Summary
Background
Although human papillomavirus detection in cervical lymph nodes of head and neck squamous cell cancers (HNSCC) of unknown primary site (UP) is indicative of a primary tumor of the oropharynx (OP), localization can remain elusive. Therefore, we investigated ultrasonography (US) for the identification of the primary tumor.
Methods
Eligible cases had HNSCC of UP after evaluation by a head and neck surgical oncologist. Controls were healthy volunteers. Transcervical and intraoral ultrasonography was performed by a standard protocol using convex (3.75–6.0 MHz and 5–7.5 MHz) transducers. US findings were compared with operative examination (exam under anesthesia, direct laryngoscopy) and biopsies. The primary outcome of interest was the presence or absence of a lesion on US.
Results
10 cases and 20 controls were enrolled. PET/CT scans were negative/nonspecific (9), or suspicious (1) for a primary lesion. On US, predominantly hypoechoic (9 of 10) lesions were visualized consistent with base of tongue (n = 7) or tonsil (n = 3) primary tumors. On operative examination, 5 of 10 were appreciated. Two additional primaries were confirmed with biopsies “directed” by preoperative US. This represents an overall diagnostic rate of 70%, which is 20% higher than our detection rate for 2008–2010. The three cases in which a suspicious lesion was visualized on US, yet remained UP despite further interventions, could represent false positives, misclassification or operator variability. No lesions were suspected among the controls.
Conclusion
Ultrasound has promise for detection of UPs of the OP and therefore warrants further investigation.
Keywords: Unknown primary, Head and neck cancer, Oropharynx neoplasm, Ultrasonography, Human papillomavirus (HPV)
Background
Head and neck squamous cell cancers (HNSCCs) with unknown primary site (UP) represent approximately 5% of HNSCCs [1]. Historically, HNSCCs of UP were recognized to be a heterogeneous clinical entity with subclinical primary tumors of the nasopharynx, hypopharynx and oropharynx. In the era of rising incidence of human papillomavirus (HPV)-related oropharyngeal squamous cell cancer (OPSCC), the majority of squamous cell carcinomas of the neck that present with an UP arise from the oropharynx [2–4]. HPV-positive OPSCCs tend to have small primary tumors and advanced nodal disease [5,6]. Therefore, when individuals present with large nodal disease and no obvious primary lesion, the primary is likely to be hidden within the cryptic lymphoepithelium of the oropharynx which is difficult to examine [7]. Although the tonsil and base of tongue (BOT) are the two most common subsites of unknown primaries [8], identification of the primary site remains elusive in up to 60% of HNSCCs of UP [9].
The traditional diagnostic paradigm comprises of comprehensive clinical examination including indirect mirror exam and fiber-optic laryngoscopy, and imaging. Contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) are recommended in the evaluation of HNSCCs of UP and primary tumor detection rates range from 9% to 20% [10,11]. PET scans demonstrate a primary tumor in 25 to 35% of cases [10,12,13]. Operative exploration of the primary site occurs in a stepwise fashion, starting with an exam under anesthesia and direct laryngoscopy with biopsy, and thereafter continuing with “blind” biopsies directed at the most common sites of UPs and bilateral palatine tonsillectomies until the UP is identified [14]. Recent case series have proposed the addition of lingual tonsillectomies to the algorithm in the event of negative biopsies and palatine tonsillectomies [15,16]. While each operative component may increase the probability of identifying the primary tumor site, each has an inherent risk (e.g., bleeding and death), prolongs time under anesthesia and could require greater than one trip to the operating room.
Successful identification of the primary tumor can have significant therapeutic implications. For example, radiotherapy fields can be limited to the oropharynx and thus reduce treatment morbidity relative to irradiation of the entire pharyngeal axis in the case of an UP. Additionally, the identification of the primary site may provide patients with therapeutic alternatives, such as definitive surgical treatment including transoral resection of the primary tumor [16,17].
In a previous study, we demonstrated that ultrasound could be used to visualize and describe anatomic characteristics of BOT malignancies [18]. With this knowledge, we sought to investigate the potential of ultrasound to detect primary tumors within the oropharynx among patients who present with HNSCC of UP.
