Abstract
Background
The effectiveness for improving the outcomes across palliative care domains remains unclear. We conducted a systematic review of different types of quality improvement interventions relevant to palliative care.
Methods
We searched PubMed, CINAHL, PsycINFO, and Cochrane for relevant articles published between 2000 and 2011.
Results
A total of 10 randomized controlled trials and 7 nonrandomized controlled trials were included. Of the 5 studies using relay of clinical information, 1 reported significant improvement in patient quality of life. Of the 5 studies targeting education and self-management, 4 found significant improvements in quality of life or patient symptoms.
Conclusion
A minority of quality improvement interventions have succeeded in improving the quality of palliative care delivery. More studies are needed on specific quality improvement types, including organizational change and multiple types of interventions.
Keywords: quality improvement, end of life, systematic review, comparative effectiveness
Introduction
Implementing evidence-based practices into health care is key to improving the quality of care.1 Quality improvement, defined as an effort to change/improve the clinical structure, process, and/or outcomes of care by means of an organizational change,2 can be effectively implemented using a variety of strategies.3 These strategies include policy changes or specific quality improvement interventions such as provider education through educational outreach4 or use of local stakeholders,5 audit and feedback of quality data to providers,6 and clinical decision support for recommended processes of care.7 Broader approaches to organizational change within institutions have also been shown to be effective in many situations; these approaches often include several types of specific quality improvement strategies.
Although the choice of a quality improvement method or combination of methods for a particular health care issue should be based on evidence,8 often insufficient evidence exists to support which approach works best for specific populations. For palliative care, or for care of patients with advanced and serious illness that focuses on quality of life,9 the complexity of illness and treatments may also require different or more multifaceted types of quality improvement than for chronic disease such as asthma or hypertension. Maximizing quality is also particularly important in palliative care. For example, these patients are frequently seriously ill or polysymptomatic,10 making delivery of timely care important; and patients often have multiple symptoms and complex care needs, increasing the need for interventions to improve patient self-management or relay patient symptom data to providers.
To help determine the most effective strategies to improve care quality for this population, we conducted a systematic review of different types of quality improvement interventions in patients with advanced and serious illness as well as interventions applying multiple types of quality improvement strategies.
Methods
Details about our methods are available in a comprehensive report covering multiple issues related to efforts to improve the quality of palliative care.11 As part of the comprehensive review on quality improvement for palliative care, we searched MEDLINE (using PubMed), CINAHL, PsycINFO, Cochrane, and DARE databases from 2000 through December 2011; for this portion of the review, we focused on prospective, controlled studies clearly addressing one or more specific types of quality improvement. Medical subheading terms included palliative care, quality improvement, and communication. Other keywords included cancer, terminally ill, hospice care, patient care planning, and quality assurance. We identified additional studies from reference lists of eligible articles and relevant reviews as well as from experts.
We defined the palliative care population as seriously ill patients and those with advanced disease (such as persons living with advanced cancer or intensive care unit patients at high risk of dying), who are unlikely to be cured, to recover, or to stabilize.9 We defined quality improvement as an effort to change/improve the clinical structure, process, and/or outcomes of care by means of an organizational change2 and included studies that fit into a taxonomy of types of quality improvement methods (Table 1) modified slightly for the purposes of palliative care from a taxonomy developed for previous systematic reviews in quality improvement.3,12 These types included provider-centered interventions such as reminder systems, facilitated relay of clinical data, audit and feedback, education, and training and support on quality improvement; patient-centered interventions such as patient/caregiver education and self-management and reminders; and organizational- and policy-level interventions such as organizational change and financial incentives and regulation. Given this definition and taxonomy, some types of interventions in palliative care were not classified as quality improvement and were not included in this portion of the review, such as palliative care consultation services and case management, comfort care order sets, or patient education and self-management or triage interventions focusing on single symptoms (eg, pain, distress).
Table 1.
