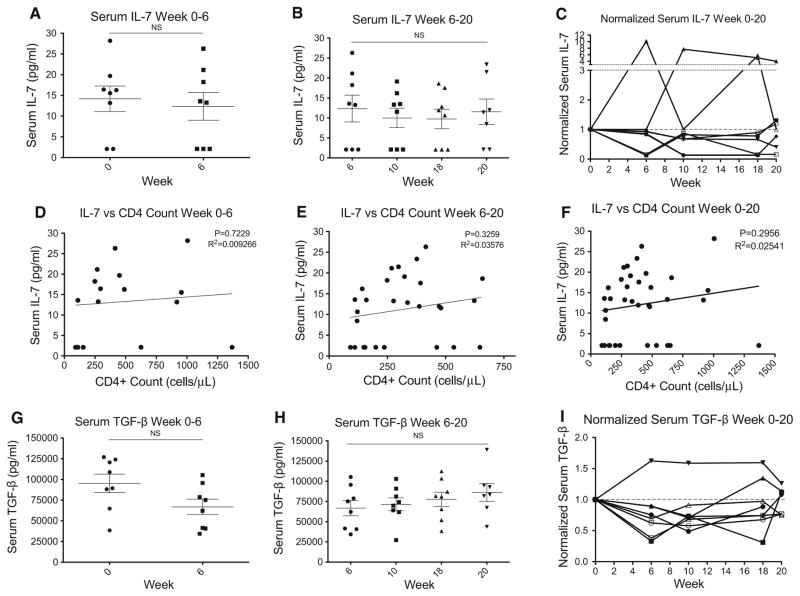
As shown in Fig. 4a, IL-7 did not increase in response to the severe CD4+ lymphopenia induced by concurrent RT/ TMZ during the initial 6 weeks of treatment. IL-7 levels were also not affected by lymphocyte reinfusion (Fig. 3b). Finally, when normalized to baseline levels, IL-7 levels did not increase over the total 20 week study period (Fig. 3c). To test whether IL-7 levels increased in response to decreased CD4+ cell counts on a patient-by-patient basis, we correlated CD4+ cell counts with IL-7 levels during the initial treatment phase (Fig. 3d), post reinfusion (Fig. 3e) and over the total study period (Fig. 3f). None of these correlations were statistically significant. The expected increase in IL-7 in response to CD4+ T cell lymphopenia was not observed [23]. In addition, we did not observe an increase in TGF-β during the first 6 weeks of treatment (Fig. 3g), and lymphocyte reinfusion had negligible effects on TGF-β levels (Fig. 3h) as TGF-β levels remained fairly constant over the entire study period (Fig. 3i).
Fig. 4.
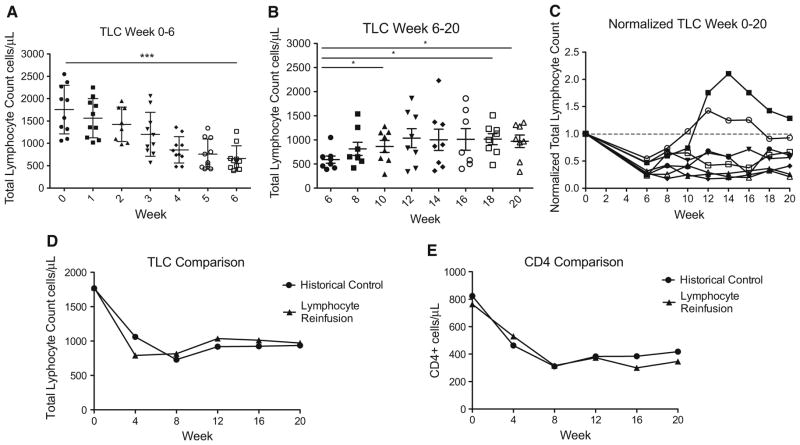
Effects of treatment and lymphocyte reinfusion on total lymphocyte counts. a Absolute total lymphocyte count (TLC) from baseline to the end of concurrent RT/TMZ (weeks 0–6); b TLC changes after lymphocyte reinfusion (weeks 6–20); c normalized TLC throughout the 20 weeks of prospective follow up, plotted individually for each patient in the study (normalized to baseline). d Total lymphocyte counts after lymphocyte reinfusion were not significantly different from matched historical controls; e CD4+ cell counts after lymphocyte reinfusion were not significantly different from matched historical controls asterisk denotes p value < 0.05. Triple asterisk denotes p value < 0.001
Fig. 3.