Methods
Cases and controls were prospectively enrolled. Eligible cases included patients with a pathological diagnosis of squamous cell cancer in a cervical lymph node without clinical identification of the primary site after examination by a head and neck surgical oncologist. Exclusion criteria included clinical suspicion of a cutaneous malignancy, prior head and neck radiotherapy, or neck surgery (incisional or excisional biopsy and/or neck dissection). Controls were healthy volunteers without known head and neck cancer. The study was approved by the Greater Baltimore Medical Center (GBMC) investigational review board and written consent was obtained from all study subjects.
Ultrasound examination was performed by investigators who were not blinded to clinical information (RGB, CF). A Toshiba ultrasound model SSA-580A was used. The transducer used for the transcervical examination was convex (3.75–6.0 MHz; Model PVQ-375A) and set at 6 MHz. For the intraoral examination, an endocavitary multifrequency convex probe (5–7.5 MHz; Model PVM-651VT) set at 7.5 MHz was used.
Ultrasound examination was performed by use of a standard protocol. Subjects were seated in an ENT exam chair. In a stepwise fashion, the following transcervical views were obtained: midline sagittal, bilateral parasagittal, and coronal. Before initiating the transoral exam, patients were sprayed with topical 1% lidocaine. The transducer surface was covered with ultrasound gel and wrapped with a disposable clingy wrap before initiating intraoral ultrasonography. To visualize each side of the pharynx (including palatine tonsils), the probe was placed on the dorsum of the tongue in superior/inferior, then medio/lateral orientation. For transcervical examination, the landmarks for the BOT were determined by identifying the central portions of the hyoid bone and the mandible and dividing this into thirds. The posterior third was considered the ultrasonographic base of tongue (Fig. 1). Doppler was used to visualize the lingual artery for tumors in the BOT and carotid artery for tumors in the tonsil.
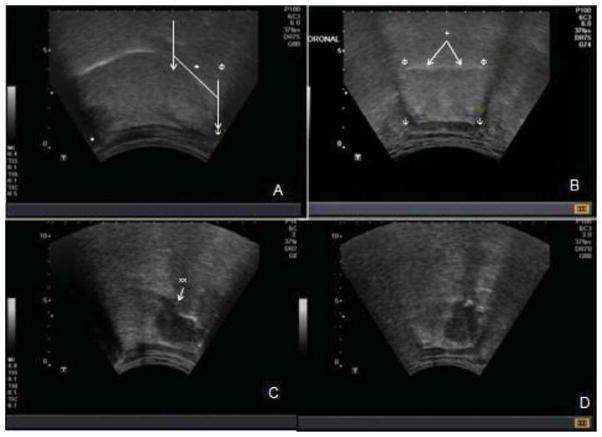
Fig. 1.
Transcervical ultrasound examination of oropharynx. Parasagittal view (A) demonstrates intact hyperechoic oral mucosa of oral and base of tongue (+). The hyoid (ψ) and mandibular (*) shadows are shown. The sonographic base of tongue is the posterior one third of the distance between the central portions of the mandible and hyoid annotated by (↓). The palatine tonsillar region is isoechoic (Φ). On coronal view (B) hyoid shadows are seen bilaterally (ψ). The oral mucosa is intact (+). The base of tongue musculature is noted to be symmetric. Panels C and D are images representative of a base of tongue lesion. On parasagittal view is a hypoechoic lesion, the majority of which is beneath the mucosal surface of the base of tongue, with a slight disruption of the mucosa (xx). The mass is also visualized in coronal view (D). Extent across midline is appreciated in coronal view.
Data were recorded using a standardized case report form at the time of ultrasound examination. An ultrasound impression was ascertained which captured the suspected location of tumor, size, anatomic extent (crossing midline, adjacent site involvement, distance to either lingual or carotid artery) and echogenic characteristics of the suspected lesion and margin (representative image reviewed in Fig. 1c and d). Ultrasound findings were shared with referring physicians before direct laryngoscopy (DL). Clinical data (including physical examination and imaging), operative examination (palpation, DL) as well as histopathology (including p16 immunohistochemistry, a surrogate of HPV tumor status) were abstracted from the medical record. Reports from PET/CT scans were reviewed and categorized as (1) “suspicious” if a lesion was definitively noted, (2) “nonspecific” if an asymmetry, question of increased physiologic uptake or any indeterminate interpretation by the radiologist and (3) “negative” if no mention of any abnormality in the oropharynx. Study data were collected and managed using REDCap (Research Electronic Data Capture), an electronic data capture tools hosted by Johns Hopkins Bloomberg School of Public Health. Data were exported to Stata 12.0 (College Station, Texas) for further analysis. The primary variable of interest was the presence or absence of a lesion on ultrasound. Descriptive characteristics were summarized. The ultrasound findings were compared with imaging, operative and histopathologic findings.
