Disseminated oligodendroglioma-like leptomeningeal neoplasm (DOLN) is a recently described entity that predominantly affects children, is slowly progressive, and exhibits little, if any, parenchymal involvement. Studies to date have demonstrated some similarities between DOLNs and adult oligodendrogliomas with respect to morphology (infiltrative, monotonous cells with round, regular nuclei and perinuclear clearing), immunohistochemistry (synaptophysin, GFAP, Olig-2 expression), and genetics (high rate of chromosome 1p deletions and some 1p19q co-deletions) [5-8]. In contrast however, no DOLNs have been shown to harbor the isocitrate dehydrogenase-1 (IDH1) R132H mutation.
BRAF abnormalities are common in pediatric low-grade CNS tumors, with the BRAF-KIAA1549 fusion/tandem duplication at 7q34 identified in approximately, 60 % of cerebellar and optic pathway pilocytic astrocytomas [4]. Others, like pleomorphic xanthoastrocytoma (PXA), ganglioglioma, and dysembryoplastic neuroepithelial tumor (DNET), have shown variable frequencies of activating BRAF V600E point mutations [1]. Because DOLNs partially overlap with these neoplasms, we assessed a series of 20 cases for BRAF alterations.
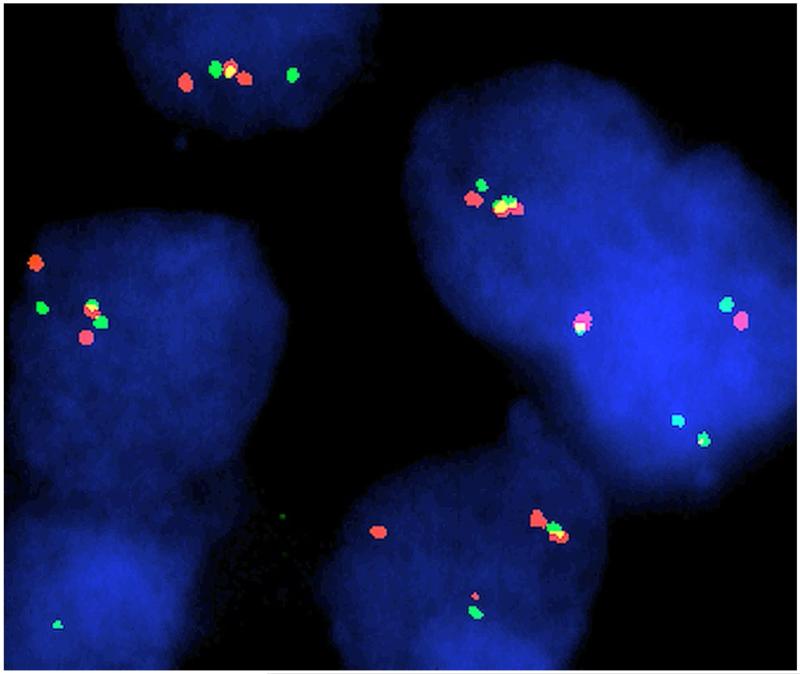
We examined 23 archival DOLNs by FISH for the BRAF-KIAA1549 fusion, and 17 cases for deletions of 1p and 19q (Abbott, North Chicago, IL, USA). One was additionally interrogated by SNP-array. Testing for BRAF V600E was also performed on nine cases. Of 15 informative cases for BRAF-KIAA1549 by FISH, 11 were fusion positive (Fig. 1). Another case was non-informative by FISH, yet harbored a duplication (ch7:138550993-140509923) by SNP-array consistent with gene fusion, for a total of 12 of 16 cases positive for BRAF-KIAA1549 (75 %). FISH revealed loss of 1p in 10/17 cases (59 %), with 3 of those being co-deleted for 19q (18 %). Of the 12 cases with BRAF fusions, 9 had 1p deletion (75 %) and 2 had 19q co-deletion (17 %). None of 9 tested specimens were positive for BRAF-V600E mutation.
Fig. 1.
DOLN nuclei with FITC-labeled probe RP11-355D18 corresponding to KIAA1549 (green) and digoxigenin-labeled probe 726N20 corresponding to BRAF (red). Yellow signals indicate fusion
These findings indicate a high rate of concurrent BRAF-KIAA1549 gene fusions and 1p deletions in DOLNs. Although BRAF fusions are typical of pilocytic astrocytomas, 1p deletions are not, confirming that DOLNs are pathologically and genetically distinct in most cases. These findings also further separate these oligodendroglioma-like tumors from adult oligodendrogliomas, although some overlap remains since 1p19q co-deletion is occasionally found in DOLN and rare BRAF fusions have been recently reported in otherwise classic, 1p19q co-deleted oligoden-droglioma [3].
DOLNs have also displayed occasional ganglion cell differentiation and areas of richly myxoid stroma, raising related possible link to gangliogliomas or DNETs, yet none of our 9 tested cases showed evidence of a BRAF V600E mutation, suggesting that DOLNs are genetically distinct from those entities as well. First-generation RAF inhibitors have been demonstrated in vitro to paradoxically activate the BRAF-KIAA1549 fusion protein [9]. Instead, MEK or mTOR inhibitors could be considered in the treatment of DOLNs, as in other fusion-positive tumors [2].