Types of Quality Improvement for Palliative Care3.
| Physician/other provider reminder systems (such as prompts in paper charts or computer-based reminders); |
| Facilitated relay of clinical data to providers (patient data transmitted by telephone call or fax, from outpatient specialty clinics to primary care physicians; would include structured documentation tools, collection of patient-reported outcomes); |
| Audit and feedback (physician performance tracking and reviews, using quality indicators and reports, comparisons with National/ State quality report cards, publicly released performance data, and benchmark outcomes data); |
| Physician/other provider education (workshops and professional conferences, educational outreach visits, distribution of educational materials); |
| Provider training/support on quality improvement skills; |
| Patient education (classes, parent and family education, pamphlets, and other media); |
| Promotion of self-management (workshops, materials such as structured prompt sheets for patients to ask physicians about palliative care issues); |
| Patient reminder systems (telephone calls or postcards from providers to their patients); |
| Organizational changes (plan-do-study-act collaboratives, multidisciplinary teams, shifting from paper-based to computer-based record keeping, long-distance case discussion between professional peers); |
| Financial incentives, regulation, and policy (performance-based bonuses and alternative reimbursement systems for physicians, positive or negative financial incentives for patients, and changes in professional licensure requirements; would include state policy, Physician Orders for Life-Sustaining Treatment [POLST] or similar programs, advance directive policy). |
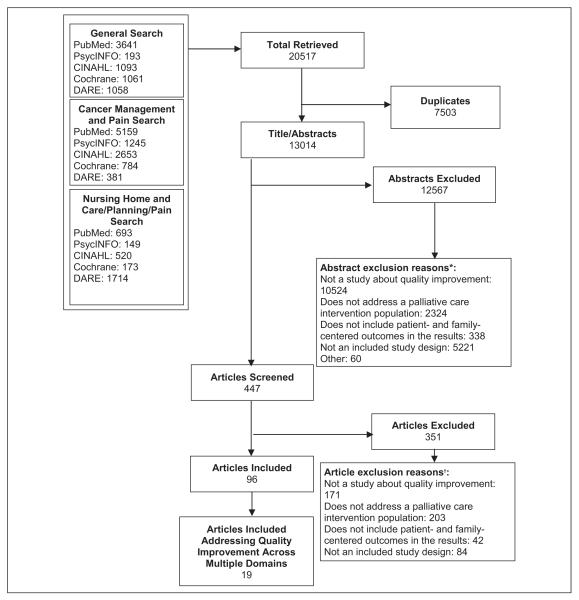
Two reviewers independently screened citations at title, abstract, and full-article levels for inclusion in the review (Figure 1). We limited our review to prospective quality improvement intervention studies that included a control group (including pre–post studies). We excluded articles if they did not report original, quantitative data, if more than 50% of intervention patients did not meet the population definition of patients with advanced and serious disease, or if the data did not include patient- or family-centered outcomes, including improvement in quality of life, quality of care, symptom management, satisfaction, or psychosocial support.
Figure 1.
Results of the literature search and article screening for applicability. *The sum of reasons for exclusion at abstract screening is greater than the total number of exclusions as each reviewer could select a different reason for exclusion. †The sum of reasons for exclusion at article screening is greater than the total number of exclusions as each reviewer could select a different reason for exclusion.
One reviewer abstracted data from included articles, which were checked by a second reviewer. We abstracted information on population characteristics, study design, setting, description of interventions, quality improvement interventions, and the effects of interventions on the patient-and family-centered outcomes. We did not conduct meta-analyses because the interventions and reporting of outcomes were too heterogeneous to allow for any pooling of data.
We evaluated the quality of individual studies using the risk of bias assessment.13 We graded the strength of the best available evidence for the outcomes of pain and quality of life in relevant studies using the GRADE Working Group criteria adapted by the Agency for Healthcare Research and Quality14 (for details, see report).11
Results
Facilitated Relay of Clinical Data to Providers
We found 6 publications on 5 studies meeting our inclusion criteria focusing on relay of clinical information to providers for quality improvement. All 5 studies were randomized controlled trials (RCTs) conducted in ambulatory settings; 4 were single-center studies treating patients with metastatic cancer, and 1 was a multicenter study of patients with lung cancer (Table 2).
Table 2.
