Abstract
Purpose
Pediatric intramedullary spinal cord tumors are exceedingly rare; in the United States, 100 to 200 cases are recognized annually, of these, most are astrocytomas. The purpose of this study is to report the outcomes in pediatric patients with spinal cord astrocytomas treated at a tertiary care center.
Methods and Materials
An institutional review board-approved retrospective single-institution study was performed for pediatric patients with spinal cord astrocytomas treated at our hospital from 1990 to 2010. The patients were evaluated on the extent of resection, progression-free survival (PFS), and development of radiation-related toxicities. Kaplan-Meier curves and multivariate regression model methods were used for analysis.
Results
Twenty-nine patients were included in the study, 24 with grade 1 or 2 (low-grade) tumors and 5 with grade 3 or 4 (high-grade) tumors. The median follow-up time was 55 months (range, 1–215 months) for patients with low-grade tumors and 17 months (range, 10–52 months) for those with high-grade tumors. Thirteen patients in the cohort received chemotherapy. All patients underwent at least 1 surgical resection. Twelve patients received radiation therapy to a median radiation dose of 47.5 Gy (range, 28.6–54.0 Gy). Fifteen patients with low-grade tumors and 1 patient with a high-grade tumor exhibited stable disease at the last follow-up visit. Acute toxicities of radiation therapy were low grade, whereas long-term sequelae were infrequent and manageable when they arose. All patients with low-grade tumors were alive at the last follow-up visit, compared with 1 patient with a high-grade tumor.
Conclusion
Primary pediatric spinal cord astrocytomas vary widely in presentation and clinical course. Histopathologic grade remains a major prognostic factor. Patients with low-grade tumors tend to have excellent disease control and long-term survival compared to those with high-grade tumors. This experience suggests that radiation therapy may enhance tumor control with an acceptably low risk of long-term sequelae in this sensitive patient population.
Introduction
Pediatric intramedullary spinal cord tumors are rare, with only 100 to 200 cases recognized annually in the United States (1). Of these, low-grade astrocytomas and other primary glial neoplasms account for the majority (2–4). In the pediatric population, the majority of spinal cord astrocytomas are low grade (5).
The presenting symptoms of intramedullary spinal cord tumors generally arise slowly and progress insidiously. They can be general or localized and may include pain, paresthesia, weakness, spinal deformity, motor regression, incontinence, and torticollis (6).
Interventions for spinal cord astrocytomas include surgery, radiation, and chemotherapy. Although surgery is the cornerstone of pediatric spinal cord astrocytoma management, the benefit of gross total resection (GTR) for low-grade astrocytomas is not clear, and higher-grade tumors are more infiltrative; therefore, a GTR is difficult to obtain (7). Radiation alters the disease course, yet is often deferred or avoided because of concerns about long-term sequelae for the pediatric patient (8). Although published reports about the use of chemotherapy are limited, it may emerge as an alternative or adjunct to surgery in an effort to delay radiation therapy (RT) in children to minimize late sequelae (7, 9).
The purpose of this study is to report the outcomes in pediatric patients with spinal cord astrocytomas treated at our institution.
Methods and Materials
An institutional review board-approved retrospective singleinstitution study was performed for pediatric patients with spinal cord astrocytomas treated at our institution from 1990 to 2010 identified from a pathology database. The inclusion criteria included age <25 years at diagnosis, intramedullary spinal cord tumor, and a tissue diagnosis of astrocytoma. All pathology reports were re-reviewed at our institution if they had been obtained from an outside institution. All World Health Organization (WHO) grades (1–4) were included and categorized as low (WHO 1–2) or high (WHO 3–4) grade.
The extent of surgical resection was determined by analysis of the operative report and postoperative radiographic imaging. GTR was defined as 90% resection or no visible tumor remaining at the end of surgery. Subtotal resection (STR) was defined as 50% to 90% resection, partial resection (PR) was defined as <50% resection, and biopsy was defined as a very limited resection intended only to reveal a histopathologic diagnosis.
Progression-free survival (PFS) was defined as the time elapsed from diagnosis to progression or recurrence. Disease control was defined as the lack of radiologic or clinical progression or recurrence at the most recent record. Patient records were assessed for the development of radiation-related toxicities. Adverse events were evaluated by the Common Terminology Criteria for Adverse Events, version 3.0 (10).
