Abstract
Human papillomavirus (HPV) is now recognized to play a role in the pathogenesis of a subset of head and neck squamous cell carcinomas (HNSCCs), particularly those that arise from the lingual and palatine tonsils within the oropharynx. High-risk HPV16 is identified in the overwhelming majority of HPV-positive tumors, which have molecular-genetic alterations indicative of viral oncogene function. Measures of HPV exposure, including sexual behaviors, seropositivity to HPV16, and oral, high-risk HPV infection, are associated with increased risk for oropharyngeal cancer. HPV infection may be altering the demographics of HNSCC patients, as these patients tend to be younger, nonsmokers, and nondrinkers. There is sufficient evidence to conclude that a diagnosis of HPV-positive HNSCC has significant prognostic implications; these patients have at least half the risk of death from HNSCC when compared with the HPV-negative patient. The HPV etiology of these tumors may have future clinical implications for the diagnosis, therapy, screening, and prevention of HNSCC.
INTRODUCTION
Oral human papillomavirus (HPV) infection, like alcohol and tobacco, is now recognized to play a role in the pathogenesis of head and neck squamous cell carcinomas (HNSCC).1-7 HPVs are DNA viruses with a specific tropism for squamous epithelia, and more than 120 different HPV types have been isolated to date. Low-risk HPVs, such as HPV6 and -11, induce benign hyperproliferations of the epithelium such as papillomas or warts. By contrast, high-risk, oncogenic types (eg, HPV16, -18, -31, -33, -35) are defined by their strong epidemiologic association with cervical cancer.8 The prototypic high-risk types -16 and -18 are capable of transforming epithelial cells derived from both the genital and upper respiratory tracts.9 The transforming potential of high-risk HPVs is largely a result of the function of two viral oncoproteins, E6 and E7, which functionally inactivate two human tumor-suppressor proteins, p53 and pRb, respectively.10 Expression of high-risk HPV E6 and E7 results in cellular proliferation, loss of cell cycle regulation, impaired cellular differentiation, increased frequency of spontaneous and mutagen-induced mutations, and chromosomal instability.10
HPV DNA PRESENCE AND EXPRESSION IN OROPHARYNGEAL CANCERS
Infection by a high-risk HPV type is now known to be necessary, although insufficient, for the development of cervical cancer. In contrast to cervical cancer, in HNSCC, HPV appears to play a pathogenic role for only a subset.1,2,6,11-18 It is clear that continued expression of the viral on cogenes is necessary for histopathologic progression and the malignant phenotype of an HPV-associated tumor19-22: HPVs are not known to work by a hit-and-run mechanism. Therefore, demonstration of HPV genomic DNA in tumors is essential for HPV to play a role in the pathogenesis of a tumor.23 In a recent meta-analysis, HPV genomic DNA was detected in approximately 26% of all HNSCC by sensitive polymerase chain reaction (PCR) -based methods.24 However, data are most strong and consistent for HPV presence in oropharyngeal cancers. In the majority of studies, 50% or more of oropharyngeal tumors contained the HPV genome.1,2,11,15,17,18,25-30 In a recent multinational study conducted by the International Agency for Research on Cancer (IARC), only 18% of oropharyngeal tumors were HPV positive, indicating that this proportion likely varies by geography.31 Regardless of the study population, high-risk HPV16 accounts for the overwhelming majority (90% to 95%) of HPV-positive tumors, whereas other high-risk types -31, -33, and -35 account for the minority.24,31 For oropharyngeal tumors, viral HPV DNA has been specifically localized to tumor cell nuclei,2,6 is frequently integrated,2,4,6,32-36 and is transcriptionally active.2,4,5,11,27,32,34,37 Furthermore, HPV is present in high copy number in tumor cell nuclei of in situ, invasive, and metastatic disease and absent in adjacent normal tissue.38 These data indicate that HPV infection is specific to tumor cell nuclei and that infection precedes histopathologic progression of the tumor. Equivalent data are not available for non-oropharyngeal tumors. For instance, HPV has not been shown to have an etiologic association with oral tongue cancers diagnosed at any age. Although HPV can infect the epithelium of the upper aerodigestive tract in general, the tonsil appears uniquely susceptible to transformation by the virus. As for the transformation zone of the cervix, the reason for this anatomic site specificity for transformation is unknown.