This report examines the role of common BRAF abnormalities in DOLNs and establishes a high frequency of concurrent BRAF-KIAA1549 fusions and 1p deletions. Although DOLNs are already clinicopathologically distinct, these findings further demonstrate fundamental genetic differences from other entities and implicate a new potential therapeutic target for patients with these otherwise challenging disseminated tumors.
Acknowledgments
The authors would like to thank Roxanne Marshall at UCSF for her excellent technical work performing FISH.
Contributor Information
Fausto J. Rodriguez, Division of Neuropathology, Department of Pathology, Johns Hopkins Hospital, Johns Hopkins University, Sheikh Zayed Tower, Room M2101, 1800 Orleans Street, Baltimore, MD 21231, USA
Matthew J. Schniederjan, Department of Pathology and Laboratory Administration, Children’s Healthcare of Atlanta, 1001 Johnson Ferry Rd NE, Atlanta, GA 30342, USA; Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Room G170, 1364 Clifton Rd NE, Atlanta, GA 30322, USA
Theo Nicolaides, Department of Pediatrics, University of California San Francisco School of Medicine, 550 16th Street, San Francisco, CA 94143, USA.
Tarik Tihan, Division of Neuropathology, Department of Pathology, University of California San Francisco School of Medicine, 505 Parnassus Avenue, San Francisco, CA 94143, USA.
Peter C. Burger, Division of Neuropathology, Department of Pathology, Johns Hopkins Hospital, Johns Hopkins University, Sheikh Zayed Tower, Room M2101, 1800 Orleans Street, Baltimore, MD 21231, USA
Arie Perry, Division of Neuropathology, Department of Pathology, University of California San Francisco School of Medicine, 505 Parnassus Avenue, San Francisco, CA 94143, USA.
References
- 1.Chappe C, Padovani L, Scavarda D, Forest F, Nanni-Metellus I, Loundou A, Mercurio S, Fina F, Lena G, Colin C, et al. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 2013;23:574–583. doi: 10.1111/bpa.12048. doi:10.1111/bpa.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasgupta T, Haas-Kogan DA. The combination of novel targeted molecular agents and radiation in the treatment of pediatric gliomas. Front Oncol. 2013;3:110. doi: 10.3389/fonc.2013.00110. doi:10.3389/fonc.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YH, Nonoguchi N, Paulus W, Brokinkel B, Keyvani K, Sure U, Wrede K, Mariani L, Giangaspero F, Tanaka Y, et al. Frequent BRAF gain in low-grade diffuse gliomas with 1p/19q loss. Brain Pathol. 2012;22:834–840. doi: 10.1111/j.1750-3639.2012.00601.x. doi:10.1111/j.1750-3639.2012.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin A, Rodriguez FJ, Karajannis MA, Williams SC, Legault G, Zagzag D, Burger PC, Allen JC, Eberhart CG, Bar EE. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. doi:10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perilongo G, Gardiman M, Bisaglia L, Rigobello L, Calderone M, Battistella A, Burnelli R, Giangaspero F. Spinal low-grade neoplasms with extensive leptomeningeal dissemination in children. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2002;18:505–512. doi: 10.1007/s00381-002-0626-8. doi:10.1007/s00381-002-0626-8. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez FJ, Perry A, Rosenblum MK, Krawitz S, Cohen KJ, Lin D, Mosier S, Lin MT, Eberhart CG, Burger PC. Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol. 2012;124:627–641. doi: 10.1007/s00401-012-1037-x. doi:10.1007/s00401-012-1037-x. [DOI] [PubMed] [Google Scholar]
- 7.Rossi S, Rodriguez FJ, Mota RA, Dei Tos AP, Di Paola F, Bendini M, Agostini S, Longatti P, Jenkins RB, Giannini C. Primary leptomeningeal oligodendroglioma with documented progression to anaplasia and t(1;19)(q10;p10) in a child. Acta Neuropathol. 2009;118:575–577. doi: 10.1007/s00401-009-0565-5. doi:10.1007/s00401-009-0565-5. [DOI] [PubMed] [Google Scholar]
- 8.Schniederjan MJ, Alghamdi S, Castellano-Sanchez A, Mazewski C, Brahma B, Brat DJ, Brathwaite CD, Janss AJ. Diffuse leptomeningeal neuroepithelial tumor: 9 pediatric cases with chromosome 1p/19q deletion status and IDH1 (R132H) immunohistochemistry. Am J Surg Pathol. 2013;37:763–771. doi: 10.1097/PAS.0b013e31827bf4cc. doi:10.1097/PAS.0b013e31827bf4cc. [DOI] [PubMed] [Google Scholar]
- 9.Sievert AJ, Lang SS, Boucher KL, Madsen PJ, Slaunwhite E, Choudhari N, Kellet M, Storm PB, Resnick AC. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci USA. 2013;110:5957–5962. doi: 10.1073/pnas.1219232110. doi:10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]