Overall survival and PFS were estimated by the Kaplan-Meier product limit method. Observations were censored at the last follow-up visit in the absence of progression or death. PFS was stratified by various covariates and compared with a log-rank test. The Cox proportional hazards method was applied to identify predictors of PFS (11). An initial model was created by adjustment for age, sex, grade, chemotherapy, radiation, and degree of resection. A final parsimonious model was generated by use of backward selection, removing 1 variable at each step and comparing the nested models using the likelihood ratio test and Akaike information criterion (12, 13). The reported P values in the results are 2-sided and considered significant when <.05. All analysis was performed with Stata Statistical Software: Release 9 and IBM SPSS Statistics, version 20 (14, 15).
Results
Demographics
There were 29 patients identified for the study. The demographic and treatment characteristics are shown in Table 1. Twenty-four patients had low-grade tumors, and 5 had high-grade tumors (including 1 glioblastoma [GBM]). Of the 29 astrocytomas, 9 were cervical, 7 were cervicothoracic, 11 were thoracic, and 2 were holocord. The median age at diagnosis was 7.1 years (range, 1.3–21.2 years). The median follow-up time was 52 months (range, 1–215 months). Patients with low-grade tumors had a longer median follow-up time of 55 months (range, 1–215 months) compared with high-grade tumor patients, who had a median follow-up of 17 months (range, 10–52 months).
Table 1.
Patient demographics
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| F | 9 | 31.0 |
| M | 20 | 69.0 |
| Pathology | ||
| Low-grade | 24 | 82.8 |
| AA | 4 | 13.8 |
| GBM | 1 | 3.4 |
| Surgery | ||
| PR/biopsy | 9 | 31.0 |
| STR | 4 | 13.8 |
| GTR | 15 | 51.7 |
| Unknown | 1 | 3.4 |
| Radiation therapy | ||
| None | 15 | 51.7 |
| Focal | 8 | 27.6 |
| CSI | 2 | 6.9 |
| Unknown | 2 | 6.9 |
| Chemotherapy | ||
| Received | 13 | 44.8 |
Abbreviations: AA = anaplastic astrocytoma; CSI = craniospinal irradiation; GBM = glioblastoma multiforme; GTR = gross total resection; PR = partial resection; STR = subtotal resection.
Pain was the most common presenting symptom, affecting 12 patients. Other prominent symptoms included weakness in 9 patients, headache in 3 patients, and urinary incontinence, torticollis, or paresthesia in 2 patients. There were 3 patients who had leptomeningeal involvement, 2 at presentation and 1 at recurrence. There were 6 patients who experienced intracranial involvement, 2 at presentation and 4 at recurrence.
Surgery, chemotherapy, and radiation therapy
All patients underwent surgical intervention at initial presentation. Fifteen patients had GTR, 4 had STR, and 9 received PR or biopsy. One patient could not be classified. Thirteen patients were treated with a variety of chemotherapy regimens and agents, including temozolomide, vinblastine, vincristine, carboplatin, and arsenic trioxide.
Twelve patients received RT to a median dose of 47.5 Gy (range, 28.6–54 Gy) (Table 2). Eight of the 24 patients with low-grade tumors received RT. Of these, 4 received adjuvant RT, whereas 4 received salvage RT after disease progression. Four patients with high-grade astrocytomas received RT, 3 of whom received adjuvant RT.
Table 2.
