Abstract
Introduction
Metastatic prostate cancer is an incurable disease that is treated with a variety of hormonal therapies targeting various nodes of the androgen receptor (AR) pathway. Invariably patients develop resistance and become castration resistant. Common treatments for castration-resistant disease include novel hormonal therapies, such as abiraterone and enzalutamide, chemotherapy, immunotherapy and radiopharmaceuticals. As this disease generally remains incurable, understanding the molecular underpinnings of resistance pathways is critical in designing therapeutic strategies to delay or overcome such resistance.
Areas covered
This review will explore the resistance mechanisms relevant to hormonal agents, such as AR-V7 expression and others, as well as discussing new approaches being developed to treat patients with castration-resistant prostate cancer that take advantage of these new insights. A literature search was performed to identify all published clinical trials related to androgen therapy mechanisms of drug resistance in metastatic castration-resistant prostate cancer.
Expert opinion
Androgen therapy resistance mechanisms are varied, and include modification of all nodes in the androgen signaling pathway. The optimal treatment for men with relapsed metastatic castration-resistant prostate cancer is uncertain at this time. The authors recommend using available clinical data to guide treatment decision making until more specific biomarkers are clinically available.
Keywords: androgen receptor, AR-V7, hormone resistance, metastatic castration-resistant prostate cancer, treatment sequence
1. Introduction
Metastatic castration-resistant prostate cancer (mCRPC) is the final common pathway for many men diagnosed with prostate cancer and leads to a significant burden of morbidity and mortality for these patients. There is an estimated incidence in the US of 233,000 cases of prostate cancer per year, representing 14% of the total cancer incidence for 2011 [1]. Most patients with mCRPC (about two-thirds or more) are diagnosed after previous treatment for localized, high-risk disease that has progressed. Only a minority of patients (about one-third or less) are diagnosed with metastatic prostate cancer at disease presentation. However, there are many new treatment options that are proven to significantly extend the survival of our patients. Currently, mCRPC is incurable, meaning that disease resistance is the primary challenge of treating prostate cancer today. Our understanding of prostate cancer biology and disease resistance mechanisms has grown over the last few decades. This has translated into improved, clinically meaningful treatment strategies for men with advanced prostate cancer. Two examples highlight this well. A recent Phase III clinical trial demonstrated a median overall survival of 32.4 months for patients receiving ‘first-line’ mCRPC therapy with enzalutamide [2], and the results of the CHAARTED [3] study demonstrated a median overall survival of 57.6 months for patients with metastatic hormone-sensitive prostate cancer (mHSPC) at disease diagnosis. This paper will discuss some of the major mechanisms of androgen pathway resistance and therapeutic strategies that have been demonstrated clinically.
2. Biology of the androgen receptor
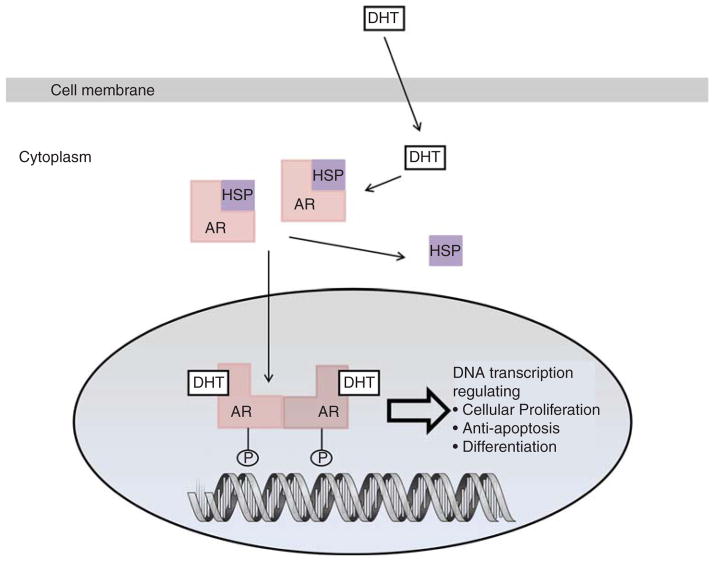
The androgen receptor is a member of the steroid hormone superfamily. It has a C-terminal region that includes the ligand-binding domain (LBD), a DNA-binding domain (DBD), and the N-terminal domain that includes the phosphorylation sites essential for transcriptional activity (Figure 1).
Figure 1. Biology of the androgen receptor signaling pathway.
AR: Androgen receptor; DHT: Dihydrotestosterone; HSP: Heat-shock protein; P: Phosphorylation.
When unbound to a steroid ligand such as dihydrotestosterone, the androgen receptor (AR) is bound to heat-shock proteins (HSP) that prevent ubiquitination and proteasomal degradation [4]. Upon ligand binding, the androgen receptor dimerizes and becomes phosphorylated, resulting in a conformational change that displaces the HSP. The dimerized ligand-bound complex then traffics into the nucleus. Ligand binding is essential for dimerization and translocation of the wild-type AR to the nucleus. After being transported to the nucleus, the AR binds to androgen response elements in promoter or enhancer regions of DNA and mediates transcription and activation of various growth signal pathways and androgen-regulated genes such as PSA. Some evidence suggests that the AR may be transported into the nucleus aboard the microtubule complex [5]. Withdrawal of the ligand in androgen-responsive prostate cells, including hormone-responsive prostate cancer, causes exportation of the AR from the nucleus [4].
3. Mechanisms of androgen resistance
3.1 Androgen receptor gene amplification and overexpression
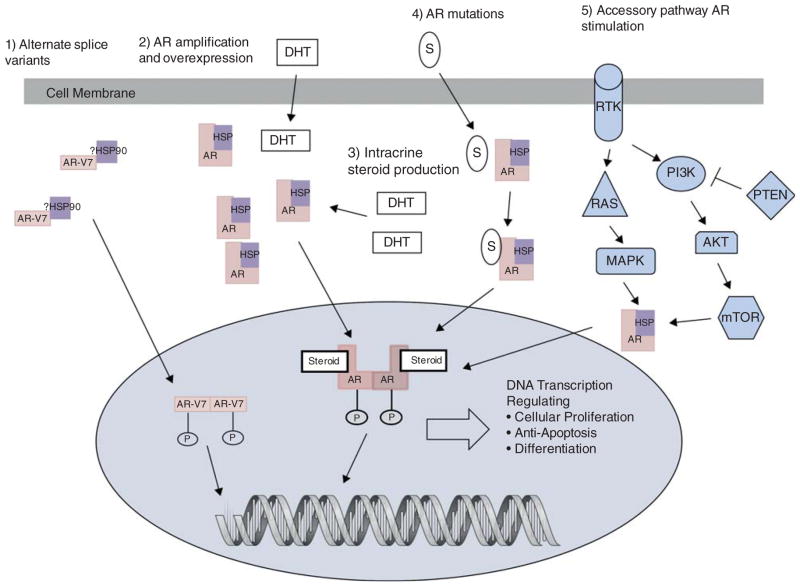
Virtually all patients with metastatic prostate cancer will eventually develop resistance and disease progression despite the treatments currently available. Resistance mechanisms have been described affecting every part of the AR axis, while androgen-independent mechanisms also exist (Figure 2).
Figure 2. Mechanisms of resistance in the AR signaling pathway.
Mechanisms of resistance to androgen deprivation therapy demonstrating AR ligand-dependent and ligand-independent mechanisms.
AR: Androgen receptor; AR-V7: Alternate splice variant 7 of the androgen receptor (it is unknown currently if HSP bind to the AR splice variants); DHT: Dihydrotestosterone; HSP: Heat-shock protein; P: Phosphorylation; RTK: Receptor tyrosine kinase; S: Other steroids (e.g., glucocorticoids).
AR gene amplification and protein overexpression are two mechanisms that have been frequently observed clinically. These changes are predominantly seen in response to treatment with androgen deprivation therapy (ADT). The frequency of AR overexpression and gene amplification has been reported to be low at baseline, but increases significantly when evaluated in the ADT-resistant population. A recent study by Edwards et al. [6] evaluated biopsy samples of twenty patients that were treated with ADT. AR gene copy numbers were compared at baseline versus at the time of disease progression in the same patients: 5 and 15% of patients in the pre-treatment and post-treatment samples demonstrated AR gene amplification, respectively. Another study [7] evaluated biopsy samples from 33 hormone-naive patients compared to biopsy samples from 13 patients with mCRPC. There was only a twofold higher expression of the AR in the mCRPC samples, showing only a small increase to AR protein overexpression. These results indicate that it is difficult to extrapolate these two mechanisms as the sole reason for disease progression on ADT. However, it does highlight the importance of continued androgen signaling in driving cell growth in mCRPC.