A role for HPV in oropharyngeal tumors is further substantiated by distinct molecular genetic alterations in HPV-positive versus HPV-negative tumors. As for many cancers, inactivation of the p53 and pRb pathways is a common event in the molecular progression of HNSCC. However, inactivation occurs by different mechanisms in HPV-positive and -negative tumors. In HPV-positive HNSCC, genetic alterations are reflective of viral oncogene function. For instance, HPV-positive tumors tend to have wild-type p53, because p53 is functionally inactivated by viral E6 oncoprotein.2,4,6,11,18,35,39,40 By contrast, HPV-negative tumors have specific p53 mutations demonstrated to be induced by carcinogens in tobacco smoke.2,4,6,11,18,35,39,40 As another example, pRb function is inactivated by viral E7 protein in the HPV-positive tumor, but in HPV-negative tumors, the pRb pathway is altered by other mechanisms, including amplification of cyclin D and inactivation of p16.3,4,6,27,29,38 More complex differences in regions of chromosomal loss and gain have been demonstrated in HPV-positive versus -negative tumors through techniques such as comparative genomic hybridization41 and microsatellite analysis.42
CLINICAL CHARACTERISTICS OF THE HPV-POSITIVE PATIENT
In addition to molecular-genetic distinctions, HPV affects the demographics, clinical presentation, and histopathology of the HNSCC patient. Patients with HPV-positive HNSCC tend to be younger by approximately 5 years, on average, when compared with HPV-negative HNSCC patients.28,30,43-45 With regard to sex, men appear to be at equal risk to women. It is clear that the majority of HPV-positive tumors arise largely from the lingual and palatine tonsils in the oropharynx, compared with other anatomic sites of the head and neck.1,2,15-18,45,46 Although some studies have found associations between advanced TNM stage at presentation and HPV positivity, this finding has been somewhat inconsistent. Histopathologically, HPV-positive tumors tend to have a poorly differentiated and frequently basaloid histology.2-5,18,27,47-50
RISK FACTORS FOR HPV-POSITIVE HNSCC
The HPV-positive patient also appears to be distinct from the HPV-negative patient with regard to alcohol and tobacco exposure history. HPV-positive HNSCC is more likely than HPV-negative HNSCC to occur in the nonsmoker and nondrinker.2,6,17,18,29,35 In a study restricted to patients with oropharyngeal cancers, nonsmokers were approximately 15-fold more likely to have a diagnosis of HPV-positive HNSCC than smokers.35 Similarly, several studies have reported an inverse association between HPV status and alcohol use.2,18,35,45,51 Although evidence suggests that HPV is associated with cancers in nonsmokers and nondrinkers,52 the degree to which oral HPV infection may combine with tobacco and/or alcohol use to increase risk of cancer is currently unclear, with some studies suggesting a synergistic effect with tobacco1 or alcohol,53 whereas others have found no such synergy.31,53
Numerous case-control studies of cervical cancer patients have indicated that HPV infection is predominantly sexually transmitted.54 Certain sexual behaviors, such as a high number of sexual partners, are associated with increased risk of cervical cancer because they serve as surrogate markers for the probability that an individual has been exposed to HPV. Consistent with this literature, several case-control studies have reported certain sexual behaviors to elevate risk of HNSCC.1,55 Risk factors among men include young age at first intercourse, number of sexual partners, and a history of genital warts.1 Risk is elevated among women with a high number of sexual partners.56 Furthermore, specific sexual behaviors have bee more strongly associated with risk of an HPV-positive tumor, including a history of performing oral sex and oral-anal contact.17,31,57
Direct measures of HPV exposure and infection have also been associated with risk of oral cancers. Seropositivity to the HPV16 viral capsid protein confers a two- to three-fold increase in risk for HNSCC.1,7,31 In case-control studies, the presence of an oncogenic, oral HPV infection has been associated with a six-fold increase in risk for oral cancer.57-59 Risk estimates are stronger when restricted to oropharyngeal cancer. In a recent study conducted in Sweden,59 oral infection by a high-risk HPV type was demonstrated to dramatically elevate odds for oropharyngeal cancer (odds ratio, 230; 95% CI, 44 to 1,200), after adjustment for alcohol and tobacco. In a nested case-control study conducted in Norway,7 HPV16-seropositive individuals had a greater than 14-fold increase in risk of subsequent oropharyngeal cancer when compared with seronegative individuals. This study was particularly important because it demonstrated that HPV exposure preceded development of clinical disease.