Given that this study was performed in the context of an exploratory study to determine the feasibility of US visualization of OP lesions, there was no comparison group of UPs undergoing diagnostic evaluation without US. This study was concurrent with a protocol to evaluate known or suspicious OPSCC lesions. To compare the detection rate of primary tumors with and without US, we determined the detection rate of primary tumor sites among patients evaluated for HNSCC of UP at Johns Hopkins Head and Neck Surgery at GBMC from January 2008 to December 2010. Any new patient presented to the multidisciplinary head and neck tumor board with a diagnosis of HNSCC of UP was identified. Operative notes, pathology results and final staging were retrospectively abstracted. Patients with a final non-SCC diagnosis or salivary gland primaries were excluded from the analysis. This served as a historical control group.
Results
Ten subjects identified as HNSCC of UP by a head and neck oncologic surgeon met eligibility criteria and were enrolled in this study. The majority of the cases were male (80%) and the mean age was 60.6 years (range 43–76). At presentation, the majority had nodal disease stage IIa or greater (80%). All 10 subjects had no clinically apparent oropharyngeal lesion on physical examination and flexible laryngoscopy. On PET/CT scans performed for staging purposes, no primary lesion was identified in 6 of 10 cases, three were nonspecific (asymmetry of unknown significance in the oropharynx) and one was suspicious.
On ultrasound examination, a candidate oropharyngeal lesion with characteristics consistent with a primary tumor site ipsilateral to the pathologic cervical node was observed in 10 of 10 cases (100%). On ultrasound assessment, suspected primary tumor sub-sites were BOT (n = 7) and tonsil (n = 3). The ultrasound characteristics of the suspected lesions are summarized in Table 1. The suspected lesions appeared hypoechoic in 90% of cases. Relative to the suspected lesions, the margins were irregularly shaped (70%, 7 of 10), hypoechoic (80%, 8 of 10) and not well circumscribed in the majority of cases (80%, 8 of 10). None of the tumors crossed the midline. Among the BOT tumors, the range of dimensions were superior–inferior (SI) 10–17.2 mm, anterior–posterior (AP) 9–17.5 mm, and medio-lateral (ML) 6.3–20.6 mm. The tonsil tumors were smaller, ranging from 6 mm to 8.5 mm (SI), 5–6.6 mm (AP) and 5–12.3 mm (ML). Representative images are displayed in Fig. 2.
Table 1.
Ultrasound characteristics of suspected primary lesions.
| n (%) | |
|---|---|
| Subsite of primary tumor | |
| BOT | 7 (70) |
| Tonsil | 3 (30) |
| Echogenicity of lesion | |
| Hypoechoic | 9 (90) |
| Isoechoic | 1 (10) |
| Echogenicity of margin | |
| Isoechoic with heterogeneous foci | 1 (10) |
| Hypoechoic | 8 (80) |
| Hyperechoic | 1 (10) |
| Shape of margin | |
| Regular | 3 (30) |
| Irregular | 7 (70) |
| Clarity of margin | |
| Well-circumscribed | 2 (20) |
| Intermediate | 8 (80) |
| Adjacent anatomic site involvement | |
| Yes | 2 (20) |
| No | 8 (80) |
| Lesion crosses midline | |
| Yes | 0 |
| No | 10 (100) |
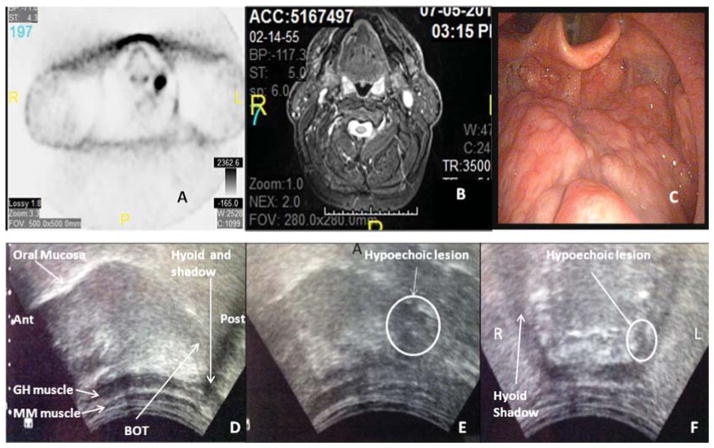
Fig. 2.