Preclinical models of mCRPC suggest that AR overexpression may be a clinically meaningful therapeutic target [8,9]. It has been proposed that supraphysiologic levels of androgens can cause lethal effects on cancer cells that overexpress the AR via double-strand DNA breaks caused by the high levels of androgens [9]. This hypothesis was recently tested in a small pilot study of 14 patients with castration-resistant prostate cancer (CRPC) [10]. Most patients (88%) had progressed on prior second-line hormonal therapy in addition to standard ADT. Patients were maintained on continuous LHRH agonist therapy to suppress endogenous testosterone production and were concurrently treated with three monthly cycles of combination intramuscular testosterone and oral etoposide (2 weeks on, 2 weeks off). Responding patients after three cycles of study drug therapy then continued on LHRH agonist therapy and monthly testosterone injections (without additional etoposide) until the time of clinical or radiographic disease progression. Serum testosterone levels were measured throughout the study. Pretreatment testosterone was suppressed at near-castration levels followed by supraphysiologic levels (mean > 1500 ng/dl) after the testosterone injections. Only 10 patients had RECIST-evaluable lesions at study enrollment. Seven patients had a PSA reduction below the baseline PSA measurement, with four patients (29%) demonstrating ≥ 50% PSA reductions. There was one complete response and four partial responses among the patients with measurable disease. Of the seven patients with PSA reductions, the median time to PSA progression was 221 days. Interestingly, all 10 patients that went on to receive an AR-directed therapy (bicalutamide, nilutamide, enzalutamide or abiraterone) following high-dose testosterone treatment had a favorable PSA response suggesting that these patients were re-sensitized to hormonal therapy by the high-dose testosterone therapy. These promising results prompted the initiation of two larger Phase II clinical trials (one of which aims to test whether men failing abiraterone or enzalutamide might become re-sensitized to these agents again after an intervening period of high-dose testosterone therapy (NCT02090114) [11]; and the other randomizing abiraterone-refractory patients to enzalutamide versus high-dose testosterone with a crossover permitted (NCT02286921) [12]. This is a novel approach with the potential advantage of reversing some resistance mechanisms of current hormonal therapies, while lengthening the time of clinical benefit for therapies that target the AR axis and improving quality of life.
3.2 Continued intracrine and paracrine androgen production
A major advance in the understanding of CRPC was the discovery of continued AR stimulation in tumors despite ‘castrate’ levels of circulating testosterone. Recent autopsy studies and tissue biopsy studies have demonstrated high levels of androgens in tumor tissue of men with CRPC [13,14]. This is thought to result from conversion of weak androgens to potent androgens such as dihydrotestosterone (DHT), or de novo production of androgens from cholesterol within the tumor tissue itself [15,16]. The role of intratumoral androgens driving CRPC cancer growth is buttressed by the results of clinical trials testing testosterone concentrations in patient tumor biopsies and in clinical trials targeting the AR axis with novel anti-hormonal therapies. Two novel hormonal agents are widely used in clinic. Enzalutamide is a pure inhibitor of the AR LBD without any known agonistic properties to the wild-type AR, resulting in decreased nuclear translocation of the AR, decreased DNA binding to androgen response elements, and decreased transcription of AR-responsive genes. Abiraterone is a selective and irreversible inhibitor of the CYP17 enzymes (17alpha-hydroxylase and C17, 20-lyase) resulting in decreased production of testosterone precursors, dehydroepiandrosterone (DHEA) and androstenedione. Both these agents have been tested in men with metastatic CRPC both pre-chemotherapy [17,18] and post-chemotherapy [19,20] with improvements in overall survival observed compared to placebo in large, Phase III clinical trials.
Two notable articles by Efstathiou et al. [21,22], demonstrate that intratumoral production of testosterone persists in the castration-resistant state. The first study [21] evaluated tissue samples in 57 patients prior to treatment with abiraterone, and again after initiation of therapy. Patient testosterone concentrations were tested from blood samples and bone marrow aspirates. CYP17 expression was evaluated in bone marrow biopsy samples. Testosterone and DHEA concentrations decreased to undetectable levels in nearly all patients in both the bone marrow and serum samples. Patients with high expression of CYP17 based on immunohistochemical staining in the pretreatment marrow samples tended to have a longer duration of benefit with abiraterone. The second study [22] was similar in design but evaluated patients being treated with enzalutamide. Testosterone levels increased as a compensatory mechanism in the blood and bone marrow, as expected from enzalutamide’s mechanism of action as a direct AR antagonist. The AR localization shifted from the nucleus to the cytoplasm in eight patients who demonstrated a PSA response to enzalutamide. Patients with overexpression of the AR or CYP17 were more likely to have clinical benefit to enzalutamide in this study.
3.3 AR splice variants
Comparing the response rates of enzalutamide in the pre-chemotherapy setting [17] (PREVAIL) to the post-chemotherapy setting [19] (AFFIRM) is instructive, and suggests that benefit to enzalutamide is blunted after prior docetaxel use. The PREVAIL study evaluated 872 patients on enzalutamide compared to 845 patients on placebo. The primary end points were overall survival and radiographic progression-free survival (PFS). OS was improved with enzalutamide therapy, 32.4 months versus 30.2 months (HR 0.71; 95% CI 0.6 – 0.84; p < 0.001). Radiographic PFS at 12 months follow-up was also improved, 65 versus 14% (HR 0.19; 95% CI 0.15 – 0.23; p < 0.001). The AFFIRM study evaluated 800 patients on enzalutamide and 399 patients on placebo. The primary end point of improved OS was achieved, 18.4 versus 13.6 (HR 0.63; 95% CI 0.53 – 0.75; p < 0.001). However, 9% of patients in the PREVAIL study and 21% of patients in the AFFIRM study demonstrated a rising PSA as their best response to enzalutamide. This increased rate of primary refractory disease likely demonstrates acquired resistance from prior treatment, and one possibility for this innate resistance is the presence of constitutively active AR splice variants.
Recent studies point towards alternate splice variants of the AR as one explanation of acquired and de novo resistance to hormonal therapies [23]. AR splice variants have a preserved N-terminal domain but have a truncated C-terminal domain, resulting in ligand-independent constitutive activation. The best studied of these variants is AR-V7. There are many pre-clinical studies testing the effect of these variants in cell line models and animal models, but until recently their clinical significance was unclear [24–27]. A recent prospective study reported clinical results of enzalutamide or abiraterone treatment in patients positive for AR-V7 compared to patients without AR-V7 expression in their circulating tumor cells (CTCs) [28]. Eighteen of the 62 patients tested positive for AR-V7 at baseline (12/31 enzalutamide-treated patients, 6/31 abiraterone-treated patients). None of these 18 AR-V7-positive patients had a PSA response with enzalutamide or abiraterone, compared to AR-V7-negative patients who had a 53 and 68% PSA response rate to enzalutamide and abiraterone respectively. Also all the patients with detectable AR-V7 at baseline remained positive for AR-V7 at the time of progression, while six patients who were AR-V7-negative at baseline converted to AR-V7-positive during the course of therapy with enzalutamide or abiraterone. These patients who converted had worse clinical outcomes compared with the cohort that remained AR-V7-negative throughout treatment with enzalutamide or abiraterone. A second study [22] evaluated men for AR-V7 at the protein level from bone marrow biopsies prior to treatment with enzalutamide and after 8 weeks of therapy, with similar results. AR-V7 protein was detected in 57% of patients (8/14), all of whom had primary refractory disease to enzalutamide (defined as disease progression within 4 months). Of the seven patients in the study who were on treatment for longer than 6 months, no patients tested positive for AR-V7 protein in bone marrow.
These data are now being confirmed within the context of a larger 5-center study in which three CTC-based AR variant assays will be compared to each other in terms of their ability to predict responses or resistance to AR-directed therapy and chemotherapy (NCT02269982) [29]. Finally, the clinical relevance of AR-V7 was also examined in the context of taxane chemotherapy, whereby 37 patients embarking on docetaxel or cabazitaxel therapy were evaluated for baseline detection of AR-V7 using the Johns Hopkins CTC-based mRNA assay described above. In this small prospective study, the presence of CTC-derived AR-V7 did not appear to be associated with primary resistance to taxane agents [30]. The PSA response rate to taxanes in the 17 patients testing positive for AR-V7 was 41 vs 65% in the 20 AR-V7 negative patients (p = 0.19). PFS estimates to taxane therapy also appeared comparable among AR-V7-positive and -negative patients. This is in contrast to preclinical data reported by Thadani-Mulero et al. where cell-line and murine model data demonstrated no growth inhibitory effects of docetaxel in AR-V7 positive prostate cancer [31]. Interestingly, some preclinical data suggest that AR-V7 activity may be regulated through the PI3K/AKT/PTEN pathway via FOXO1 [32]. This emphasizes the complex interactions between the signaling pathways in prostate cancer, making treatment outcomes difficult to predict from preclinical models alone.
3.3.1 Androgen receptor point mutations
Point mutations in the AR have previously been demonstrated to alter the effect of AR signaling. Such point mutations are more commonly found in tumors that are castration-resistant compared with hormone-sensitive tumors, with a recent review of 27 previous reports finding an incidence of 10 – 50% compared with 0 – 44%, respectively [33]. The total number of patients in each of these 27 studies is small, with the number of patients ranging from 5 – 54. Mutations in the AR have a variety of effects including loss of function, no change, increased and decreased AR signaling, with most mutations studied resulting in loss of function when studied in cell culture models [34]. Point mutations to the AR in prostate cancer are somatic events, with most being located in the LBD [35]. Gain-of-function mutations result in nonspecific activation of the AR by weak androgens, progesterones, glucocorticoids, estrogens and anti-androgens. These mutations seem to arise in consequence to specific treatment pressure that is not necessarily cross-resistant between hormonal therapies, but can be. Many mutations have been reported previously. Three examples are highlighted here.