Radiation therapy characteristics
| Pt | Age at dx (y) | Total F/U (mo) | Site | Grade | Surgery | RT type | Total dose/fraction (cGy) | RT after failure | Failure after RT (mo) | Salvage tx | Status at last F/U |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.8 | 57 | T | 2 | GTR | Conv | 5040/180 | N | N/A | N/A | S |
| 2 | 5.3 | 153 | T | 2 | PR | CSI | 4640/160 | N | N/A | N/A | S |
| 3 | 6.3 | 117 | C | 2 | PR | 3D CRT | 5400/180 | N | N/A | N/A | S |
| 4 | 3.8 | 215 | C | 1–2 | PR | Conv | 4910/180–190 | Y | N/A | N/A | S |
| 5 | 6.8 | 143 | T | 1 | PR | 3D CRT | 4500/180 | N | 28 | Surgery, chemo | S |
| 6 | 7.0 | 52 | CT | 1 | GTR | IMRT | 4860/180 | Y | N/A | N/A | S |
| 7 | 12.8 | 193 | CT | 1 | PR | 3D CRT | 2860/180 | Y | N/A | N/A | S |
| 8 | 2.2 | 143 | CT | 1 | PR | 3D CRT | 4600/200 | Y | N/A | N/A | S |
| 9 | 6.9 | 21 | T | 3 | GTR | Conv | 4500/180 | Y | 17 | Hospice | D |
| 10 | 10.8 | 10 | C | 4 | STR | CSI | 5400/180 | N | 2 | Chemo | D |
| 11 | 16.0 | 17 | CT | 3 | GTR | * | * | Y | 11 | Surgery | S |
| 12 | 11.6 | 12 | C | 3 | GTR | * | * | N | 11 | Surgery, chemo | D |
Abbreviations: 3D=3-dimensional; C=cervical; Chemo=chemotherapy; Conv=conventional radiation therapy; CSI=craniospinal irradiation; CT=cervicothoracic; D=deceased; dx=diagnosis; F/U=follow-up; GTR=gross total resection; IMRT=intensity modulated radiation therapy; N/A=not applicable; PR=partial resection; Pt=patient; RT=radiation therapy; S=stable; STR=subtotal resection; T=thoracic; tx=treatment.
Information unavailable.
Overall survival and progression-free survival
The overall survival of the 29 patients is shown in Figure 1A. At the time of the last follow-up visit, all 24 patients with low-grade spinal astrocytomas were alive, whereas only 1 of 5 patients with high-grade spinal astrocytomas was alive. The median PFS was 23 months for low-grade astrocytomas (range 1–153 months), compared with 14 months (range, 3–45 months) for high-grade astrocytomas (Fig. 1B). Fifteen patients with low-grade astrocytomas experienced recurrence after initial therapy, and all highgrade astrocytomas recurred or progressed.
Fig. 1.
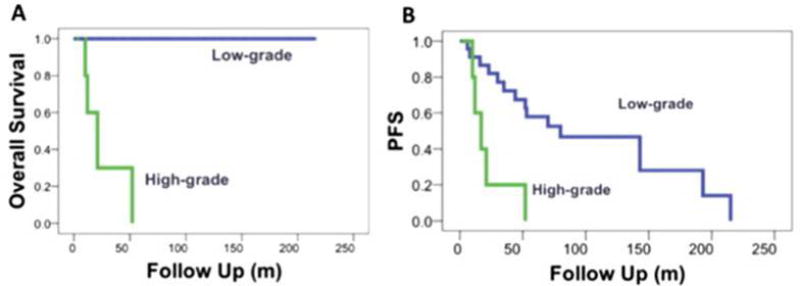
(A) Overall survival for high-grade and low-grade tumors. (B) Progression-free survival (PFS) for high-grade versus low-grade tumors (P=.13).
Of the 21 patients with low-grade tumors who had longer than 6 months of follow-up, 15 had stable disease at the last follow-up visit. Six patients with low-grade astrocytomas experienced at least 2 recurrences. The median time to second recurrence among patients with low-grade tumors was 40.5 months from the date of diagnosis (range, 12–47 months). One patient with a low-grade astrocytoma experienced 3 recurrences and was stable at the last follow-up visit, 64 months after the final recurrence. One patient with a high-grade astrocytoma had stable disease at the last follow-up visit, and all others had died at a median time to death of 16.5 months (range, 10–52 months). No patient with a highgrade astrocytoma received salvage treatment after the first recurrence. A multivariate regression model failed to identify any correlation between patient variables (sex, age, extent of resection, presence of intracranial disease, RT, or tumor grade) that correlated with PFS.