Serum IL-7 and TGF-β levels after RT/TMZ therapy and lymphocyte reinfusion, suggesting a lack of correlation between serum IL-7 and peripheral CD4+ cell counts. a Serum IL-7 levels (pg/mL) at baseline and 6 weeks (at the end of the RT/TMZ) showing no significant change; b serum IL-7 levels from week 6 to 20 after lymphocyte reinfusion again showing no significant change; c normalized serum IL-7 levels from week 0 to 20 during the study period (normalized to baseline), plotted individually for each patient in the study; d scatterplot showing a lack of correlation between serum IL-7 levels (y axis; pg/mL) and absolute peripheral CD4+ cell counts (x axis; cells/μL) in weeks 0 to 6 in patients who were under concurrent RT/TMZ treatment; e scatterplot showing a lack of correlation between serum IL-7 levels (y axis; pg/mL) and absolute peripheral CD4+ cell counts (x axis; cells/μL) in weeks 6–20 after lymphocyte reinfusion; f scatterplot showing a lack of correlation between serum IL-7 levels (y axis; pg/mL) and absolute peripheral CD4+ cell counts (x axis; cells/μL) in 20 weeks of prospective follow up; g serum TGF-β levels (pg/mL) at baseline and 6 weeks (at the end of the RT/TMZ) showing no significant change; h serum TGF-β levels from week 6 to 20 after lymphocyte reinfusion again showing no significant change; i normalized TGF-β levels from week 0 to 20 during the study period (normalized to baseline), plotted individually for each patient in the study
Effects of treatment and lymphocyte reinfusion on total lymphocyte counts
At baseline, all ten patients had a normal TLC with a median of 1980 cells/mm3 (range 1060–2550 cells/mm3). However, after the completion of 6 weeks of RT/TMZ, the median TLC fell by 69 % to 605 cells/mm3 (range 390–1270 cells/ mm3) (Fig. 4a). The effects of reinfusion on TLC are shown in Fig. 4b. 4 weeks after reinfusion the TLC had increased by an average of 274 cells/mm3 in the eight patients who were reinfused. The primary endpoint of this study was to determine if 50 % of treated patients could achieve an increase in TLC of ≥300 cells/mm3 4 weeks post reinfusion. Three out of the eight patients who received reinfusion (38 %; 90 % CI 11–71 %) reached this mark. However, over the 14 week observation period, seven of eight patients (87.5 %) had an absolute increase of TLC ≥ 300 cell/mm3 and in four of these the increased TLC (57 %; 90 % CI 23–87 %) was sustained through the study (Fig. 4b).
Comparison of total lymphocyte counts with matched controls
As shown in Fig. 4c, normalized data suggested that lymphocyte reinfusion might be associated with a partial recovery of total lymphocyte counts, at least in some patients. To further explore that hypothesis, we retrospectively compared the current dataset to those acquired from a similar group of RT/TMZ treated HGG patients followed without reinfusion on a prior observational study [5]. Twenty-three patients were selected as matched historical controls. Patients were matched by age, performance status, histology, baseline TLC and first month of TLC counts after the treatment was started. As shown in Fig. 4d and e, this post hoc comparison suggested that the lymphocyte harvest and reinfusion protocol did not significantly augment either TLC or CD4+ cell recovery.
Discussion
In recent years, the frequency, severity, duration and consequences of TRL have been increasingly appreciated [5–9]. This association of TRL with survival appears to be independent of tumor histology, pre-treatment prognostic factors, dexamethasone use, or the chemotherapy regimen administered. The single common modality is the use of radiation.