The F876L mutation, as demonstrated in cell line models [36,37] and from patient tissue samples [38] arises in the setting of enzalutamide therapy and converts this agent into an agonist. In the laboratory, induction of resistance to enzalutamide has been shown in LNCaP cells by Korpal et al. [36]. The authors created enzalutamide resistant clones spontaneously by culturing the cells with enzalutamide for an extended time (> 1.5 months). The authors used an RNA gene expression array assay to test for resistance mechanisms, discovering that all resistant clones expressed the AR F876L mutation. This mutation was shown to be sufficient for development of enzalutamide resistance through inducible gene expression assays. This mutation has been demonstrated clinically as well. Azad et al. [39] studied 62 patients progressing on abiraterone, enzalutamide or other therapy. Cell-free circulating DNA from patient plasma was tested for known mechanisms of drug resistance. Missense AR mutations on exon 8 (the location of the LBD) were present in 11 of 62 (18%) of patients. Six different mutations were identified, including F876L which was present in two patients progressing on enzalutamide. The F876L mutation does not confer resistance to all AR antagonists. Korpal et al. found that the ARF876L cells retained sensitivity to bicalutamide despite resistance to enzalutamide in their in vitro assays. There is some cross-resistance though among AR antagonists to F876L expression. In the Azad study [39], the F876L mutation was also found in patients progressing on ARN-509 which is structurally very similar to enzalutamide. Additionally, Joseph et al. [38] found that enzalutamide and ARN-509 have agonistic activity in F876L engineered LNCaP cell lines. They also tested circulating tumor DNA from plasma samples in patients treated with ARN-509. Three of 29 patients had induced expression of F876L after treatment with ARN-509, compared with no expression prior to treatment.
The T878A mutation is another type of AR mutation, which arises in the setting of androgen synthesis inhibitor treatment. As a consequence of CYP17 inhibition, the production of DHEA and testosterone is suppressed but upstream progesterone production (converted from pregnenolone) is increased. This increased progesterone concentration may select for the T878A mutation. This mutation confers agonistic activity of progesterone on the AR. Chen et al. [40] analyzed metastatic tumor tissue biopsy samples using targeted sequencing of the AR in 18 patients with mCRPC who progressed on CYP17 inhibitors (17 abiraterone and 1 patient on ketoconazole). Three of the 18 were found to express the T878A mutation at a high allele frequency. This mutation is not pan-resistant to all antiandrogens, as bicalutamide demonstrated preserved efficacy in one patient identified with a T878A mutation who previously progressed on flutamide [41].
Different AR point mutations can also result in glucocorticoid activation of the AR [42,43]. The L702H mutation, for example, has been identified in patients taking abiraterone with dexamethasone or prednisone. Carreira et al. [44] sequenced the coding regions of the AR using cell-free circulating DNA in 16 patients prior to treatment with abiraterone, during treatment, and after disease progression with abiraterone. Two of 16 patients were found to express the L702H mutation. One patient, who expressed this mutation prior to treatment with abiraterone, had primary refractory disease to subsequent treatment with abiraterone. The authors also note that using an in vitro luciferase reporter assay confirmed that these cells were also resistant to enzalutamide, and demonstrated AR signaling activation by prednisolone.
Currently, there are no FDA approved treatment approaches taking advantage of AR mutational status. However, active research is implementing these techniques in clinical trials. For example, there is an ongoing clinical trial (NCT01949337) [45] combining abiraterone and enzalutamide based on pre-clinical data that a combination approach will overcome some resistance secondary to AR mutations. If this work of using blood-based tests to identify AR mutations in CRPC is clinically validated, then clinicians to may have accessible biomarkers for individual treatment selection and perhaps improve the clinical outcome for our patients.
3.4 Interplay between PI3K/AKT/mTOR pathway and AR axis
Complementary signaling pathways may also drive prostate cancer growth in conjunction with the AR. For instance, the PI3K-AKT-mTOR pathway and the RAS/RAF pathway have been shown to be mutated frequently in CRPC. PI3K is an intracellular kinase that is activated by G-protein coupled receptors or receptor tyrosine kinases. Activation of PI3K leads to phosphorylation of AKT and subsequently activation of mTOR, a serine/threonine kinase. This leads to downstream effects including cellular proliferation, survival and angiogenesis. An evaluation of 218 prostate tumor samples found inactivating mutations along the PI3K pathway in 42% of localized tumors compared with 100% of meta-static tumors. In addition, 43% of localized tumors demonstrated RAS/RAF signaling activating mutations compared with 90% in metastatic tumors [46]. Mouse models and cell line studies using LNCaP cells have shown that alterations in PI3K and PTEN activity using either targeted drugs or gene knock-out techniques demonstrate changes to AR expression and AR transcriptional activity [47–49]. Because of this association, there has been intensive investigation in therapy co-targeting these signaling pathways. There are many other AR-independent mechanisms of resistance. However, this paper will evaluate the PI3K pathway and angiogenesis as illustrative examples since these two areas are well studied clinically in prostate cancer.
Kruczek et al. [50] evaluated the effect of temsirolimus, an mTOR inhibitor, in 21 patients with chemotherapy-naïve CRPC. The primary outcome was a composite of overall response rate plus stable disease. The secondary end points evaluated were the percentage of patients with a ≥ 50% PSA decline and time to radiographic or PSA progression. Overall clinical benefit was seen in 10 of 15 patients (67%), with 8 of those patients experiencing stable disease and none having a complete response. The median time to progression was 2 months and only one patient having a ≥ 50% PSA reduction. The study was terminated early since no significant signal of activity was seen. Single-agent temsirolimus is unlikely to be of major clinical benefit in CRPC despite good pre-clinical evidence for mTOR as a target. Similar unimpressive clinical outcomes were observed in a study with perifosine, an AKT inhibitor, as single-agent therapy in patients with non-metastatic, hormone-sensitive disease after local therapy. Only 5 of 24 patients had a PSA reduction in that trial [51].
Temsirolimus has also been studied in 11 chemotherapy refractory CRPC patients [52]. The primary outcome was a reduction in the number of circulating tumor cells with secondary end points of overall survival, PFS and PSA/ radiographic response rates. This was a heavily pre-treated population with over half of the patients progressing on two or more lines of chemotherapy. Most of the men in the study did not have a favorable decline in the number of circulating tumor cells. The median PFS was unimpressive at 1.9 months. The authors concluded that temsiroliums as a single agent has poor clinical activity in chemotherapy-pretreated patients with CRPC.
Angiogenesis is noted to be important to progression of metastatic disease in many cancers, including prostate cancer [53,54]. Sunitinib is a multi-target kinase inhibitor, including VEGFR with preclinical studies showing marked growth inhibition of prostate cancer in cell line models [55]. Recently, a large randomized Phase III clinical trial reported results using sunitinib in patients with chemotherapy-resistant mCRPC [56]. 873 patients who previously progressed on docetaxel were treated with sunitinib plus prednisone (n = 584) versus prednisone plus placebo (n = 289). The primary end point was overall survival, with secondary end points of radiographic PFS, objective response rate and safety. OS was not different between the groups, 13.1 months (95% CI 12.0 – 14.1 months) for sunitinib vs 11.8 months (95% CI 10.8 – 14.2 months) placebo with a hazard ratio of 0.914 (p = 0.168). However, the PFS was slightly longer with sunitinib versus placebo, 5.6 months and 4.1 months, respectively (p < 0.001). Similar to many trials with single-agent targeted therapy, no complete responses were observed. The adverse events were as expected from sunitinib. Because of the multi-targeted effect of sunitinib, it is not clear at this time if the failure of this drug to achieve a meaningful improvement in OS is related to failure to adequately suppress activity of the VEGFR pathway or some other pathway, such as PDGFR or c-Kit.
Why have the clinical trials using single-agent targeted therapies been disappointing thus far? This could be explained by clinical trial designs using unselected patient populations rather than selecting for patients with genetic alterations in the intended target. Also, single-agent targeted therapy may not be as effective in advanced tumors with multiple concurrent genetic aberrations. There is some suggestion that combination therapy may be more effective. In a Phase I study evaluating bicalutamide in combination with the mTOR inhibitor ridaforolimus [57], there was a modest signal of activity. Three of ten patients had a ≥ 50% decline in PSA, with four men (40%) experiencing primary resistance (i.e. PSA progression as the best response to therapy). This is in contrast to a study [58] in 38 patients with chemotherapy-resistant CRPC who were treated with single-agent ridaforolimus. In that study, no objective responses were observed. Twenty-nine patients (78%) had primary resistance with respect to PSA changes. Although these are different patient populations (with the former study performed in chemotherapy-naive patients), the result of potentially increased activity when used in combination is intriguing.