Although natural history studies have not been conducted, it is presumed that oral HPV infection precedes development of an HPV-positive HNSCC. Therefore, risk factors for oral HPV infection are likely, by extension, to be risk factors for HPV-positive HNSCC. Oral HPV infection has recently been associated with sexual behavior, in particular with number of oral sex partners.55,60 Other factors associated with elevated risk of oral HPV infection include increasing age, male sex, history of sexually transmitted disease, HIV infection, and severity of immunosuppression.60,61
Because the majority of tonsillar cancers are HPV positive, risk for tonsillar cancer may be a reasonable surrogate for risk of an HPV-associated HNSCC. Individuals at increased risk of tonsillar cancer include those with a history of HPV-associated malignancy,62 women over the age of 50 years with a history of in situ cervical cancer, and husbands of women with in situ and invasive cervical cancer.63 HIV-seropositive and immunosuppressed transplant patients have also been recognized to be at increased risk for tonsillar and oropharyngeal cancer, as well as all other HPV-associated malignancies.64 A recent report has suggested that the 500- to 700-fold increase in risk of HNSCC among patients with Fanconi anemia may be attributable to an increased genetic susceptibility to HPV-mediated tumorigenesis.65,66
As stated previously, HPV-positive patients tend to be younger than patients with HPV-negative tumors. Consistent with this finding, high-risk sexual behaviors were more prevalent among HNSCC patients younger than 55 years when compared with those older than 55 years in a recent case-control study.58 In the United States, herpes simplex-2 seroprevalence, a validated marker for high-risk sexual behaviors, increased by 30% between the periods 1976 through 1980 to 1988 through 1994, and the relative increase was more pronounced as age declined.67 Studies from Scandinavia68 indicate that HPV16 sero-prevalence also significantly increased over time, between 1968 and 1990. According to Surveillance, Epidemiology, and End Results (SEER) data, the incidence of base-of-tongue and tonsil cancers increased by 2.1% and 3.9% per year, respectively, from 1973 to 2001 among white men and women ages 20 to 44 years, whereas the incidence at other sites declined.69,70 Additional analysis indicates tonsillar cancer incidence increased annually by approximately 2% to 3% among African American and white men younger than 60 years through 1998.69,71 These data are consistent with a role for HPV in the etiology of tonsillar cancers. HPV may therefore be having a significant impact on the demographics of the HNSCC patient.
It is now clear from numerous lines of evidence that individuals exposed to HPV are at increased risk for oropharyngeal cancer. However, data are currently insufficient to estimate with precision the magnitude of this risk. The potential utility of HPV detection for oral cancer screening is largely unexplored. Because of the absence of this data, there are currently no commercially available, US Food and Drug Administration–approved tests for HPV serology or for detection of oral HPV infection.
CLINICAL SIGNIFICANCE OF AN HPV-POSITIVE TUMOR
HPV detection may have future implications for the diagnosis, prognosis, therapeutics, and prevention of HNSCC. For diagnostic purposes, HPV detection in cervical lymph nodes of patients presenting with an occult primary may be used to establish with high specificity, the location of the primary within the oropharynx.38 Tonsillectomy has been shown in retrospective analyses to identify the primary site of cervical metastases as the contralateral72,73 or ipsilateral74,75 tonsil in approximately 30% and 10% of cases, respectively.
With regard to prognosis, patients with HPV-positive tumors have improved prognosis when compared with patients with HPV-negative tumors in the majority of studies.2,15,17,28,35,76-78 Studies to date have suggested that HPV-positive patients may have as much as 60% to 80% reduction in risk of dying from their cancer when compared with the HPV-negative patient.2,77,78 Negative studies may be explained by inadequate sample size, follow-up time, and residual confounding by other prognostically significant variables.12-14,17,18,40,45,51 The reason for the improved survival is unclear; however, improved radiation responsiveness, immune surveillance to viral antigens, and the absence of field cancerization in these patients who tend to be nonsmokers, have been postulated.28,35,79,80 In addition, E6-related degradation of p53 in HPV-positive cancers may be functionally inequivalent to HPV-negative p53 mutations,81,82 and therefore, HPV-positive tumors may have an intact apoptotic response to radiation and chemotherapy.79,83
Possible therapeutic implications of an HPV-positive diagnosis are an active area of investigation. This includes selection of patients for organ preservation therapy, which may be more successful in patients with HPV-HNSCC. The impact of HPV presence on oropharygeal organ preservation is currently being evaluated in an Eastern Cooperative Oncology Group protocol that has met its target enrollment. A clinical trial of an HPV16-specific therapeutic vaccine is also currently actively recruiting patients at The Johns Hopkins Hospital (J.H.H.; Baltimore, MD). The vaccine is administered in the adjuvant setting and is intended to enhance the cytotoxic T-cell response to the HPV16 oncoproteins.84 With regard to prevention, a prophylactic vaccine composed of the HPV16 viral capsid protein has recently been shown to prevent persistent HPV16 infection and the development of cervical dysplasia in phase three randomized controlled trials.85,86 US Food and Drug Administration approval is anticipated within the next few years. However, the clinical trials have not included an evaluation of the impact of the vaccine on oral HPV infection. Data on oral HPV infections in the setting of immunization are limited to canine and hamster models, which have shown a protective effect and a reduction in the development of HPV-related oral lesions.87,88 The vaccine does have the potential to have a greater impact on HNSCC incidence than for cervical cancer incidence, given that the current vaccines are largely targeted to HPV16.