Clinical and radiographic images of a patient with head and neck squamous cell cancer of unknown primary. PET scan (A), MRI (B) and fiberoptic laryngoscopy (C) do not demonstrate any evidence of a primary lesion in the oropharynx. On transcervical ultrasound, right parasagittal view (D) posterior and anterior are labeled “post” and “ant”. Normal base of tongue (BOT) without evidence of a tonsillar mass is shown in panel D. The mucosa of the oral and base of tongue is intact. The myelohoid (MM) and geniohyoid (GH) muscles are visualized and intact. On left parasagittal view (E) a hypoechoic lesion, relative to the isoechoic normal tongue is appreciated and is consistent with a suspected base of tongue mass. This is confirmed on coronal view (F), whereby a small hypoechoic lesion is similarly appreciated.
After palpation and DL, 5 of 10 cases had clinically discernable masses which were histopathologically confirmed (Table 2). With biopsies directed at the ultrasound area of suspicion, the primary sites of two additional cases were histologically confirmed. Three cases remained UPs after DL and biopsies. These subjects with persistent UPs underwent variable additional procedures (Table 2). Subject H underwent ipsilateral lingual tonsillectomy (specimen measurements: 24 × 15 × 4 mm). Subject I underwent ipsilateral lingual (specimen measurements: 29 × 19 × 9 mm) and palatine tonsillectomy. Subject J underwent bilateral palatine tonsillectomies without lingual tonsillectomy (due to airway concerns). All three cases remained HNSCC of UP.
Table 2.
Clinical and ultrasound findings.
| Subject | PET/CT in oropharynx |
PET/CT subsite |
HPV tumor status |
Ultrasound subsite |
Ultrasound measurement of mass |
DL€ findings | EUA- palpation* |
Additional procedures performed | Histologic confirmed subsite |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S–I | A–P | M– L |
|||||||||
| A | Suspicious | BOT¥ | Positive | BOT | 12.1 | 17.3 | 6.3 | BOT lesion | – | BOT | |
| B | Negative | Positive | BOT | 17.2 | 9 | 16.1 | BOT lesion | – | BOT | ||
| C | Nonspecific | BOT | Positive | BOT | 10 | 11.4 | 20.6 | No lesion | BOT lesion | BOT | |
| D | Negative | Positive | BOT | 12.1 | 12.1 | 12.9 | No lesion | No lesion | Initial biopsies negative, deep biopsies of BOT positive | BOT | |
| E | Nonspecific | BOT or tonsil | Positive | Tonsil | 6.6 | 5.8 | 12.3 | No lesion | No lesion | Biopsies of BOT, tonsil, glossotonsillar sulcus | Tonsil |
| F | Nonspecific | Positive | Tonsil | 8.5 | 6.6 | 6.6 | Tonsil lesion | – | Tonsil | ||
| G | Negative | Positive | Tonsil | 6 | 5 | 5 | Tonsil lesion | – | Tonsil | ||
| H | Negative | Positive | BOT | 13.1 | 12.1 | 19.2 | No lesion | Firm GTψ sulcus | Ipsilateral lingual tonsillectomy | UPΦ | |
| I | Negative | Positive | BOT | 16.4 | 17.5 | 11.8 | Induration tonsil | – | Ipsilateral palatine and lingual tonsillectomies | UP | |
| J | Negative | Positive | BOT | 14 | 12.6 | 12.6 | No lesion | Firm GT sulcus | Bilateral palatine tonsillectomy | UP | |
For lesions not visualized on DL;
direct laryngoscopy;
glossotonsillar;
base of tongue;
unknown primary.
Given that HPV-positive tumor status localizes HNSCCs of UP to the oropharynx [2], we used tumor HPV status as a surrogate of primary tumor site. 100% of these HNSCCs of UP were HPV-positive, and we therefore assumed that all ten arose from an oropharyngeal primary site. Operative examination in this series captured 70% of the tumors expected to be in the oropharynx, whereas ultrasound had increased sensitivity with a suspected oropharyngeal tumor visualized in 100% of cases. Given the lack of HPV-negative tumors and negative ultrasound studies, the sensitivity and specificity of ultrasound could not be calculated.