Radiation as a single agent has been known to produce severe prolonged lymphopenia since the 1970s [24, 25]. We have reported that TRL is not seen in patients with non-small cell lung cancer during neoadjuvant chemotherapy but does occur immediately after the initiation of radiation therapy [7]. Recent computer modeling studies demonstrated that a typical 60-Gy radiation treatment plan for patients with HGG delivers sufficient radiation to severely injure 95 % of circulating lymphocytes [18]. These data suggest that radiation is likely to play a major role in TRL.
Consistent with our previous findings, severe lymphopenia was found after 6 weeks of RT/TMZ in this study [5]. Following lymphocyte reinfusion, TLC levels appeared to be higher as 88 % of patients (7/8) had an absolute increase of TLC ≥ 300 cell/mm3 during the 14 week observation period post reinfusion. Fifty-seven percent of these patients (4/7) had sustained increases at the end of the study. However, only moderate increases in CD4+ cell counts were observed. For comparison, we retrospectively constructed a matched historical control of 23 subjects who received identical treatment without lymphocyte reinfusions. Although the sample sizes are small, the average change in TLC was not statistically different in patients who did and did not receive the lymphocyte infusions. These data suggest that a single lymphocyte harvest and reinfusion might not be sufficient to correct. To be noted, the dexamethasone use was not matched between the research subjects and historical controls. Our experience in evaluating treatment-related lymphopenia in patients with other cancers such as pancreatic cancer, head and neck squamous carcinoma and lung cancer where dexamethasone is not routinely used provides results that are remarkably similar to the glioblastoma data. In these patient cohorts, 40–50 % of patients develop grade III–IV lymphopenia and this is related to survival in a multivariate analysis. In these tumor types, dexamethasone was not routinely used [6–8, 10]. There is also abundant data from the 1960s and 1970s that radiation by itself induces profound lymphopenia [18]. Therefore, patients in the control and research group were not matched for dexamethasone use.
Similar to a previous report, we found CD4+ count, CD8+ count and the ratio of CD4+/CD8+ were significantly decreased after RT/TMZ [26]. The CD4+/CD8+ ratio was not restored by lymphocyte reinfusion. These data suggest that CD4+ cells are more likely to be damaged by RT/TMZ and that this lymphocyte loss is not significantly modified by reinfusing autologous lymphocytes collected with one apheresis. Our data also demonstrated significant decreases in the NK cell but not Treg numbers. NK cells appeared to be increased slightly by lymphocyte reinfusion while Tregs remained unaffected. Unfortunately, the matched controls we were using for the total lymphocyte comparisons did not include data on lymphocyte subtypes.
IL-7 is the essential homeostatic driver of peripheral T cell numbers and its level is inversely correlated with CD4+ cell counts [15, 27, 28]. TGF-β expression is increased after exposure to irradiation and it is thought to be a negative regulator for IL-7 levels by down-regulating stromal IL-7 production [16, 29, 30]. We were expecting compensatory changes in IL-7 as patients developed TRL. Although TLC and CD4+ cell counts dropped significantly after RT/TMZ, serum IL-7 levels were not increased. The mechanisms underlying the lack of a compensatory increase in serum IL-7 are unknown [16]. Our data also did not reveal a correlation between IL-7 and TGF-β. In addition, TGF-β levels were not up-regulated by radiation as previously reported [29]. TGF-β levels were not impacted by lymphocyte reinfusion. These findings suggest that the observed lack of compensatory increases in IL-7 in response to TRL were not likely to be mediated by TGF-β. Taken together, these data suggest that IL-7 does not increase in response to severe TRL and that cytokine levels are not significantly affected by either treatment or lymphocyte reinfusion. The lack of a compensatory increase in IL-7 in response to severe TRL raises the possibility that reinfused lymphocytes may not proliferate in an IL-7 deficient host. Exogenous administration of IL-7 could be considered to aid lymphocyte recovery in these patients.