HOW TO MAKE THE DIAGNOSIS OF HPV-HNSCC
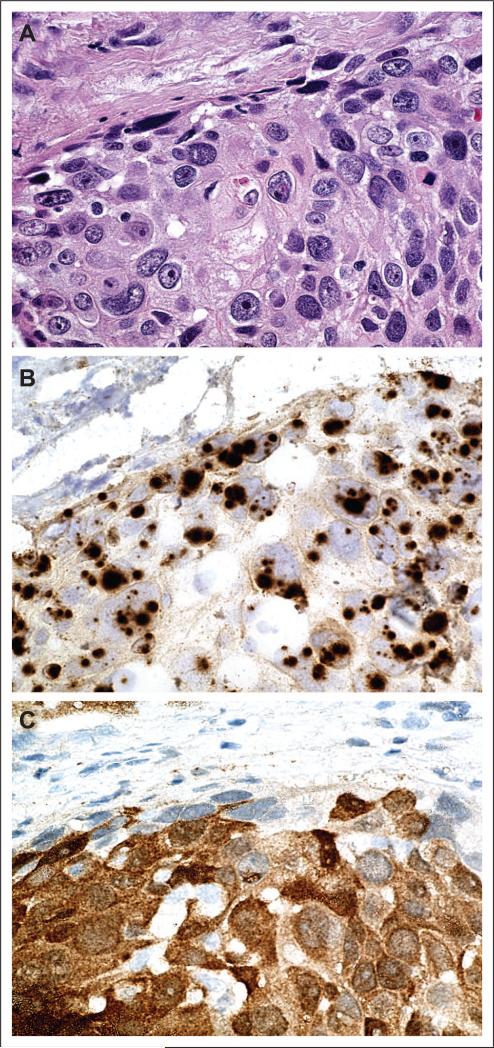
The diagnosis of HPV-HNSCC should be considered in all squamous cell carcinomas that arise from the lingual and palatine tonsils. Suspicion should be high for cancers in nonsmokers and nondrinkers, patients with basaloid or poorly differentiated histology, the young patient, the immunosuppressed patient and the patient with Fanconi anemia. The only clinically useful test to confirm the diagnosis of HPV is in situ hybridization (ISH), and is currently only available at a limited number of tertiary referral centers. The JHH Pathology Department uses the GenPoint system (DAKO, Carpinteria, CA) with a DAKO HPV16 probe with a sensitivity of one to two copies of integrated HPV16.89 (Tumor testing is available at JHH http://pathology.jhu.edu/labservices/hpv.cfm.) p16 immunohistochemistry may serve as a reasonable surrogate marker for high-risk HPV because strong correlations have been found between diffuse nuclear and cytoplasmic p16 staining, HPV DNA by ISH38 and real-time PCR29(Fig 1). For research purposes only, real-time PCR can be performed on microdissected tumor DNA normalized to a single-copy human gene to demonstrate one or more viral copies per tumor cell.29,90,91 Possible future diagnostic tests that would likely have high specificity but low sensitivity for a diagnosis of HPV-associated HNSCC will include the detection of HPV16 DNA in plasma,92 fluorescence ISH or ISH on papanicolou smears obtained directly from tumors,6,93 and HPV16 E6 and E7 seroreactivity.94,95 These are all active areas of investigation.
Fig 1.