There is some evidence that in appropriately selected patients the outcomes will be even more significant. Templeton et al. [59] studied the mTOR inhibitor everolimus, in an unselected population of 37 chemotherapy-naive patients with CRPC. The primary outcome of PFS at 12 weeks was observed in only 35% of patients. However, a subset analysis of the patients with PTEN deletion compared to those men with PTEN expression demonstrated a trend towards longer PFS, HR of 2.58 (p = 0.07). Currently there are no ongoing Phase III clinical trials with agents targeting the PI3K/AKT/mTOR pathway in prostate cancer, though there are additional early-phase clinical trials ongoing. This may be an approach that is clinically beneficial in the future.
3.5 Post-translational modification of the AR
Extensive research has been done over the last few decades to understand the clinical effects resulting from post-translational modifications to the AR through acetylation, methylation, ubiquitination, SUMOylation and phosphorylation. The molecular effects of these changes include decreased apoptosis and increased transcriptional activity to the androgen responsive genes. The biochemical mechanisms and effects have been well-described previously [60], so only the clinical implications will be reviewed here. Various intracellular kinases have been demonstrated to phosphorylate the AR. Aurora kinase is one example [61] and is the most extensively studied clinically. A Phase II trial in unselected patients with mCRPC using danusertib was reported previously. A total of 60 patients were randomized between two different treatment schedules. All patients had previously progressed on docetaxel and had not received abiraterone or enzalutamide as these agents were not yet available. Only two patients had a PSA response, and the best radiographic response was stable disease in 27% of patients. The median PFS was 12 weeks in both arms. Given the disappointing results, no further research of this compound is being pursued. Previous reports indicate that aurora kinase is upregulated in anaplastic, or neuroendrocrine prostate cancer [62]. More recent clinical trials using these agents are targeting the small cell and anaplastic populations of prostate cancer (NCT01799278 and NCT01848067) [63,64]. Research is actively continuing for these targets, though no late-Phase clinical trials are currently planned or ongoing.
3.6 Coregulators
As reviewed previously [60], transcriptional activity of AR responsive genes is increased through many mechanisms including receptor tyrosine kinases such as EGFR, alternate signaling pathways such as MAPK and PI3K pathways, and through modification by coactivators and corepressors to increase or decrease AR pathway transcriptional activity. The transcriptional co-activator p300 is one example of this pathway that has been well-described and is upregulated in patients receiving androgen deprivation therapy [65,66]. Now it is understood that many co-activators are involved in augmenting the transcriptional activity of AR-responsive genes. The p160 SRC family (SRC-1 or NCOA1, SRC-2 or NCOA2 and SRC-3 or NCOA3) family is one group of co-activators that has been shown to be important [67]. Other coregulators have been described, such as Oct1 and p300 as previously mentioned [68]. Research is ongoing to identify appropriate drugs for these targets, but to date no clinical trials have been conducted that specifically alter coregulator activity. However, there is clinical data demonstrating that some of these coregulators are negative prognostic markers [67,68].
4. Sequencing of agents in CRPC
4.1 Chemotherapy efficacy after novel hormonal therapy
It is clear that tumors develop resistance to therapy over time, raising the possibility of cross-resistance between therapies. Is there an optimal sequence of therapies to maximize the clinical benefit for our patients? Although there are currently no definite answers, we can glean some insights from the available clinical studies. For example, a retrospective analysis of docetaxel activity after ketoconazole showed no significant abrogation of docetaxel’s effect [69]. In that analysis, 728 men had no prior ketoconazole compared to 277 men with prior exposure to ketoconazole. There was no difference in the baseline characteristics between the groups. Also there was no difference between the two groups with respect to overall survival (median 21.1 months vs 22.3), PFS (median 8.1 months vs 8.6), the objective response rate (39 vs 43%) and the proportion with a PSA decline ≥ 50% (61 vs 66%). However, this is in contrast to a retrospective single-arm evaluation of 35 men with mCRPC receiving docetaxel after abiraterone [70]. In this small study, it appeared that the docetaxel response was attenuated due to prior receipt of abiraterone. The authors reported that the eight patients who failed to achieve a PSA reduction of ≥ 50% on abiraterone were also primary refractory to docetaxel chemotherapy. Overall, in the 35 patients in this study, there was a PSA reduction of ≥ 50% in 26% of the patients and a median OS of 12.5 months. Concordant findings were recently reported from another retrospective analysis of patients receiving docetaxel after abiraterone [71]. In this evaluation, 24 patients had received abiraterone prior to docetaxel compared with 95 docetaxel-treated patients who had not previously received abiraterone. The baseline characteristics of the two groups were similar except for a worse baseline ECOG performance status (ECOG 2 = 34 vs 0%, p = 0.04) and higher baseline PSA levels (815.7 vs 245.9, p = 0.05) in the docetaxel-only cohort compared with the abiraterone-docetaxel cohort. However, the abiraterone-docetaxel group had an increased rate of visceral metastasis (lung 40 vs 17%, p = 0.04; liver 20 vs 11%, p > 0.05) and a higher burden of bone disease (> 10 foci: 52 vs 0%). The duration of response to docetaxel was shorter in the abiraterone-docetaxel cohort compared to the docetaxel-only cohort, with a median PFS of 4.4 months (95% CI, 3.1 – 6.7 months) versus 7.6 months (95% CI, 6.2 – 8.4 months), respectively (p = 0.003). This suggests that docetaxel has an abrogated response after abiraterone for some patients. However, what is not clear from this information is if the blunted efficacy of docetaxel post-abiraterone is secondary to acquired drug resistance mechanisms from prior hormonal therapy, or if the results are because of more advanced disease and an overall decreased tolerance to therapy. For example, it is conceivable that inferior outcomes could be explained by impaired patient tolerance to chemotherapy and increased side effects seen in a more advanced patient population. However, this is not necessarily supported by all of the evidence reported in the studies. Given the differences in outcomes between the ketoconazole study and the abiraterone studies, the decreased efficacy of docetaxel after hormonal therapy is likely agent specific, with taxane cross-resistance developing to some, but not all hormonal therapies.
4.2 Novel hormonal therapy efficacy after chemotherapy
Other retrospective sequencing studies have demonstrated decreased effectiveness of the second therapeutic agent after receiving first-line treatment for CRPC. Badrising et al. [72] retrospectively evaluated 61 CRPC patients for enzalutamide efficacy after previously progressing on docetaxel and abiraterone. Patients received a median of 8 cycles of docetaxel and 18 weeks of abiraterone prior to starting enzalutamide. The median OS was 31.6 weeks, and the median PFS was 12 weeks. Only 21% of patients had a PSA response of ≥ 50% to enzalutamide. Thirty percent of patients had a PSA rise as the best PSA response to enzalutamide (i.e., demonstrated primary resistance). This compares poorly to the results of the AFFIRM (enzalutamide post-chemotherapy) study where the median OS was 18.4 months and the proportion of patients with a PSA decline of ≥ 50% was 54%. However, there are baseline differences between these two studies including prior abiraterone use, higher baseline PSA, a higher proportion of visceral metastatic disease, and lower ECOG PS score in the Badrising study compared with the AFFIRM study. However, the number of prior docetaxel cycles was similar in the two studies (8 vs 8.5 cycles). The worse outcomes with enzalutamide in this case could potentially be explained by the development of cross-resistance between abiraterone and enzalutamide in the Badrising study compared to AFFIRM, or the results might possibly relate to more advanced disease in the Badrising study as evidenced by the worse performance status, more visceral disease and higher median PSA.
A second retrospective analysis also showed a decreased response to enzalutamide in 35 CRPC patients post-chemotherapy and post-abiraterone [73]. A similar duration of chemotherapy was noted with a median number of eight cycles, and a median of nine months of abiraterone therapy prior to enzalutamide. Forty percent of patients had PSA progression as the best response to therapy, nearly double the proportion of patients that were primary refractory to enzalutamide treatment compared with the AFFIRM study. These data suggest that newer hormonal therapies perform less well after chemotherapy and after prior novel AR-directed therapy. A recent retrospective study reported by Zafeiriou et al. attempted to delineate the optimal sequencing of docetaxel and abiraterone in men with mCRPC treated at the Royal Marsden Hospital [74]. In their report, 161 patients received docetaxel first followed by abiraterone, and 37 men received abiraterone first followed by docetaxel. Their primary outcome measures were the mean duration on each treatment as well as the OS. There was no difference in OS based on the sequence used, with a median OS of 31.4 months in the docetaxel-first group (95% CI 28.3 – 34.4) and 38.6 months in the abiraterone-first cohort (95% CI 30.3 – 46.9). One of the major limitations affecting the interpretation of this study was the fact that patients did not necessarily receive the two agents sequentially (i.e., without intervening treatments). Baseline characteristics, including LDH and albumin, were similar between the two groups except that hemoglobin was lower (11.93 vs 12.68, p = 0.038) in the docetaxel-first group and mean PSA was numerically higher (786.29 vs 118.72, p = 0.072) in the docetaxel-first group. Gleason score, extent of visceral disease and extent of bony metastatic disease were not reported. Therefore, at this time, the optimal sequencing of AR-directed therapies and taxane chemotherapies in men with mCRPC remains undetermined, and this should be the subject of prospective clinical trials or (at least) multi-institutional retrospective studies.