Characteristics of Studies of Interventions to Improve the Quality of Palliative Care.
| Author, Year | Study design |
Sample size | Setting | Intervention |
|---|---|---|---|---|
| Facilitated relay of clinical data | ||||
| Detmar, 200215 | RCT | 214 | Ambulatory | Preconsultation quality-of-life survey |
| Mills, 200916 | RCT | 115 | Ambulatory | Patient-held quality-of-life diary |
| Rosenbloom, 200717 | RCT | 213 | Ambulatory | Quality-of-life survey with interpretive assistance |
| Taenzer, 200018 | RCT | 53 | Ambulatory | Preconsultation quality-of-life survey |
| Velikova, 2004,19 Velikova, 201020 | RCT | 286 | Ambulatory | Preconsultation quality-of-life survey |
| Provider education | ||||
| Keay, 200321 | Non-RCT | 176 | Nursing home | Half-day physician education with audit and feedback |
| Audit and feedback | ||||
| Campion, 201122 | Non-RCT | 973 practices |
Ambulatory | Evaluation and feedback to practices |
| Jacobs, 200223 | Non-RCT | 194 | Hospital | Evaluative feedback to individual physicians |
| Patient/caregiver education and self- management |
||||
| Sherwood, 200524 | RCT | 124 | Ambulatory | Cognitive–behavioral intervention for symptoms |
| Sikorskii, 200725 | RCT | 435 | Home | Nurse-assisted symptom management |
| Rummans, 200626 | RCT | 115 | Ambulatory | Structured multidisciplinary quality-of-life intervention |
| Meyers, 201127 | RCT | 476 | Ambulatory/home | Problem solving with patient–caregiver dyads |
| McMillan, 2006,28 200729 | RCT | 709 | Ambulatory/home | Creativity, optimism, planning, expert information intervention |
| Organizational change | ||||
| Holt, 201030 | Non-RCT | 292 | Ambulatory | Clinic providing more accessible, efficient palliative radiotherapy |
| Multiple quality improvement types | ||||
| Curtis, 200831 | Non-RCT | 590 | ICU | Education, local champions, feedback, system support |
| Curtis, 201132 | RCT | 822 | ICU | Same as above |
| Hanson, 200533 | Non-RCT | 458 | Nursing home | Plan-do-study-act with education, training, feedback, leadership |
Abbreviation: RCT, randomized controlled trial; ICU, intensive care unit.
Only 1 of the 5 studies demonstrated a significant effect of the intervention on quality of life. Velikova et al conducted a 3-arm RCT, randomizing patients to (1) complete a touch screen health-related quality-of-life survey that provided feedback to physicians, (2) complete the quality-of-life survey without feedback to physicians; or (3) not complete a survey. After 3visits postrandomization, a higher number of symptoms were mentioned during the clinical encounter in the intervention group (where patients completed the survey that provided feedback to the physician) than in the other 2 control groups (estimate effect: 4.5; P = .03). The intervention group showed an improved quality of life when compared to the control group (estimate effect: 8.0, P = .006).19 In an analysis of secondary outcomes of evaluations of care, patients in the intervention group reported higher ratings for communication (P = .03) but not for 2 other subscales or for satisfaction with care (Table 3).20 Mills et al studied the use of a structured patient-held quality-of-life diary at home, weekly for 16 weeks; patients were encouraged to share it with their health care providers. The study found no significant differences between the groups for the primary quality-of-life measure and the groups for satisfaction. However, some of the secondary quality-of-life outcomes were worse in the intervention group compared to the control group. Most patients did not give feedback to their providers (Table 3).16 Detmar et al used a quality-of-life questionnaire among patients undergoing palliative chemotherapy at 3 consecutive visits to determine the effect on patient–physician communication. Ten physicians were enrolled in a randomized, crossover study to receive a graphic summary of responses to the questionnaire prior to consultation. After 3 visits, patients in the intervention group reported significantly greater communication on quality-of-life issues with their physicians than those in the control group (mean: 4.7 vs 3.7; P = .01), but there were no significant differences in secondary outcomes of quality of life or patient satisfaction (Table 3).15 Two studies found no differences in any reported outcomes between their intervention and control groups.17,18 Rosenbloom et al evaluated the effect of quality-of-life screening with physician-interpretive assistance on quality-of-life outcomes and satisfaction among patients with metastatic cancer. This 3-arm study randomized 213 patients to complete a quality-of-life survey with follow-up interview and discussion, complete a quality-of-life survey without follow-up, or receive usual care. After follow-up at 3 and 6 months, the study showed no significant improvement in quality of life or satisfaction among any of the groups.17 Taenzer et al evaluated the effect of a computerized quality-of-life survey on physician behavior and patient satisfaction. This 2-armed randomized study did not demonstrate significant differences in patient satisfaction or physician documentation between the intervention and the control groups (Table 3).18
Table 3.