Conclusion
In summary, lymphocyte harvest and reinfusion is feasible in patients with HGG treated with radiation and temozolomide. This approach was not associated with noticeable toxicity during lymphocyte harvest or reinfusion. Overall, an increase in TLC ≥ 300 cells/mm3 was seen in 88 % of reinfused patients during the 14-week observation period post reinfusion. However, in this small series no significant difference for TLC or CD4 cell counts was observed when compared with matched historical controls. Of particular note is our finding that IL-7 levels did not increase in response to lymphopenia as might be have been expected. Future studies involving the administration of IL-7 with or without lymphocyte reinfusion should be considered as an option to modify TRL in this patient population. If IL-7, with or without lymphocyte harvesting and reinfusion, does promote lymphocyte recovery the stage would be set to design and conduct trials to determine if this would have an impact on survival in patients with severe TRL.
Acknowledgments
This study was supported by philanthropic contributions under the auspices of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.
Footnotes
Conflict of interest: None.
References
- 1.Hughes MA, Parisi M, Grossman SA, et al. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balmanoukian A, Ye X, Herman J, et al. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small cell lung cancer. Cancer Invest. 2013;31:183–188. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wild AT, Ye X, Ellsworth S, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2013;38(3):259–265. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Campian JL, Ye X, Sarai G, et al. The association between severe treatment-related lymphopenia and progression free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36:1747–1753. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monforte Ad, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grulich AE. Cancer: the effects of HIV and antiretroviral therapy, and implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4:183–187. doi: 10.1097/COH.0b013e328329c5b2. [DOI] [PubMed] [Google Scholar]
- 14.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sportés C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J, Nuccie BL, Ritterman I, et al. TGF-beta down-regulates stromal IL-7 secretion and inhibits proliferation of human B cell precursors. J Immunol. 1997;159:117–125. [PubMed] [Google Scholar]
- 17.Okonogi N, Shirai K, Oike T, et al. Topics in chemotherapy, molecular-targeted therapy, and immunotherapy for newly-diagnosed glioblastoma multiforme. Anticancer Res. 2015;35:1229–1235. [PubMed] [Google Scholar]
- 18.Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 20.Rapoport AP, Stadtmauer EA, Aqui N, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of Costimulated autologous T cells. Clin Cancer Res. 2009;15:4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Gast GC, Vyth-Dreese FA, Nooijen W, et al. Reinfusion of autologous lymphocytes with granulocyte-macrophage colony-stimulating factor induces rapid recovery of CD4+ and CD8+ T cells after high-dose chemotherapy for metastatic breast cancer. J Clin Oncol. 2002;20:58–64. doi: 10.1200/JCO.2002.20.1.58. [DOI] [PubMed] [Google Scholar]
- 22.Gladstone DE, Davis-Sproul J, Campian J, et al. Infusion of cryopreserved autologous lymphocytes using a standard peripheral i.v. catheter. Bone Marrow Transplant. 2014;49:1119–1120. doi: 10.1038/bmt.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llano A, Barretina J, Gutiérrez A, et al. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J Virol. 2001;75:10319–10325. doi: 10.1128/JVI.75.21.10319-10325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer KK. Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch Surg. 1970;101:114–121. doi: 10.1001/archsurg.1970.01340260018003. [DOI] [PubMed] [Google Scholar]
- 25.Raben M, Walach N, Galili U, et al. The effect of radiation therapy on lymphocyte subpopulations in cancer patients. Cancer. 1976;37:1417–1421. doi: 10.1002/1097-0142(197603)37:3<1417::aid-cncr2820370324>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Ellsworth E, Balmanoukian A, Kos F, et al. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3:e27357. doi: 10.4161/onci.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–2990. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 28.Lundström W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Semin Immunol. 2012;24:218–224. doi: 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 30.Hodge SJ, Hodge GL, Reynolds PN, et al. Increased production of TGF-beta and apoptosis of T lymphocytes isolated from peripheral blood in COPD. Am J Physiol Lung Cell Mol Physiol. 2003;285:L492–L499. doi: 10.1152/ajplung.00428.2002. [DOI] [PubMed] [Google Scholar]