4.3 Sequential use of novel hormonal therapies
There is ample evidence suggesting that progression on prior hormonal therapy does predict for a worse response to a subsequent hormonal agent. Noonan et al. [75] evaluated 30 patients treated with abiraterone after progression on prior enzalutamide. In this study, the baseline characteristics and the treatment response were compared at the time of enzalutamide therapy to subsequent abiraterone therapy in the same patient cohort. The response to abiraterone was markedly worse than the prior responses to enzalutamide. For comparison, 60 and 70% of patients had a 50 and 30% PSA decline respectively, while on enzalutamide. This is in contrast to 3 and 11% of patients who had a 50 and 30% PSA decline while on subsequent abiraterone. Of note, the baseline characteristics of these patients were much better when starting enzalutamide compared to later in their disease course when starting abiraterone. The proportion of patients with an ECOG PS of 0 – 1 was 86 versus 70% for the time of enzalutamide initiation versus abiraterone initiation. Prognostic laboratory values were also worse at the time of abiraterone therapy as noted by decreased hemoglobin levels and elevation of LDH and alkaline phosphatase. The PFS was modest in this evaluation with a median of 15.4 weeks (95% CI 10.7 – 20.2 weeks) while on abiraterone treatment. Even though the overall response rate was modest at best, some patients did respond to abiraterone following enzalutamide. It is unclear if the poorer than expected outcome for abiraterone is due to more advanced disease/larger tumor burden or acquired AR-related resistance mechanisms. A separate analysis performed by Azad et al. [76] compared enzalutamide after abiraterone in those who were docetaxel-naïve to those previously receiving docetaxel. 68 patients were previously treated with docetaxel and 47 patients had not received docetaxel. There was no difference in the PSA response rates, median time to progression or median OS. There was limited benefit to all patients with enzalutamide with a median time on treatment of only 4.1 months. This data suggests enzalutamide and abiraterone share some common mechanisms of resistance, reinforcing the need to identify these mechanisms with better biomarkers for appropriate treatment selection.
4.4 Subsequent treatment after first-line novel hormonal therapy: chemotherapy or a second hormonal therapy?
Another question that arises in the clinic is the optimal selection of treatment after failure of one novel AR-directed therapy: should such patients proceed to a second AR-targeting therapy or taxane chemotherapy? There is one retrospective single-institution study that attempted to answer this question by comparing the use of enzalutamide versus docetaxel in men with mCRPC progressing on abiraterone [77]. In this analysis, 30 abiraterone-refractory patients were treated with subsequent enzalutamide while 31 received subsequent docetaxel. The outcomes of PFS and time-to-PSA-progression were similar between the two groups. For example, the median PFS was 4.7 versus 4.4 months in the enzalutamide and docetaxel groups respectively. There were differences in the baseline clinical characteristics between these two groups. The docetaxel group had a larger disease burden as suggested by an increased number of bone-metastatic lesions (> 10 lesions in 54 vs 0%), more visceral disease (48 vs 30%) and a higher baseline PSA level (median 196 vs 26 ng/ml). Multivariate modeling was performed to adjust for these differences: the PFS was still similar between the enzalutamide and the docetaxel groups after such an adjustment, with a hazard ratio of 1.44 (95% CI, 0.53 – 3.92, p = 0.47). This suggests that chemotherapy and subsequent AR-directed therapy can be equally effective in patients after progression on first-line CRPC therapy. However, the AR-V7 biomarker studies discussed previously [28,30] did suggest superior treatment responses to taxanes compared to AR-targeting therapies in the context of AR-V7-positive disease. Therefore, a potential clinical utility of the AR-V7 biomarker might be in patients progressing after one line of novel hormonal therapy (e.g., abiraterone or enzalutamide) and may help select the subsequent therapy in this setting (perhaps a second hormonal therapy might be appropriate in AR-V7-negative men, while a taxane might be preferable in AR-V7-positive patients). Further prospective clinical studies are being designed to answer this question. For example, the PRIMCAB study (NCT02379390) will prospectively enroll 274 men with primary resistance to either abiraterone or enzalutamide and will randomize patients (1:1) to either cabazitaxel chemotherapy or the alternative novel AR-directed therapy; AR-V7 testing will be incorporated prospectively into this trial [78].
5. Future directions
Ongoing clinical trials are focusing on novel methods to overcome drug resistance in mCRPC (Table 1). One area of active research involves immunotherapy, either using immune checkpoint blockade or active prostate cancer vaccines. Ipilimumab, a CTLA-4 inhibitory antibody inducing activation of cytotoxic T cells, was tested recently in a large Phase III randomized, placebo-controlled clinical trial in patients with taxane-resistant mCRPC [79]. In the final analysis of that trial, there was no statistical improvement in overall survival, the primary endpoint. Median OS was 11.2 months versus 10.0 months for ipilimumab and placebo arms, respectively (HR 0.85, 95% CI 0.72 – 1.00, p = 0.053). Although the results were not statistically significant, there was a clear trend towards clinical benefit with ipilimumab, which was made even more apparent when considering only men without visceral (liver) metastases. This has given some promise to the field of immunotherapy and further studies are awaiting results, including the pre-chemotherapy study of ipilimumab versus placebo (NCT01057810) [80] which should report results soon. Other rational approaches include PD-1 inhibitors, such as nivolumab or pembrolizumab, and studies employing these agents in mCRPC are currently being designed (NCT02312557 and NCT01688492) [81,82]. Vaccine-based approaches (NCT02111577 and NCT01881867) [83,84] that stimulate and augment the immune response to prostate cancer cells are also being investigated. The most relevant example is the ProstVac-VF randomized trial (NCT00078585) [85], which is fully enrolled and should report results in the next 1 – 2 years. These approaches have the theoretical benefit of being effective despite many of the AR-related resistance mechanisms discussed herein.
Table 1.
Selected clinical trials with AR-directed therapies.
| Investigational agents | Clinical trial Phase | Description | Primary outcome(s) | NTC identifier |
|---|---|---|---|---|
| Abiraterone | ||||
| Abiraterone, BEZ235 (PI3K inhibitor), BKM120 (PI3K inhibitor) | Phase Ib | Non-randomized study of abiraterone with either BEZ235 or BKM120 after progression on abiraterone | Safety | NCT01634061 |
| Abiraterone, Docetaxel | Phase I | Single-arm trial of the combination in mCRPC | Safety | NCT01400555 |
| Abiraterone, Cabozantinib (c-MET and VEGFR inhibitor) | Phase I | Open-label trial of the combination in mCRPC | Safety | NCT01574937 |
| Abiraterone, Ipilimumab (CTLA-4 inhibitor) | Phase I/II | Single-arm trial of the combination in mCRPC in patients who are chemotherapy naive | Safety and PFS. | NCT01688492 |
| Abiraterone, AT13387 (HSP90 inhibitor) | Phase I/II | Randomized, open-label trial of AT13387 with/without abiraterone in men with mCRPC after progression on abiraterone | Safety, PSA and Radiographic Response Rate | NCT01685268 |
| Abiraterone, Alisertib (Aurora Kinase Inhibitor) | Phase I/II | Open-label, single-arm trial of the combination after disease progression on abiraterone in mCRPC | Safety, PFS | NCT01848067 |
| Abiraterone, Dasatinib (BCR-ABL, c-KIT and Src-family tyrosine kinase inhibitor) | Phase II | Randomized, open-label trial of abiraterone with/without dasatinib in mCRPC | PFS | NCT01685125 |
| Abiraterone, Olaparib (PARP inhibitor) | Phase II | Randomized, placebo-controlled trial of abiraterone with/without olaparib in mCRPC, chemotherapy resistant | Safety and rPFS | NCT01972217 |
| Abiraterone, Cabazitaxel | Phase II | Randomized, open-label trial of abiraterone with/without cabazitaxel in mCRPC | rPFS | NCT02218606 |
| Abiraterone, GDC-0980 (PI3K/mTOR inhibitor) | Phase II | Randomized, open-label trial of abiraterone with/without GDC-0068 or GDC-0980 in men with PTEN loss, after progressing on docetaxel | Safety and rPFS | NCT01485861 |
| Abiraterone, Veliparib (PARP inhibitor) | Phase II | Randomized, open-label trial of abiraterone with or without veliparib in mCRPC | PSA response rate | NCT01576172 |
| Abiraterone, Enzalutamide | Phase II | Randomized, open-label study of the optimal treatment sequence in mCRPC, chemotherapy naive | PSA response rate | NCT02125357 |
| Abiraterone, Enzalutamide | Phase II | Single-arm study evaluating the combination in mCRPC | Safety | NCT01650194 |
| Abiraterone, AMG386 (angiopoietin inhibitor) | Phase II | Randomized open-label trial of abiraterone with/without AMG386 in mCRPC | PFS | NCT01553188 |