A human papilloma virus (HPV) –positive tumor by (A) hematoxylin and eosin, (B) in situ hybridization (ISH), and (C) p16 immunohistochemistry. ISH provides localized detection of HPV DNA in the context of preserved tissue architecture. HPV hybridization signal is seen as punctate nuclear staining. The corresponding p16 immunohistochemistry stain is strong and diffuse in part C.
CONCLUSION
Strong epidemiologic and molecular data support the conclusion that HPV is responsible for a unique subset of HNSCCs. Sexually acquired oral HPV infection may be altering the epidemiology and demographics of head and neck cancer. Currently, the diagnosis of HPV-positive HNSCC is clinically relevant for prognostication. A diagnosis of an HPV-positive malignancy may have future diagnostic and therapeutic implications, as well as implications for prevention and screening.
Acknowledgments
Supported in part by National Institute for Dental and Craniofacial Research Grant No. DE016631-02.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Maura L. Gillison
Financial support: Maura L. Gillison
Administrative support: Carole Fakhry
Manuscript writing: Carole Fakhry, Maura L. Gillison
Final approval of manuscript: Maura L. Gillison
REFERENCES
- 1.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58:5–13. [PubMed] [Google Scholar]
- 4.Wiest T, Schwarz E, Enders C, et al. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21:1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 5.van Houten VM, Snijders PJ, van den Brekel MW, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer. 2001;93:232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 6.Hafkamp HC, Speel EJ, Haesevoets A, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer. 2003;107:394–400. doi: 10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 7.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 8.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 9.McDougall JK. Immortalization and transformation of human cells by human papillomavirus. Curr Top Microbiol Immunol. 1994;186:101–119. doi: 10.1007/978-3-642-78487-3_6. [DOI] [PubMed] [Google Scholar]
- 10.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 11.Balz V, Scheckenbach K, Gotte K, et al. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63:1188–1191. [PubMed] [Google Scholar]
- 12.Pintos J, Franco EL, Black MJ, et al. Human papillomavirus and prognoses of patients with cancers of the upper aerodigestive tract. Cancer. 1999;85:1903–1909. doi: 10.1002/(sici)1097-0142(19990501)85:9<1903::aid-cncr4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Snijders PJ, Scholes AG, Hart CA, et al. Prevalence of mucosotropic human papillomaviruses in squamous-cell carcinoma of the head and neck. Int J Cancer. 1996;66:464–469. doi: 10.1002/(SICI)1097-0215(19960516)66:4<464::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Brandwein M, Zeitlin J, Nuovo GJ, et al. HPV detection using “hot start” polymerase chain reaction in patients with oral cancer: A clinicopathological study of 64 patients. Mod Pathol. 1994;7:720–727. [PubMed] [Google Scholar]
- 15.Paz IB, Cook N, Odom-Maryon T, et al. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer. 1997;79:595–604. doi: 10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Fouret P, Monceaux G, Temam S, et al. Human papillomavirus in head and neck squamous cell carcinomas in nonsmokers. Arch Otolaryngol Head Neck Surg. 1997;123:513–516. doi: 10.1001/archotol.1997.01900050063008. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 18.Haraf DJ, Nodzenski E, Brachman D, et al. Human papilloma virus and p53 in head and neck cancer: Clinical correlates and survival. Clin Cancer Res. 1996;2:755–762. [PubMed] [Google Scholar]
- 19.Nasseri M, Gage JR, Lorincz A, et al. Human papillomavirus type 16 immortalized cervical keratinocytes contain transcripts encoding E6, E7, and E2 initiated at the P97 promoter and express high levels of E7. Virology. 1991;184:131–140. doi: 10.1016/0042-6822(91)90829-z. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz E, Freese UK, Gissmann L, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 21.Howley PM. Role of the human papillomaviruses in human cancer. Cancer Res. 1991;51:5019s–5022s. [PubMed] [Google Scholar]
- 22.Crook T, Morgenstern JP, Crawford L, et al. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. Embo J. 1989;8:513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillison ML, Shah KV. Chapter 9: Role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr. 2003:57–65. doi: 10.1093/oxfordjournals.jncimonographs.a003484. [DOI] [PubMed] [Google Scholar]