Outcomes of Studies of Interventions to Improve Quality of Palliative Care.
| Author, Year | Study design |
Sample size |
Outcomes |
||||
|---|---|---|---|---|---|---|---|
| Facilitated relay of clinical data | Quality of life |
Satisfaction | Quality of care |
Psychosocial support |
Symptoms | ||
| Detmar, 200215 | RCT | 214 | NS | NS | |||
| Mills, 200916 | RCT | 115 | NS | NS | |||
| Rosenbloom, 200717 | RCT | 213 | NS | NS | |||
| Taenzer, 200018 | RCT | 53 | NS | NS | |||
| Velikova, 2004,19 Velikova, 201020 |
RCT | 286 | S | NS | |||
| Provider education | |||||||
| Keay, 200321 | Non-RCT | 176 | S | ||||
| Audit and feedback | |||||||
| Campion, 201122 | Non-RCT | S (10/15 measures) |
|||||
| Jacobs, 200223 | Non-RCT | 194 | NS (9/10 items) | ||||
| Patient/caregiver education and self-management |
|||||||
| Rummans, 200626 | RCT | 115 | S | NS | |||
| Sherwood, 200524 | RCT | 124 | S | ||||
| Sikorskii, 200725 | RCT | 435 | NS | ||||
| McMillan, 2006,28 200729 | RCT | 709 | S | S | NS pain, S distress |
||
| Meyers, 201127 | RCT | 476 | NS patient, S caregiver |
||||
| Organizational change | |||||||
| Holt, 201030 | Non-RCT | 292 | S | ||||
| Multiple quality improvement types |
|||||||
| Curtis, 200831 | Non-RCT | 590 | NS | NS | |||
| Curtis, 201132 | RCT | 822 | NS | NS | |||
| Hanson, 200533 | Non-RCT | 458 | S | ||||
Abbreviations: RCT, randomized controlled trial; NS, not significant; S, significant; QOL, quality of life.
Audit and Feedback
We identified 2 studies that focused on audit and feedback, one small single-center non-RCT found no significant improvement in quality measures, and a large multicenter non-RCT found significant improvements in multiple quality measures.22, 23 In a single-center non-RCT, physicians received 3 biannual palliative care reports on patients where death was likely, including patient/family satisfaction and reported symptom relief and timeliness of advance directive discussions. The study found no difference in quality of care on 10 items evaluated through chart reviews. However, 2 important limitations of this study are that physicians may not have reviewed their reports, and feedback may have been delayed too long (between 3 and 9 months after care) to make a significant difference on the outcome measures (Table 3).23
A recent study by Campion et al evaluated the impact of the Quality Oncology Practice Initiative (QOPI) on palliative care quality indicators in outpatient oncology practice. The QOPI is a consortium of oncology practices, which voluntarily reports key quality-of-care performance measures and receives feedback for quality improvement purposes. Oncology practices that participated in multiple cycles of reporting and feedback reported significantly higher quality of care on multiple palliative care performance measures than those practices that had just started participating in QOPI, including all 4 measures for pain management, 2 of 3 dyspnea measures, and 4of 7 measures on hospice and palliative care discussions and referrals; there was no difference in chemotherapy use in the last 2 weeks of life. For example, patient pain was appropriately assessed more frequently (66% vs 47%, P < .001) and dyspnea was more often addressed appropriately (71% vs 61%, P =.005) prior to death. In addition, practices reporting in multiple cycles were more likely to address hospice or palliative care appropriately (65% vs 55%, P .005) and enroll patients in hospice (53% vs 44%, P = .03; Table 3).22
Patient/Caregiver Education and Self-Management
We identified 6 publications on 5 studies that met inclusion criteria, focusing on patient/caregiver education and self-management addressing multiple symptoms. In all, 4 of the studies focused on reducing symptom severity, and 1 focused on maintaining quality of life. All were single-center RCTs in patients with advanced cancer using external providers to deliver the intervention. Of these 5 studies, 4 had statistically significant findings for at least 1 key outcome, although 1 was significant only at 1 of the 3 time points (Tables 2 and 3).