| Abiraterone, Radium-223 | Phase III | Randomized, blinded trial of abiraterone with/without Radium-223 in chemotherapy naive men with mCRPC | Symptomatic skeletal event free survival | NCT02043678 |
| Enzalutamide | ||||
| Enzalutamide, Abiraterone, Afuresertib (AKT inhibitor) | Phase I | Non-randomized Open-label trial of afuresertib with either abiraterone or enzalutamide in men with mCRPC and PSA only progression on abiraterone or enzalutamide | Safety | NCT02380313 |
| Enzalutamide, GSK2636771 (PI3K inhibitor) | Phase I | Single-arm trial on the safety of the combination in mCRPC patients progressing on enzalutamide who have PTEN loss | Safety | NCT02215096 |
| Enzalutamide, Crizotinib (ALK and ROS-1 inhibitor) | Phase I | Single-arm trial of the combination in mCRPC | Safety | NCT02207504 |
| Enzalutamide, BI836845 (inhibitory antibody to the IGF ligand) | Phase I/II | Randomized, open-label trial of enzalutamide with/without BI836845 in mCRPC patients previously progressing on abiraterone and docetaxel | Safety, PSA response rate, rPFS | NCT02204072 |
| Enzalutamide, Abiraterone Cabazitaxel | Phase II | Randomized, open-label trial of cabazitaxel or a second hormonal therapy after progression on the first hormonal agent in mCRPC patients | rPFS | NCT02379390 |
| Enzalutamide, LY3023414 (dual PI3K/mTOR inhibitor) | Phase II | Double-blinded, placebo-controlled trial of enzalutamide with/without LY3032414 in men previously progressing on abiraterone | PFS | NCT02407054 |
| Enzalutamide, PROSTVAC | Phase II | Randomized, open-label trial of enzalutamide with/without PROSTVAC | Time to Progression | NCT01867333 |
| Novel Androgen Synthesis Inhibitors | ||||
| VT-464 (lyase specific androgen synthesis inhibitor) | Phase I/II | Non-randomized single arm trial of VT-464 | Safety | NCT02012920 |
| VT464 | Phase II | Non-randomized trial of VT464 in chemotherapy naive and chemotherapy resistant disease | PSA response and rPFS | NCT02130700 |
| CFG920 (androgen synthesis inhibitor) | Phase I/II | Non-randomized, open-label trial of CFG920 in mCRPC | Safety, PSA response rate | NCT01647789 |
| Direct AR Antagonists | ||||
| ARN-509, Everolimus | Phase I | Open-label trial of the combination after progression on abiraterone | Safety | NCT02106507 |
| ARN-509, Abiraterone (AR antagonist) | Phase I | Non-randomized study of the combination of Abiraterone with ARN-509 in mCRPC | Safety | NCT02123758 |
| ARN-509 | Phase III | Double-blinded, placebo-controlled study in men with non-metastatic CRPC | Metastasis-free survival | NCT01946204 |
| EPI-506 (N-terminal domain inhibitor) | Phase I/II | Single-arm trial in men with mCRPC after progression on enzalutamide or abiraterone | Safety | Planned, not currently enrolling |
| Galeterone (AR antagonist and androgen synthesis inhibitor) [ARMOR2] | Phase II | Single-arm trial in men with metastatic and non-metastatic CRPC | Safety, PSA response rate | NCT01709734 |
| Galeterone Enzalutamide [ARMOR3] | Phase III | Randomized, open-label trial of enzalutamide or galeterone in treatment-na-ve, AR-V7 positive mCRPC | rPFS | Planned, not currently enrolling |
| ODM-201 (AR antagonist) | Phase III | Double-blind, placebo-controlled trial of ODM-201 in non-metastatic CRPC | Metastasis-free survival | NCT02200614 |
mCRPC: Metastatic castrate-resistant prostate cancer; PFS: Progression-free survival.
Epigenetic approaches are also being studied. The BET bromodomain family of proteins (BRD2, BRD3, BRD4 and BRDT) is associated with acetylated chromatin during interphase, and may help to regulate cell division. These proteins have conserved regions of bromodomains which have functional significance. Clinical inhibitors of these BET proteins are being studied in Phase I clinical trials, including in patients with mCRPC (NCT02259114) [86]. Preclinical studies show a strong effect of targeting this pathway in advanced prostate cancer, and these drugs may possibly have activity in AR-V7-mediated disease [87,88]. This approach also may be effective despite many of the resistance mechanisms previously described.
Galeterone is a novel AR-directed agent in clinical trials that has multi-targeted effects including CYP17-lyase inhibition, direct AR antagonism resulting in decreased AR nuclear translocation, and enhanced degradation of the AR via phosphorylation of MDM2 (E3 ubiquitin ligase) [89–91]. Results from the Phase II ARMOR2 trial were recently reported [92]. In this study, 108 patients with CRPC were treated with daily oral galeterone and evaluated for PSA response. This was a diverse group of patients that included four cohorts: non-metastatic treatment-naive CRPC, meta-static treatment-naïve CRPC, as well as abiraterone-resistant and enzalutamide-resistant CRPC patients. Overall 68% of patients had a ≥ 30% PSA decrease, and 59% of patients had a ≥ 50% PSA decrease. CTC analysis was also performed in this study. Seven patients had AR-LBD loss in the presence of N-terminal AR domain positivity (suggesting AR variant expression) using a CTC-based immunohistochemistry assay for AR protein detection. Six out of seven patients had a PSA decrease of ≥ 50%, suggesting that galeterone may have activity in prostate cancer expressing truncated forms of AR (possibly including AR splice variants). This clinical data corroborates preclinical work showing that galeterone has activity against AR variants, including AR-V7 [89]. These findings have led to the design of the ARMOR3 trial in which men with treatment-naïve AR-V7-positive mCRPC will be randomized (1:1) to receive either enzalutamide or galeterone, with a primary end point of radiographic PFS (the trial is currently not open to accrual yet) [93].
Finally, targeting of the N-terminal domain of AR may ultimately be the most promising way to completely extinguish AR signaling. Because the N-terminal is present in both the full-length AR and all of the constitutively active AR splice variants (including AR-V7), targeting of this region may represent the ultimate AR-directed strategy in CRPC. The EPI family of small molecule compounds has been shown to covalently bind and inhibit the AR N-terminus. In the LNCaP cell line, the EPI family of compounds has been shown to selectively bind to AR and inhibit growth. This was also demonstrated in mice xenograft models [94,95]. These compounds may also work synergistically with docetaxel in mCRPC as suggested by results from cell-line and animal xenograft models. A Phase I/II clinical trial is currently being planned using EPI-506 in men with mCRPC who have received prior enzalutamide or abiraterone [96].
6. Expert opinion
Common clinical practice in men with mCRPC is to delay the use of chemotherapy for a significant time by using sequential hormonal therapies and other approved non-chemotherapy agents first. However, there is some speculative data to suggest that this may not be the most effective sequencing strategy. The recently reported CHAARTED study [3] highlights the role for early chemotherapy in conjunction with hormonal therapy in newly diagnosed mHSPC. This trial reported a median OS of 52.7 months for the combination group versus 42.3 months in the hormonal-only group (which might be considered as the ‘deferred chemotherapy’ group). Subgroup analyses of patients with a large baseline tumor burden seem to suggest a more significant magnitude of benefit compared with those at a lower tumor burden. It is instructive to remember that the primary analysis was applied to the entire study population involving all enrolled patients with newly diagnosed mHSPC, including low disease burden and high disease burden patients. However, this study does not address the most common population of men for which docetaxel chemotherapy is usually considered, mCRPC. What is unclear in this patient population is how to proceed with sequencing or combining therapy for optimal clinical benefit and maximal survival.
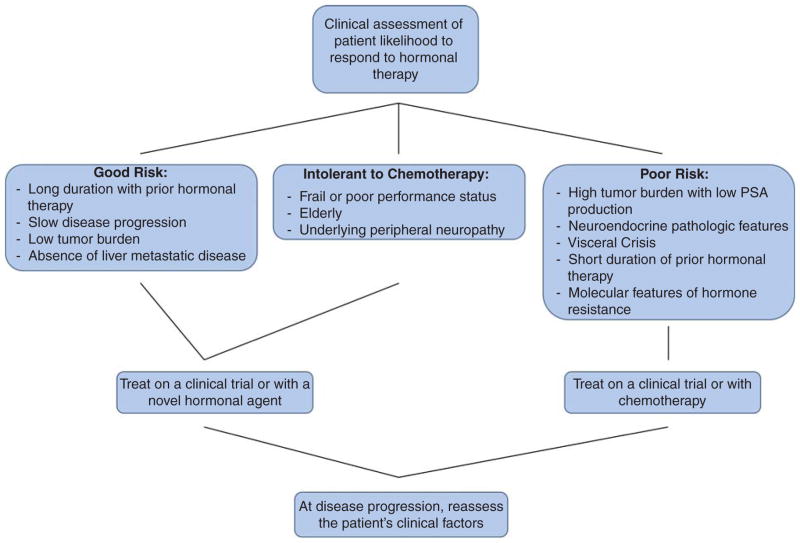
Our preferred approach to such patients with mCRPC is to consider participation in clinical trials aiming to prospectively answer these questions of timing, sequencing and combination therapy. Treatment decisions such as when to choose chemotherapy over hormonal therapy or vice versa might be based on pathologic and clinical features, such as extent and distribution of metastases, number and types of prior therapies received, clinical intuition and patient preferences (Figure 3). One common practice at our institution is to consider chemotherapy prior to subsequent novel AR-directed therapy especially in patients with progression on one novel AR-directed therapy. Additional types of patients that may be preferentially steered towards taxane chemotherapy might include those with high-risk pathologic or molecular features; a very short duration of effect with primary ADT; presence of rapidly progressing visceral disease (especially in the liver); expression of neuroendocrine markers on pathology such as chromogranin A or neuron-specific enolase; or those with a large tumor burden but only a small PSA production. These patients are less likely to benefit from additional hormonal therapy in our opinion.