Among the twenty controls, there were no suspicious tumors on ultrasound examination.
To understand whether the addition of ultrasound knowledge improved our detection rate in this prospective case series, we determined the detection rate in the two years prior to our implementation of this study. Nineteen HNSCCs of UP presented between 2008 and 2010. PET/CT results were available for 16 of 19 patients. 10 (62.5%) of 16 were negative, 2 (12.5%) of 16 were suspicious and 4 (25%) of 16 were non-specific. This was a similar distribution of PET/CT results when compared with the UP in the present cohort that underwent ultrasound (p = 0.95). Nine oropharyngeal sites were histologically confirmed (47.4%) after EUA/DL with biopsy (19 of 19, 100%) and bilateral tonsillectomy (9 of 19, 28%). 10 patients (53.6%) remained with unknown primary tumor sites. Among the nine patients who underwent bilateral tonsillectomy and EUA/DL with biopsy, 4 (44.4%) primary sites were determined. HPV tumor status was available for 14 of 19 cases and the majority were HPV-positive (12 of 14, 85.7%).
Discussion
This is the first report in the literature (to our knowledge) to use ultrasound for the evaluation of HNSCCs of unknown primary site. The successful identification of primary sites has been reported to range from 17% to 40% using traditional modalities [9]. In our small series of ten HNSCCs of UP, 70% of primary sites were histologically confirmed with EUA, DL and biopsy, while the remaining 30% were visualized by ultrasound without operative or histologic confirmation despite lingual tonsillectomies (±palatine tonsillectomies). This detection rate is 20% higher than the institutional historical detection rate without ultrasound knowledge between 2008 and 2010.
Review of our data suggests that ~50% of the primaries may have been discovered by traditional diagnostic management (EUA and DL). However, this exploratory study design did not capture how ultrasound knowledge biased operative examinations. Patients were referred by their head and neck surgeons for a pre-operative US assessment of a potential primary site. This may have influenced operative examinations and overestimated the diagnostic yield of DLs, which is higher than that reported in the literature (17–40%) [1,8,9,14,19]. Two additional cases were confirmed based upon the knowledge of ultrasound and therefore increased the diagnostic yield by 20%. These data are promising and provide compelling evidence to evaluate the role of ultrasound in the detection of UPs with a stronger, randomized study design.
An overall 70% diagnostic rate was achieved without palatine or lingual tonsillectomies and simply relied on biopsies which are significantly less invasive than a tonsillectomy. This is commensurate with a recent case series, in which 7 of 22 patients (31.8%) underwent diagnostic TORS (lingual and palatine tonsillectomy) after negative DLs [16] to identify the primary site. In our series, patients with histologic confirmation of the primary site had limited exposure to potential morbidity, which is in contrast to the aforementioned diagnostic approach.
For the three cases that remained unconfirmed histologically, potential pitfalls can be identified that may explain the persistent UP classification. All three were suspected lesions of the BOT. In two cases (“H” and “I”), the depth of dissection was superior to the suspected level of the lesion, even after accounting for retraction of a surgical specimen. In the third case “J”, a “directed biopsy” was unlikely to reach the suspected 1 cm endophytic lesion which appeared adjacent to the hyoid, and a lingual tonsillectomy was not performed. Therefore, the relevant epithelium was likely not sampled in these procedures. Alternatively, the ultrasound may have misclassified the primary tumor location. Indeed, lingual lymphoid tissue is relatively hypoechoic and therefore can be mistaken for a lesion if the examiner does not appreciate the symmetric nature of the lingual tonsils on US. These three cases were performed by one surgeon, suggesting that operator variability may have affected the DLs. Lastly, we must also consider that the ultrasound assessments may have been falsely positive and that US may be a highly sensitive modality with low specificity and/or positive predictive value. Given the sample size and distribution of case outcomes, these characteristics could not be evaluated.
As compared with PET scans which were nonspecific or negative for 9 cases, 6 of 9 lesions were visualized by ultrasound and confirmed histologically. This represents an improvement in the preoperative visualization of primary tumors of the oropharynx that are otherwise not appreciable clinically or radiographically. To validate this visualization technique, future diagnostic interventions (e.g., DL biopsies) should be performed under ultrasound guidance to document that the ultrasound visualized lesion is indeed the one sampled at the time of biopsy and to determine agreement between ultrasound and histology. This may improve the diagnostic yield of HNSCCs of UPs. For example, the three cases which remained UPs may have been histologically confirmed had the biopsy been performed with real-time ultrasound guidance. In thyroid ultrasonography, biopsies are routinely performed under ultrasound guidance and entry of the needle into the lesion of interest is visualized by ultrasound.