One study randomized 124 patients receiving chemotherapy to either standard of care or standard of care plus a cognitive behavioral intervention targeted to decrease the severity of symptoms. Experienced oncology nurses delivered 5 contact sessions over an 8-week time period aimed at teaching problem-solving techniques to reduce symptom severity. At 20 weeks of follow-up, individuals participating in the intervention experienced significantly lower symptom severity than the control group. From a pooled, baseline symptom severity score of 31.2 (standard deviation [SD] = 18.3), patients in the intervention group had a mean symptom severity score of 22.1 (SD = 15.2) versus 28.2 (SD = 19.6) in the control group (P = .02), at 20 weeks.24
The second study randomized 437 patients with cancer undergoing chemotherapy to either nurse-assisted symptom management (NASM) or automated telephone symptom management (ATSM). The study compared the impact of an 8-week, 6-contact ATSM intervention delivered through an automated system with an NASM intervention (which had previously been found to improve outcomes compared to standard care) delivered by experienced cancer nurses. This study looked at reducing the severity of 17 common symptoms experienced by patients receiving chemotherapy but found no significant differences between the NASM and the ATSM groups postintervention.25 The third study randomized caregivers of hospice patients to the creativity, optimism, planning, expert (COPE) information intervention with 3 home visits and 2 interim calls to assist with symptom management, compared to standard hospice care.29 For the primary outcomes, which were caregiver outcomes, the study found an impact on caregiver quality of life (estimate 0.096, P = .04) and task burden (estimate 0.01, P = .04) as well as the burden of patient symptoms. For patient symptoms, the study found no impact on dyspnea or pain but did find an impact on distress (estimate 0.101, P = .009).28 The fourth study also used the COPE intervention for patient–caregiver dyads for patients with advanced cancer participating in clinical trials, over 3 educational sessions. The study found statistically significant improvements in caregiver (P = .02) but not patient quality of life, but neither patients nor caregivers showed any change in problem-solving skills.27
The last study randomized 115 patients with advanced cancer to either 8 structured multidisciplinary sessions or usual care. Eight structured sessions lasted for 90 minutes each and addressed the domains of quality of life, including cognitive, physical, emotional, spiritual, and social functioning. Participants in the intervention group also received a manual reviewing material covered in the 8 multidisciplinary sessions. The study used the Spitzer Quality of Life Uniscale for the primary outcome measure, overall quality of life, and the Linear Analog Scale of Assessment of quality of life for 12 secondary outcome measures. After the 4-week intervention, the quality of life among patients in the intervention group showed a 3-point increase over baseline, while patients in the control group showed a 9-point decrease from baseline (P = .009). For patients in the control group, quality-of-life measures=returned to baseline at 8 weeks’ and 27 weeks’ follow-up, while quality-of-life measures in the intervention group maintained the initial increase over baseline at each subsequent follow-up (Table 3).26
Provider Education
We identified 1 non-RCT addressing only this quality improvement type that met the inclusion criteria. Keay et al conducted a half-day education seminar for 5 nursing homes and provided quality improvement suggestions targeting medical directors and physicians with the majority of patients. A before–after evaluation found statistically significant improvements in symptom control during dying for quality indicators for terminal care in nursing homes (19% to 45%, P < .001).21
Organizational Change
We identified 1 non-RCT that focused on organizational change (Table 2).30 Holt et al conducted a before–after study of a rapid-response clinic for patients referred for palliative radiotherapy to reduce wait times and have patients seen same day, if possible. They found a statistically significant increase in single-fraction treatment (which is guideline-recommended care; 65% vs 42%, P −.002) and a statistically significant reduction in time-to-treatment (<24 hours; 74% vs 27%, P < .001) but found no improvement in waiting times for consultation (Table 3).30
Multiple Quality Improvement Types
We identified 3 studies that focused on multiple quality improvement types and multiple outcomes targeted for improvement. Only 1 study found a significant improvement in patient- or caregiver-reported outcomes.