- 24.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 25.Niedobitek G, Pitteroff S, Herbst H, et al. Detection of human papillomavirus type 16 DNA in carcinomas of the palatine tonsil. J Clin Pathol. 1990;43:918–921. doi: 10.1136/jcp.43.11.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snijders PJ, Cromme FV, van den Brule AJ, et al. Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer. 1992;51:845–850. doi: 10.1002/ijc.2910510602. [DOI] [PubMed] [Google Scholar]
- 27.Wilczynski SP, Lin BT, Xie Y, et al. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol. 1998;152:145–156. [PMC free article] [PubMed] [Google Scholar]
- 28.Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–304. [PubMed] [Google Scholar]
- 29.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strome SE, Savva A, Brissett AE, et al. Squamous cell carcinoma of the tonsils: A molecular analysis of HPV associations. Clin Cancer Res. 2002;8:1093–1100. [PubMed] [Google Scholar]
- 31.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 32.Snijders PJ, Meijer CJ, van den Brule AJ, et al. Human papillomavirus (HPV) type 16 and 33 E6/E7 region transcripts in tonsillar carcinomas can originate from integrated and episomal HPV DNA. J Gen Virol. 1992;73(Pt 8):2059–2066. doi: 10.1099/0022-1317-73-8-2059. [DOI] [PubMed] [Google Scholar]
- 33.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated to better prognosis in tonsillar cancer. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 34.Steenbergen RD, Hermsen MA, Walboomers JM, et al. Integrated human papillomavirus type 16 and loss of heterozygosity at 11q22 and 18q21 in an oral carcinoma and its derivative cell line. Cancer Res. 1995;55:5465–5471. [PubMed] [Google Scholar]
- 35.Lindel K, Beer KT, Laissue J, et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: A radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Koskinen WJ, Chen RW, Leivo I, et al. Prevalence and physical status of human papillomavirus in squamous cell carcinomas of the head and neck. Int J Cancer. 2003;107:401–406. doi: 10.1002/ijc.11381. [DOI] [PubMed] [Google Scholar]
- 37.Ke LD, Adler-Storthz K, Mitchell MF, et al. Expression of human papillomavirus E7 mRNA in human oral and cervical neoplasia and cell lines. Oral Oncol. 1999;35:415–420. doi: 10.1016/s1368-8375(99)00015-9. [DOI] [PubMed] [Google Scholar]
- 38.Begum S, Gillison ML, Ansari-Lari MA, et al. Detection of human papillomavirus in cervical lymph nodes: A highly efffective strategy for localizing site of tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2003;9:6469–6475. [PubMed] [Google Scholar]
- 39.Brachman DG, Graves D, Vokes E, et al. Occurrence of p53 gene deletions and human papilloma virus infection in human head and neck cancer. Cancer Res. 1992;52:4832–4836. [PubMed] [Google Scholar]
- 40.Chiba I, Shindoh M, Yasuda M, et al. Mutations in the p53 gene and human papillomavirus infection as significant prognostic factors in squamous cell carcinomas of the oral cavity. Oncogene. 1996;12:1663–1668. [PubMed] [Google Scholar]
- 41.Smeets SJ, Braakhuis BJ, Abbas S, et al. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2005 Nov 28; doi: 10.1038/sj.onc.1209275. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Braakhuis BJ, Snijders PJ, Keune WJ, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96:998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 43.Sisk EA, Soltys SG, Zhu S, et al. Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck. 2002;24:841–849. doi: 10.1002/hed.10146. [DOI] [PubMed] [Google Scholar]
- 44.Cruz IB, Snijders PJ, Steenbergen RD, et al. Age-dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Eur J Cancer B Oral Oncol. 1996;32B:55–62. doi: 10.1016/0964-1955(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 45.Ringstrom E, Peters E, Hasegawa M, et al. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–3192. [PubMed] [Google Scholar]
- 46.Brandsma JL, Abramson AL. Association of papillomavirus with cancers of the head and neck. Arch Otolaryngol Head Neck Surg. 1989;115:621–625. doi: 10.1001/archotol.1989.01860290079018. [DOI] [PubMed] [Google Scholar]
- 47.Klussmann JP, Weissenborn SJ, Wieland U, et al. Human papillomavirus-positive tonsillar carcinomas: A different tumor entity? Med Microbiol Immunol (Berl) 2003;192:129–132. doi: 10.1007/s00430-002-0126-1. [DOI] [PubMed] [Google Scholar]
- 48.Zoltan S, Szabolcs O, Miklos K. Indications for molecular detection of human papillomavirus (HPV) [in Hungarian]. Magy Onkol. 2002;46:235–237. [PubMed] [Google Scholar]