Figure 3. Treatment selection flow chart.
Conversely, hormonal therapy might be preferred over chemotherapy for those men with a worse ECOG performance status given the higher rate of side effects with chemotherapy, or in the elderly frail population, or patients with prolonged treatment responses to previous hormonal therapies. Although these clinical parameters are helpful in identifying subgroups more likely to respond to one class of agent over another, molecular biomarkers are more likely to provide meaningful treatment selection criteria. We strongly encourage the enrollment of patients on clinical trials to evaluate the prognostic and predictive utility of treatment-selection biomarkers. For instance, there is now an ongoing prospective biomarker trial (NCT02269982) [29] enrolling mCRPC patients with high-risk features who are embarking on therapy with abiraterone or enzalutamide, with the goal of prospectively collecting and evaluating CTC-derived AR-V7 using three different platforms for AR-V7 detection as an essential next step towards clinically validating the utility of this bio-marker in routine clinical practice.
Article highlights.
All nodes in the androgen receptor (AR) pathway are involved in hormonal therapy drug resistance.
AR splice variant AR-V7 is strongly associated with abiraterone and enzalutamide drug resistance.
The AR point mutations F867L, T878A and L702H are common activating mutations that paradoxically cause enzalutamide, progesterone or glucocorticoids to induce transcriptional activitiy, respectively.
Increased co-activator activity, AR phosphorylation, post-translational modifications to the AR, and other events result in increased transcription of AR regulated genes.
The optimal sequence of chemotherapy and hormonal therapy is not currently known. Biomarker studies are ongoing to clarify these treatment-selection questions.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The authors were partially funded by a grant from the National Institute of Health (NIH grant P30 CA006973). ES Antonarakis is a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Medivation and Essa. He has received research funding from Janssen, Johnson & Johnson, Sanofi, Dendreon, Aragon, Exelius, Genentech, Novartis and Tokai. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2012) www.seer.cancer.gov/popdata. released March 2014. [Google Scholar]
- 2.Beer MT, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweeney C, Chen Y-H, Carducci MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. J Clin Oncol. 2014;32:5s. [Google Scholar]
- 4.Roy AK, Tyagi RK, Song CS, et al. Androgen Receptor: Structural Domains and Functional Dynamics after Ligand-Receptor Interaction. Ann N Y Acad Sci. 2001;949:44–57. doi: 10.1111/j.1749-6632.2001.tb04001.x. [DOI] [PubMed] [Google Scholar]
- 5.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-Induced Blockade to Nuclear Accumulation of the Androgen Receptor Predicts Clinical Responses in Metastatic Prostate Cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards J, Krishna NS, Mukherjee R, et al. Amplification of the androgen receptor may not explain the development of androgen-independent prostate cancer. BJU Int. 2001;88:633–7. doi: 10.1046/j.1464-410x.2001.02350.x. [DOI] [PubMed] [Google Scholar]
- 7.Linja MJ, Savinainen KJ, Saramaki OR, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 8.Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–75. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haffner MC, De Marzo AM, Meeker AK, et al. Transcription-induced DNA double strand breaks: both oncogenic force and potential therapeutic target? Clin Cancer Res. 2011;17:3858–64. doi: 10.1158/1078-0432.CCR-10-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Sci Transl Med. 2015;7:269ra2. doi: 10.1126/scitranslmed.3010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RE-sensitizing with supraphysiologic testosterone to overcome resistance to abiraterone and enzalutamide (The RESTORE Study) Available from: https://clinicaltrials.gov/ct2/show/NCT02090114.
- 12.Testosterone Revival Abolishes Negative Symptoms, Fosters Objective Response and Modulates Enzalutamide Resistance (TRANSFORMER) Available from: https://clinicaltrials.gov/ct2/show/NCT02286921.
- 13••.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Can Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. This paper compares androgen levels in hormone sensitive and resistant tumors from patients, providing strong evidence for continued androgen production and dependence even in men with mCRPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohler JL, Gregory CW, Ford OH, et al. The androgen axis in recurrent prostate cancer. Clin Can Res. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 15.Knuuttila M, Yatkin E, Kallio J, et al. Castration induces up-regulation of intratumoral androgen biosynthesis and androgen receptor expression in an orthotopic VCaP human prostate cancer xenograft model. Am J Pathol. 2014;184:2163–73. doi: 10.1016/j.ajpath.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 16•.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Can Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. This paper provides compelling pre-clinical data that intratumoral production of androgens is a significant source of AR signaling and cancer growth. [DOI] [PubMed] [Google Scholar]
- 17.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Eng J Med. 2014;37:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;10:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 20.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of Abiraterone Acetate on Androgen Signaling in Castrate-Resistant Prostate Cancer. JCO. 2011;6:637–43. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;1:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonarakis ES. Predicting treatment response in castration-resistant prostate cancer: could androgen receptor variant-7 hold the key? Expert Rev Anticancer Ther. 2014;15:143–5. doi: 10.1586/14737140.2015.999044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadiminty N, Tummala R, Liu C, et al. NF-κB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12:1629–37. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Chan SC, Brand LJ, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. The first clinical data reported confirming AR splice variants are associated with resistance to novel hormonal therapy treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Development of circulating molecular predictors of chemotherapy and novel hormonal therapy benefit in men with metastatic castration resistant prostate cancer (mCRPC) Available from: https://clinicaltrials.gov/ct2/show/NCT02269982.
- 30••.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncology. 2015 doi: 10.1001/jamaoncol.2015.1341. This is the first clinical trial data regarding taxane response in men with detection of AR-V7, demonstrating that taxane therapy remains efficacious. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thadani-Mulero M, Portella L, Sun S, et al. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Can Res. 2014;74:2270–82. doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mediwala SN, Sun H, Szafran AT, et al. The activity of the androgen receptor variant AR-V7 is regulated by FOXO1 in a PTEN-PI3K-AKT-dependent way. Prostate. 2013;73:267–77. doi: 10.1002/pros.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koochekpour S. Androgen receptor signaling and mutations in prostate cancer. Asian J Androl. 2010;12(5):639–57. doi: 10.1038/aja.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwan IJ, Hay CW. The impact of point mutations in the human androgen receptor: classification of mutations on the basis of transcriptional activity. PLoS One. 2012;7(3):e32514. doi: 10.1371/journal.pone.0032514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottliet B, Beitel LK, Nadarajah A, et al. The Androgen receptor gene mutations database: 2012. Hum Mut. 2012;33:887–94. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 36.Korpal M, Korn JM, Gao X, et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide) Cancer Discov. 2013;3:1030–43. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 37.Balbas MD, Evans MJ, Hosfield DJ, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Joseph JD, Qian J, Sensintaffar J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–9. doi: 10.1158/2159-8290.CD-13-0226. This research clearly shows in pre-clinical and clinical data that the F876L mutation confers resistance against enzalutamide and ARN-509, both novel anti-androgen therapies. [DOI] [PubMed] [Google Scholar]
- 39.Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Can Res. 2015;21(10):2315–24. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 40••.Chen EJ, Sowalsky AG, Gao S, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21:1273–80. doi: 10.1158/1078-0432.CCR-14-1220. This paper identifies the progesterone-activating T878A mutation in tissue samples from patients previously treated with abiraterone. This mutation was previously shown to be a mechanism of resistance to older-generation androgen therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joyce R, Fenton MA, Rode P, et al. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol. 1998;159:149–53. doi: 10.1016/s0022-5347(01)64039-4. [DOI] [PubMed] [Google Scholar]
- 42•.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid Receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. Glucocorticoid receptor overexpression has also been shown to drive prostate cancer growth via steroid signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–82. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carreira S, Romanel A, Goodall J, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014;6:254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enzalutamide with or without abiraterone and prednisone in treating patients with castration-resistant metastatic prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01949337.