Two studies evaluated the same intervention, first in a non-RCT and then in a multicenter RCT (Table 2).31,32 Curtis et al conducted the integrating palliative care in the intensive care unit (ICU) study. The multiple quality improvement types included clinician education, local champions, academic detailing, feedback to clinicians, and system support. The non-RCT of the intervention found no significant impact on family-completed Quality of Dying and Death scale or satisfaction.31 However, the median length of stay in the ICU was significantly lower in the intervention group compared to the control group (3.8 vs 3.1 days, P = .01). In the multicenter RCT in 12 hospitals, there were no significant differences in any of these outcomes (Table 3).32 Hanson et al conducted a quality improvement non-RCT over a 6-month period in 9 nursing homes. The intervention used a plan-do-study-act structure with feedback of performance data at 3 time points. The quality improvement types studied included recruitment and training of palliative care leadership in each facility, in-depth education and technical assistance meetings for team members as well as educational sessions for nursing staff. Postintervention, there was a significant increase in hospice enrollment from 4% to 6.8% (P = .01) and in the use of pain assessments (18%-60%, P < .001) and advance care planning discussions (4%-17%, P < .001); but the use of pain interventions did not change. The nursing homes were relatively diverse, and all volunteered to participate in this study.33
We found no published studies that focused on reminder systems for patients or providers, training and support for providers, financial incentives, or regulation and policy.
All studies, both RCTs and non-RCTs, were of medium quality (details of quality assessment are provided elsewhere).11 Given the small number of studies for each quality improvement type, the variability in outcomes measured, and lack of statistically significant improvements in many studies, strength of evidence was low overall across the quality improvement types.
Discussion
In this review of quality improvement interventions among patients with advanced and serious illness, we found low strength of evidence supporting the possible effectiveness of specific types of quality improvement for palliative care outcomes, mainly due to the small number of studies for each quality improvement type, variability in outcomes evaluated, and inconsistent findings among studies. The most common intervention types that we identified were facilitated relay of clinical information, patient and caregiver education and self-management, audit and feedback to providers, and multiple quality improvement types. We found few studies focusing on provider education or training, reminders, organizational change, or regulatory or policy interventions that met our inclusion criteria for prospective studies that measured patient-centered outcomes.
For facilitated relay of clinical data to providers, only 1 of 5 studies that we identified, all randomized trials in ambulatory oncology care, found a significant improvement in quality of life, and none found an impact for satisfaction. These findings are less promising than the results for this type of intervention across other patient populations; the most recent systematic review34 (through 2007) of the impact of providing patient-reported outcomes information to health care professionals in daily clinical practice found that almost half had an impact on patient outcomes. The studies in patients with advanced illness have many of the same limitations and potential explanations for lack of impact as studies of this quality improvement strategy in other populations. Behavior change usually requires more than simply providing additional information. In addition, clinicians or patients may not change the way that they interact to use standardized symptom or quality-of-life data; may not prioritize these issues in consultations that often focus on treatment options; or may not know how best to use the information.35
Of the 5 studies targeting patient education and self-management for a range of symptoms, 4 showed significant improvement in outcomes (quality of life and patient symptoms) compared to control groups. This quality improvement type has also been effective for a variety of other conditions, such as asthma.36 Although only 1 of the 3 studies including multiple quality improvement types showed a significant improvement in patient-reported outcomes, these studies were conducted in complex settings (ICUs and nursing homes) with competing priorities, and more experience may be needed to implement these types of interventions. Finally, audit and feedback is an intervention type that appears promising for future study; 1 of 2 audit and feedback studies employed a multifaceted, voluntary intervention that targeted key quality-of-care outcomes and provided feedback for meaningful quality improvement within a large number of individual practices, resulting in significantly higher quality of care across most measures.
Our review has a number of limitations. Our focus on a relatively narrow definition of quality improvement excluded a number of studies and interventions such as palliative care consultation and case management, although these have been reviewed elsewhere.37 Our focus on studies in patients with advanced and serious illness also excluded some studies using organizational change in broader populations that also include palliative care patients, such as general nursing home populations. Importantly, many quality improvement projects are not conducted as research or in academic centers (particularly hospice-based studies) and are not published in the peer-reviewed literature and therefore could not be included in this review. Finally, due to the diversity of interventions, outcomes measured, and reporting of results (including infrequent reporting of effect sizes), we were limited to reporting whether the studies had statistically significant effects and were unable to perform quantitative syntheses.