- 49.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: A distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Poetsch M, Lorenz G, Bankau A, et al. Basaloid in contrast to nonbasaloid head and neck squamous cell carcinomas display aberrations especially in cell cycle control genes. Head Neck. 2003;25:904–910. doi: 10.1002/hed.10301. [DOI] [PubMed] [Google Scholar]
- 51.Portugal LG, Goldenberg JD, Wenig BL, et al. Human papillomavirus expression and p53 gene mutations in squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:1230–1234. doi: 10.1001/archotol.1997.01900110084011. [DOI] [PubMed] [Google Scholar]
- 52.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363:1488–1489. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 53.Smith EM, Ritchie JM, Summersgill KF, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 54.Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: Evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev. 2001;10:101–106. [PubMed] [Google Scholar]
- 55.Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 56.Rajkumar T, Sridhar H, Balaram P, et al. Oral cancer in Southern India: The influence of body size, diet, infections and sexual practices. Eur J Cancer Prev. 2003;12:135–143. doi: 10.1097/00008469-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Smith EM, Ritchie JM, Summersgill KF, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108:766–772. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 58.Maden C, Beckmann AM, Thomas DB, et al. Human papillomaviruses, herpes simplex viruses, and the risk of oral cancer in men. Am J Epidemiol. 1992;135:1093–1102. doi: 10.1093/oxfordjournals.aje.a116209. [DOI] [PubMed] [Google Scholar]
- 59.Hansson BG, Rosenquist K, Antonsson A, et al. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1337–1344. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 60.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 61.Coutlee F, Trottier AM, Ghattas G, et al. Risk factors for oral human papillomavirus in adults infected and not infected with human immunodeficiency virus. Sex Transm Dis. 1997;24:23–31. doi: 10.1097/00007435-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Frisch M, Biggar RJ. Aetiological parallel between tonsillar and anogenital squamous-cell carcinomas. Lancet. 1999;354:1442–1443. doi: 10.1016/S0140-6736(99)92824-6. [DOI] [PubMed] [Google Scholar]
- 63.Hemminki K, Jiang Y, Dong C. Second primary cancers after anogenital, skin, oral, esophageal and rectal cancers: Etiological links? Int J Cancer. 2001;93:294–298. doi: 10.1002/ijc.1319. [DOI] [PubMed] [Google Scholar]
- 64.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 65.Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 66.Lowy D, Gillison ML. New link between Fanconi Anemia and human papillomavirus associated malignancies. J Natl Cancer Inst. 2003;95:1648–1650. doi: 10.1093/jnci/djg125. [DOI] [PubMed] [Google Scholar]
- 67.Fleming DT, McQuillan GM, Johnson RE, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 68.Af Geijersstam V, Wang Z, Lewensohn-Fuchs I, et al. Trends in seroprevalence of human papillomavirus type 16 among pregnant women in Stockholm, Sweden, during 1969-1989. Int J Cancer. 1998;76:341–344. doi: 10.1002/(sici)1097-0215(19980504)76:3<341::aid-ijc10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 69.Frisch M, Hjalgrim H, Jaeger AB, et al. Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control. 2000;11:489–495. doi: 10.1023/a:1008918223334. [DOI] [PubMed] [Google Scholar]
- 70.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: Increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 71.Canto MT, Devesa SS. Oral cavity and pharynx cancer incidence rates in the United States, 1975-1998. Oral Oncol. 2002;38:610–617. doi: 10.1016/s1368-8375(01)00109-9. [DOI] [PubMed] [Google Scholar]
- 72.Koch WM, Bhatti N, Williams MF, et al. Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg. 2001;124:331–333. doi: 10.1067/mhn.2001.114309. [DOI] [PubMed] [Google Scholar]
- 73.McQuone SJ, Eisele DW, Lee DJ, et al. Occult tonsillar carcinoma in the unknown primary. Laryngoscope. 1998;108:1605–1610. doi: 10.1097/00005537-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Mendenhall WM, Mancuso AA, Parsons JT, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Head Neck. 1998;20:739–744. doi: 10.1002/(sici)1097-0347(199812)20:8<739::aid-hed13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 75.Lapeyre M, Malissard L, Peiffert D, et al. Cervical lymph node metastasis from an unknown primary: Is a tonsillectomy necessary? Int J Radiat Oncol Biol Phys. 1997;39:291–296. doi: 10.1016/s0360-3016(97)00321-0. [DOI] [PubMed] [Google Scholar]