- 46.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinkade CW, Castillio-Martin M, Puzio-Kuter A, et al. Targeting AKT/ mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Zhu J, Efferson CL, et al. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a PTEN-deficient mouse model of prostate cancer. Cancer Res. 2009;69:7466–72. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
- 49.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kruczek K, Ratterman M, Tolzien K, et al. A phase II study evaluating the toxicity and efficacy of single-agent temsirolimus in chemotherapy-naïve castration-resistant prostate cancer. Br J Cancer. 2013;109:1711–16. doi: 10.1038/bjc.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chee KG, Longmate J, Quinn DI, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5:433–7. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong AJ, Shen T, Halabi S, et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clin Genitourin Cancer. 2013;11:397–406. doi: 10.1016/j.clgc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Stefanou D, Batistatou A, Kamina S, et al. Expression of vascular endothelial growth factor (VEGF) and association with microvessel density in benign prostatic hyperplasia and prostate cancer. In Vivo. 2004;18(2):155–60. [PubMed] [Google Scholar]
- 54.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- 55.Park HS, Hong SK, Oh MM, et al. Synergistic Antitumor effect of NVP-BEZ235 and sunitinib on docetaxel resistant human castration-resistant prostate cancer cells. Anticancer Res. 2014;34:3457–68. [PubMed] [Google Scholar]
- 56.Michaelson MD, Oudard S, Ou YC, et al. Randomized, placebo controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 57.Meulenbeld HJ, de Bono J, Tagawa ST, et al. Tolerability, safety and pharmacokinetics of ridaforolimus in combination with bicalutamide in patients with asymptomatic, metastatic castration-resistant prostate cancer (CRPC) Cancer Chemother Pharmacol. 2013;72:909–16. doi: 10.1007/s00280-013-2250-6. [DOI] [PubMed] [Google Scholar]
- 58.Amato RJ, Wilding G, Bubley G, et al. Safety and preliminary efficacy analysis of the mTOR inhibitor ridaforolimus in patients with taxane-treated, castration-resistant prostate cancer. Clin Genitourin Cancer. 2012;10:232–8. doi: 10.1016/j.clgc.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Templeton AJ, Dutoit V, Cathomas R, et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08) Eur Urol. 2013;64:150–8. doi: 10.1016/j.eururo.2013.03.040. The subset analysis of patients with loss of PTEN had improved outcomes in this study with everolimus treatment compared to those with PTEN expression. This provides some evidence that appropriately selected patients may benefit from targeted therapy. [DOI] [PubMed] [Google Scholar]
- 60.Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. J Endocrinol. 2012;215:221–37. doi: 10.1530/JOE-12-0238. [DOI] [PubMed] [Google Scholar]
- 61.Meulenbeld HJ, Bleuse JP, Vinci EM, et al. Randomized phase II study of danusertib in patients with metastatic castration-resistant prostate cancer after docetaxel failure. BJU Int. 2013;111(1):44–52. doi: 10.1111/j.1464-410X.2012.11404.x. [DOI] [PubMed] [Google Scholar]
- 62.Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–95. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.A Phase II Trial of MLN8237 in patients with metastatic castrate resistant and neuroendocrine prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01799278.
- 64.Alisertib, abiraterone acetate and prednisone in treating patients with hormone-resistant prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01848067.
- 65.Heemers HV, Sebo TJ, Debes JD, et al. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Res. 2007;67:3422–30. doi: 10.1158/0008-5472.CAN-06-2836. [DOI] [PubMed] [Google Scholar]
- 66.Santer FR, Hoschele PP, Oh SJ, et al. Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Mol Cancer Ther. 2011;10:1644–55. doi: 10.1158/1535-7163.MCT-11-0182. [DOI] [PubMed] [Google Scholar]
- 67.Qin J, Lee HJ, Wu SP, et al. Androgen deprivation-induced NCoA2 promotes metastatic and castration-resistant prostate cancer. J Clin Invest. 2014;124:5013–26. doi: 10.1172/JCI76412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obinata D, Takayama K, Urano T, et al. Oct1 regulates cell growth of LNCaP cells and is a prognostic factor for prostate cancer. Int J Cancer. 2012;130:1021–8. doi: 10.1002/ijc.26043. [DOI] [PubMed] [Google Scholar]
- 69.Aggarwal R, Halabi S, Kelly WK, et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance) Cancer. 2013;119:3636–43. doi: 10.1002/cncr.28285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mezynski J, Pezaro C, Bianchini D, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23:2943–7. doi: 10.1093/annonc/mds119. [DOI] [PubMed] [Google Scholar]
- 71•.Schweizer MT, Zhou XC, Wang H, et al. The Influence of Prior Abiraterone Treatment on the Clinical Activity of Docetaxel in Men with Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2014;66:646–52. doi: 10.1016/j.eururo.2014.01.018. This study highlights the likelihood of cross-resistance between chemotherapy and abiraterone, a novel hormonal therapy. The duration of clinical response to docetaxel was compared between men previously receiving abiraterone to those who were abiraterone naïve. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120:968–75. doi: 10.1002/cncr.28518. [DOI] [PubMed] [Google Scholar]
- 73.Schrader AJ, Boegemann M, Ohlmann C-H, et al. Enzalutamide in Castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Europ Urol. 2014;65:30–6. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 74.Zafeiriou Z, Ferraldeshi R, Omlin AG, et al. Sequencing of docetaxel and abiraterone acetate for metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2014;25(Suppl 4):6360. [Google Scholar]
- 75.Noonan KL, Sorth S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–7. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 76.Azad AA, Eigl BJ, Murray N, et al. Efficacy of Enzalutamide Following Abiraterone Acetate in Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer Patients. Eur Urol. 2015;67:23–9. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 77.Suzman DL, Luber B, Schweizer MT, et al. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate. 2014;74:1278–85. doi: 10.1002/pros.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cabazitaxel versus the switch to alternative AR targeted therapy enzalutamide or abiraterone in metastatic castration-resistant prostate cancer (mCRPC) primary resistant patients to abiraterone or Enz (PRIMCAB) Available from: https://clinicaltrials.gov/ct2/show/NCT02379390.
- 79••.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicenter, randomized, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. doi: 10.1016/S1470-2045(14)70189-5. This phase III clinical trial of ipilimumab failed to show statistical significance in overall survival; however, PSA responses and objective responses were seen. This trial highlights the likely activity of immune-therapy in prostate cancer treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phase 3 study of immunotherapy to treat advanced prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01057810.
- 81.Pembrolizumab in treating patients with metastatic castration resistant prostate cancer previously treated with enzalutamide. Available from: https://clinicaltrials.gov/ct2/show/NCT02312557.
- 82.Combining ipilimumab with abiraterone acetate plus prednisone in chemotherapy and immunotherapy-naïve patients with progressive metastatic castration-resistant prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01688492.
- 83.Phase III study of DCVAC added to standard chemotherapy for men with metastatic castration resistant prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT02111577.
- 84.CYT107 with or without vaccine therapy in treating patients with metastatic hormone-resistant prostate cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01881867.
- 85.PROSTVAC®-VF/TRICOM™ vaccine for the treatment of metastatic prostate cancer after failing hormone therapy. Available from: https://clinicaltrials.gov/ct2/show/NCT00078585.
- 86.A Phase IB Trial With OTX015, a small molecule inhibitor of the bromodomain and extra-terminal (BET) proteins, in patients with selected advanced solid tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT02259114.
- 87.Wuyce A, Degenhardt Y, Bai Y, et al. Inhibition of BET bromodomain proteins as a therapeutic approach in prostate cancer. Oncotarget. 2013;4:2419–29. doi: 10.18632/oncotarget.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asangani IA, Dommeti VL, Wang X, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17 {alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7:2348–57. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Purushottamachar P, Godbole AM, Gediya LK, et al. Systematic structure modifications of multitarget prostate cancer drug candidate galeterone to produce novel androgen receptor down-regulating agents as an approach to treatment of advanced prostate cancer. J Med Chem. 2013;56:48880–98. doi: 10.1021/jm400048v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.kwegyir-Afful AK, Ramalingam S, Purushottamachar P, et al. Clinical candidate galeterone (VN/124-1 or TOK-001) induces the degradation of full-length and splice variant androgen receptors in human prostate cancer cell lines via PI3K-Akt-Mdm2 pathway: implications for prostate cancer therapy. Cancer Res. 2013;73(8 Suppl):1322. [Google Scholar]
- 92•.Taplin ME, Chi KN, Chu F, et al. Galeterone in 4 patient populations of men with CRPC: Results from ARMOR2. Annals of Oncology. 2014;25(suppl 4):iv255–79. This ongoing clinical trial has reported clinical efficacy of galeterone in men with splice variant production. There are agents in the drug-development pipeline that demonstrate activity even in tumors with resistance mechanisms described in this review. [Google Scholar]
- 93.Roberts J, Dhillon R, Dransfield D, et al. Androgen Receptor Modulation Optimized for Response in Splice Variant (ARMOR3-SV): Randomized, open-label, multicenter, controlled study of galeterone versus enzalutamide (enz) in men expressing AR-V7 splice variant (SV), metastatic castrate resistant prostate cancer (mCRPC) J Clin Oncol. 2015;33(Suppl 7):abstr 259. [Google Scholar]
- 94.Myung J-K, Banuelos CA, Fernandez JG, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123:2948–60. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin SK, Banuelos CA, Sadar MD, et al. N-terminal targeting of androgen receptor variant enhances response of castration resistant prostate cancer to taxane chemotherapy. Mol Oncol. 2015;9:628–39. doi: 10.1016/j.molonc.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montgomery RB, Antonarkis ES, Hussain M, et al. A phase 1/2 open-label study of safety and antitumor activity of EPI-506, a novel AR N-terminal domain inhibitor, in men with metastatic castration-resistant prostate cancer (mCRPC) with progression after enzalutamide or abiraterone. J Clin Oncol. 2015;33(Suppl):abstr TPS5072. [Google Scholar]