This proof of concept study demonstrates that ultrasound is a feasible means to identify primary oropharyngeal tumors and provided a means for the investigators to learn the ultrasonographic appearance of the oropharynx. This study did not account for the inherent learning curve for a novel application of ultrasound, which likely biased our results. Indeed, enrollment of UPs was not staggered from that of patients with clinically evident OPSCCs in which we evaluated the characteristics of the normal OP and known malignant lesions. Therefore, during the time of this study, we were building our knowledge for a technique that is widely accepted to be experience and operator-dependent.
Ultrasound has potential treatment implications. Small primary lesions may be localized which may render patients eligible for transoral surgery or de-intensification trials when they otherwise may have received irradiation of the entire pharyngeal axis. These data also have exciting implications for future screening studies. Given the success of ultrasound in visualizing lesions of the oropharynx as small as 5 mm that were unappreciable clinically and by conventional imaging, this could provide a potential means of visualizing and sampling suspicious lesions in high-risk populations and therefore warrants further study.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Strojan P, Ferlito A, Medina JE, Woolgar JA, Rinaldo A, Robbins KT, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck. 2013;35:123–32. doi: 10.1002/hed.21898. [DOI] [PubMed] [Google Scholar]
- 2.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008;2:163–8. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vent J, Haidle B, Wedemeyer I, Huebbers C, Siefer O, Semrau R, et al. p16 Expression in carcinoma of unknown primary: diagnostic indicator and prognostic marker. Head Neck. 2013 doi: 10.1002/hed.23190. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 5.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch WM. Clinical features of HPV-related head and neck squamous cell carcinoma: presentation and work-up. Otolaryngol Clin North Am. 2012;45:779–93. doi: 10.1016/j.otc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Cianchetti M, Mancuso AA, Amdur RJ, Werning JW, Kirwan J, Morris CG, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119:2348–54. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- 9.Haas I, Hoffmann TK, Engers R, Ganzer U. Diagnostic strategies in cervical carcinoma of an unknown primary (CUP) Eur Arch Otorhinolaryngol. 2002;259:325–33. doi: 10.1007/s00405-002-0470-1. [DOI] [PubMed] [Google Scholar]
- 10.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101:2641–9. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 11.Strojan P, Ferlito A, Langendijk JA, Corry J, Woolgar JA, Rinaldo A, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: II. A review of therapeutic options. Head Neck. 2013;35:286–93. doi: 10.1002/hed.21899. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ibraheem A, Buck A, Krause BJ, Scheidhauer K, Schwaiger M. Clinical Applications of FDG PET and PET/CT in head and neck cancer. J Oncol. 2009;2009:208725. doi: 10.1155/2009/208725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009;19:731–44. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch WM, Bhatti N, Williams MF, Eisele DW. Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg. 2001;124:331–3. doi: 10.1067/mhn.2001.114309. [DOI] [PubMed] [Google Scholar]
- 15.Mehta V, Johnson P, Tassler A, Kim S, Ferris RL, Nance M, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123:146–51. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- 16.Durmus K, Rangarajan SV, Old MO, Agrawal A, Teknos TN, Ozer E. Transoral robotic approach to carcinoma of unknown primary. Head Neck. 2013 doi: 10.1002/hed.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein GS, Quon H, Newman HJ, Chalian JA, Malloy K, Lin A, et al. Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch Otolaryngol Head Neck Surg. 2012;138:628–34. doi: 10.1001/archoto.2012.1166. [DOI] [PubMed] [Google Scholar]
- 18.Blanco RG, Califano J, Messing B, Richmon J, Liu J, Quon H, et al. Transcervical ultrasonography is feasible to visualize and evaluate base of tongue cancers. PLoS One. 2014;9:e87565. doi: 10.1371/journal.pone.0087565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McQuone SJ, Eisele DW, Lee DJ, Westra WH, Koch WM. Occult tonsillar carcinoma in the unknown primary. Laryngoscope. 1998;108:1605–10. doi: 10.1097/00005537-199811000-00004. [DOI] [PubMed] [Google Scholar]