We also identified several key gaps in the literature. Few studies evaluating regulatory or policy interventions met our inclusion criteria for prospective studies with a control group using patient-centered outcomes and focusing on advanced and serious disease. More rigorous studies on policy interventions such as Physicians Orders for Life-Sustaining Treatment and studies on hospital policies on end-of-life care (such as changes in do-not-resuscitate policies) are needed. Few studies frequently used quality improvement approaches in other populations, such as reminders, collaboratives, or plan-do-study-act interventions. Finally, few included studies were conducted in hospices or nursing homes.
In conclusion, we found low strength of evidence to support quality improvement strategies for palliative care, although there were few studies for any given quality improvement type, and interventions and outcomes were heterogeneous. Results were most significant for interventions focusing on patient and caregiver education and self-management for multiple symptoms, although outcomes varied, and a few studies on organizational change, audit and feedback, and multiple quality improvement types also found statistically significant improvement in outcomes. Although few quality improvement evaluations have been published in this population, there is significant need for better quality care and quality improvement.
Often, patients undergo aggressive treatment during the final weeks of life, delaying palliative care until the final few days of life, resulting in palliative care that is simply used to manage death rather than to palliate symptoms.38 Uncoordinated or ineffective care creates an undue burden on the already physically, mentally, and emotionally taxing experience of suffering with advanced and serious illness. In hospice care in particular, external barriers such as the lack of resources and models for quality improvement, lack of evidence for best practices, and concerns that traditional models of quality improvement may not work well for hospice have all been reported,39 emphasizing the additional need for research in this setting. More research is needed to better understand how to improve access to palliative care, increase the use of symptom data in practice, change provider and system behavior, and implement and measure the impact of quality improvement in patients with advanced and serious illness.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded under contract 290-2007-10061-1-EPC3 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Qaseem A, Snow V, Shekelle P, Casey DE, Jr, Cross JT, Jr, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Dallas P, Dolan NC, Forciea MA, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(2):141–146. doi: 10.7326/0003-4819-148-2-200801150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Danz MS, Rubenstein LV, Hempel S, et al. Identifying quality improvement intervention evaluations: is consensus achievable? Qual Saf Health Care. 2010;19(4):279–283. doi: 10.1136/qshc.2009.036475. [DOI] [PubMed] [Google Scholar]
- 3.Shojania KG, M. K, Wachter RM, Owens DK. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Agency for Healthcare Research and Quality; Rockville, MD: 2004. Report nr 1. [PubMed] [Google Scholar]
- 4.O’Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;(4):CD000409. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flodgren G, Parmelli E, Doumit G, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011;(8):CD000125. doi: 10.1002/14651858.CD000125.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamtvedt G, Young JM, Kristoffersen DT, O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;(2):CD000259. doi: 10.1002/14651858.CD000259.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Shojania KG, Jennings A, Mayhew A, Ramsay C, Eccles M, Grimshaw J. Effect of point-of-care computer reminders on physician behaviour: a systematic review. CMAJ. 2010;182(5):E216–E225. doi: 10.1503/cmaj.090578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shojania KG, Grimshaw JM. Evidence-based quality improvement: the state of the science. Health Aff (Millwood) 2005;24(1):138–150. doi: 10.1377/hlthaff.24.1.138. [DOI] [PubMed] [Google Scholar]
- 9.National Consensus Project for Quality Palliative Care . Clinical Practice Guidelines for Quality Palliative Care. 2nd ed Pittsburgh, PA: USA: 2009. [Google Scholar]
- 10.Institute of Medicine . Improving Palliative Care for Cancer. National Academy Press; Washington DC: 2001. [Google Scholar]
- 11.Dy SM, Aslakson R, Wilson RF, et al. Improving Health Care and Palliative Care for Advanced and Serious Illness. Closing the Quality Gap: Revisiting the State of the Science. Evidence Report No. 208. 9Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290-2007-10061-I. AHRQ Publication No. 12(13)-E014-EF. Agency for Healthcare Research and Quality; Rockville, MD: Oct, 2012. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [Google Scholar]
- 12.Cochrane effective practice and organization of care review group EPOC Taxonomy. 2010 http://www.epoc.cochrane.org.