- 76.Li W, Thompson CH, O'Brien CJ, et al. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer. 2003;106:553–558. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz SR, Yueh B, McDougall JK, et al. Human papillomavirus infection and survival in oral squamous cell cancer: A population-based study. Otolaryngol Head Neck Surg. 2001;125:1–9. doi: 10.1067/mhn.2001.116979. [DOI] [PubMed] [Google Scholar]
- 78.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 79.DeWeese TL, Walsh JC, Dillehay LE, et al. Human papillomavirus E6 and E7 oncoproteins alter cell cycle progression but not radiosensitivity of carcinoma cells treated with low-dose-rate radiation. Int J Radiat Oncol Biol Phys. 1997;37:145–154. doi: 10.1016/s0360-3016(96)00448-8. [DOI] [PubMed] [Google Scholar]
- 80.Mellin Dahlstrand H, Lindquist D, Bjornestal L, et al. P16(INK4a) correlates to human papillomavirus presence, response to radiotherapy and clinical outcome in tonsillar carcinoma. Anticancer Res. 2005;25:4375–4383. [PubMed] [Google Scholar]
- 81.Butz K, Whitaker N, Denk C, et al. Induction of the p53-target gene GADD45 in HPV-positive cancer cells. Oncogene. 1999;18:2381–2386. doi: 10.1038/sj.onc.1202557. [DOI] [PubMed] [Google Scholar]
- 82.Huang H, Li CY, Little JB. Abrogation of P53 function by transfection of HPV16 E6 gene does not enhance resistance of human tumour cells to ionizing radiation. Int J Radiat Biol. 1996;70:151–160. doi: 10.1080/095530096145148. [DOI] [PubMed] [Google Scholar]
- 83.Ferris RL, Martinez I, Sirianni N, et al. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): A natural disease model provides insights into viral carcinogenesis. Eur J Cancer. 2005;41:807–815. doi: 10.1016/j.ejca.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 84.Hsu KF, Hung CF, Cheng WF, et al. Enhancement of suicidal DNA vaccine potency by inking mycobacterium tumberculosis heat shock protein 70 to an antigen. Gene Ther. 2001;8:376–383. doi: 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- 85.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 86.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 87.Maeda H, Kubo K, Sugita Y, et al. DNA vaccine against hamster oral papillomavirus-associated oral cancer. J Int Med Res. 2005;33:647–653. doi: 10.1177/147323000503300606. [DOI] [PubMed] [Google Scholar]
- 88.Johnston KB, Monteiro JM, Schultz LD, et al. Protection of beagle dogs from mucosal challenge with canine oral papillomavirus by immunization with recombinant adenoviruses expressing codon-optimized early genes. Virology. 2005;336:208–218. doi: 10.1016/j.virol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 89.Lizard G, Demares-Poulet MJ, Roignot P, et al. In situ hybridization detection of single-copy human papillomavirus on isolated cells, using a catalyzed signal amplification system: GenPoint. Diagn Cytopathol. 2001;24:112–116. doi: 10.1002/1097-0339(200102)24:2<112::aid-dc1020>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 90.Gravitt PE, Peyton C, Wheeler C, et al. Reproducibility of HPV 16 and HPV 18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003;112:23–33. doi: 10.1016/s0166-0934(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 91.Ha PK, Pai SI, Westra WH, et al. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8:1203–1209. [PubMed] [Google Scholar]
- 92.Capone RB, Pai SI, Koch WM, et al. Detection and quantitation of human papillomavirus (HPV) DNA in the sera of patients with HPV-associated head and neck squamous cell carcinoma. Clin Cancer Res. 2000;6:4171–4175. [PubMed] [Google Scholar]
- 93.Veltman JA, Hopman AH, Bot FJ, et al. Detection of chromosomal aberrations in cytologic brush specimens from head and neck squamous cell carcinoma. Cancer. 1997;81:309–314. doi: 10.1002/(sici)1097-0142(19971025)81:5<309::aid-cncr9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 94.Herrero R, Catellsague X, Munoz N, et al. Multicentric case-control study of HPV and oral cancer.. Presented at the 20th International Papillomavirus Conference; Paris, France. October 4-9, 2002. [Google Scholar]
- 95.Zumbach K, Kisseljov F, Sacharova O, et al. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in cervical-carcinoma patients from Russia. Int J Cancer. 2000;85:313–318. doi: 10.1002/(sici)1097-0215(20000201)85:3<313::aid-ijc3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]