- 13.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. version 5.0.2 The Cochrane Collaboration; 2009. [Google Scholar]
- 14.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions–agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–523. doi: 10.1016/j.jclinepi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288(23):3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 16.Mills ME, Murray LJ, Johnston BT, Cardwell C, Donnelly M. Does a patient-held quality-of-life diary benefit patients with inoperable lung cancer? J Clin Oncol. 2009;27(1):70–77. doi: 10.1200/JCO.2008.17.5687. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology. 2007;16(12):1069–1079. doi: 10.1002/pon.1184. [DOI] [PubMed] [Google Scholar]
- 18.Taenzer P, Bultz BD, Carlson LE, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology. 2000;9(3):203–213. doi: 10.1002/1099-1611(200005/06)9:3<203::aid-pon453>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 20.Velikova G, Keding A, Harley C, et al. Patients report improvements in continuity of care when quality of life assessments are used routinely in oncology practice: secondary outcomes of a randomised controlled trial. Eur J Cancer. 2010;46(13):2381–2388. doi: 10.1016/j.ejca.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Keay TJ, Alexander C, McNally K, Crusse E, Eger RE. Nursing home physician educational intervention improves end-of-life outcomes. J Palliat Med. 2003;6(2):205–213. doi: 10.1089/109662103764978452. [DOI] [PubMed] [Google Scholar]
- 22.Campion FX, Larson LR, Kadlubek PJ, Earle CC, Neuss MN. Advancing performance measurement in oncology. Am J Manag Care. 2011;17(suppl 5):SP32–SP36. [PubMed] [Google Scholar]
- 23.Jacobs LG, Bonuck K, Burton W. Can “palliative care reports” improve end-of-life care for hospitalized patients? J Pain Symptom Manage. 2002;24(3):299–311. doi: 10.1016/s0885-3924(02)00492-x. [DOI] [PubMed] [Google Scholar]
- 24.Sherwood P, Given BA, Given CW, et al. A cognitive behavioral intervention for symptom management in patients with advanced cancer. Oncol Nurs Forum. 2005;32(6):1190–1198. doi: 10.1188/05.ONF.1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikorskii A, Given CW, Given B, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34(3):253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006;24(4):635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 27.Meyers FJ, Carducci M, Loscalzo MJ, Linder J, Greasby T, Beckett LA. Effects of a problem-solving intervention (COPE) on quality of life for patients with advanced cancer on clinical trials and their caregivers: Simultaneous Care Educational Intervention (SCEI): linking palliation and clinical trials. J Palliat Med. 2011;14(4):465–473. doi: 10.1089/jpm.2010.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan SC, Small BJ, Weitzner M, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer. 2006;106(1):214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- 29.McMillan SC, Small BJ. Using the COPE intervention for family caregivers to improve symptoms of hospice homecare patients: a clinical trial. Oncol Nurs Forum. 2007;34(2):313–321. doi: 10.1188/07.ONF.313-321. [DOI] [PubMed] [Google Scholar]
- 30.Holt TR, Yau VK. Innovative program for palliative radiotherapy in australia. J Med Imaging Radiat Oncol. 2010;54(1):76–81. doi: 10.1111/j.1754-9485.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 31.Curtis JR, Treece PD, Nielsen EL, et al. Integrating palliative and critical care: evaluation of a quality-improvement intervention. Am J Respir Crit Care Med. 2008;178(3):269–275. doi: 10.1164/rccm.200802-272OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis JR, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183(3):348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanson LC, Reynolds KS, Henderson M, Pickard CG. A quality improvement intervention to increase palliative care in nursing homes. J Palliat Med. 2005;8(3):576–584. doi: 10.1089/jpm.2005.8.576. [DOI] [PubMed] [Google Scholar]
- 34.Valderas JM, Kotzeva A, Espallargues M, et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17(2):179–193. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- 35.Greenhalgh J. The applications of PROs in clinicalpractice: whatare they, do they work, and why? Qual Life Res. 2009;18(1):115–123. doi: 10.1007/s11136-008-9430-6. [DOI] [PubMed] [Google Scholar]
- 36.Bravata DM, Sundaram V, Lewis R, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Agency for Healthcare Research and Quality; Rockville, MD: 2007. Report nr 5. [PubMed] [Google Scholar]
- 37.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299(14):1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 38.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durham DD, Rokoske FS, Hanson LC, Cagle JG, Schenck AP. Quality improvement in hospice: adding a big job to an already big job? Am J Med Qual. 2011;26(2):103–109. doi: 10.1177/1062860610379631. [DOI] [PubMed] [Google Scholar]