Of the 4 patients with low-grade tumors who received adjuvant radiation, 3 did not experience recurrence or progression for the duration of follow-up at a median of 116 months (range, 52–138 months), whereas the fourth patient experienced recurrence 30 months after the initial diagnosis and was able to achieve disease control for the remaining 113 months of follow-up after salvage treatment. By contrast, only 6 of the 20 patients with low-grade tumors who did not receive adjuvant RTwere free of disease progression or recurrence for the duration of follow-up. All 4 patients who received salvage RT after an initial treatment failure had well-controlled disease thereafter and did not experience recurrence or progression.
All 3 of the patients with high-grade tumors who received adjuvant RT experienced recurrence at a median of 14 months (range, 12–20 months). One patient with a high-grade tumor received salvage RT and experienced recurrence after 3 months, and 1 patient did not receive RT and experienced recurrence after 45 months.
Toxicity
Of the 12 patients who received RT, 6 experienced acute toxicities, including nausea/vomiting (4 patients), desquamation (3 patients), skin hyperpigmentation (2 patients), and pruritus (2 patients). All acute toxicities were grade 1–2. Two patients experienced long-term sequelae, potentially caused by RT. One patient received 45 Gy to the whole spine and experienced hypothyroidism. A patient with a low-grade cervical astrocytoma received 49.1 Gy focal radiation at 5 years of age and experienced a Hürthle-cell neoplasm, thyroid cysts, and hypothyroidism. Ten patients received spinal fusion for kyphoscoliosis after surgical resection of tumor.
Discussion
Owing to the rarity of this condition, pediatric astrocytomas of the spinal cord are often analyzed in studies that include many distinct spinal tumors (16–19). This study focused solely on spinal astrocytomas and encompasses the full spectrum of low- and highgrade neoplasms. As with all retrospective studies, caution must be exercised when drawing conclusions because treatment modalities cannot be directly compared, and the lack of randomization leaves the data vulnerable to bias.
Our study demonstrates that multimodality therapy for low-grade spinal astrocytomas results in excellent survival rates with minimal treatment sequelae. All toxicities, acute and chronic, observed in this report were manageable. Further, some of the sequelae observed in this case series, such as in the patient in whom a Hürthle-cell neoplasm developed, arose in unusual circumstances, such as cervical radiation received by a young child. By contrast, our study affirms the dismal prognosis of highgrade spinal astrocytomas similar to their intracranial counterparts. The results from our study are consistent with those from other recent reports. The Hospital for Sick Children in Toronto published a case series of 29 pediatric patients with low-grade gliomas, 86% of which were grade 1, over a 22-year period (7). They found a male predominance and a peak in diagnosis at age 2. Although our present study did not find a similar age distribution, we also observed a male predominance. Similar to our experience with low-grade tumors, all patients were alive at the last follow-up visit, albeit with significant long-term sequelae, including kyphoscoliosis, hemiparesis, paraparesis, and neurogenic bladder. The authors did not find a relationship between the extent of surgery, radiation, or chemotherapy and functional outcome. Of the 5 patients who received radiation, 4 received radiation at recurrence and 1 received adjuvant radiation, reflecting the approach of delaying radiation to spare potential developmental complications.
A recent case series, from St. Jude Children’s Research Hospital, highlights the aggressive nature of, and unsatisfactory treatments available for, high-grade pediatric spinal cord tumors (20). Among the 17 patients evaluated, 7 had GBM and 8 had anaplastic astrocytoma. The GBM patients experienced worse outcomes than did the others, and the 3 long-term survivors had grade 3 astrocytomas. All patients received RT, and 7 received craniospinal irradiation. The poor outcomes in this study mirror our institution’s experience and other recent reports (21, 22).
Although our sample size is limited, the results of RT are encouraging for patients with low-grade tumors. Seven of 8 patients who received adjuvant or salvage RT never experienced recurrence or further progression. Even more notably, all 4 patients who had experienced 1 to 3 recurrences before receiving RT exhibited disease control for the remainder of the follow-up time. Therefore, delaying RT until disease recurrence rather than irradiating at presentation for patients with low-grade astrocytomas may spare the adverse effects of radiation on development without sacrificing tumor control.
Overall, radiation treatment was well tolerated in this cohort. The variety of radiation techniques patients received, including craniospinal irradiation and focal therapies using conventional RT, 3-dimensional conformal RT, and intensity modulated RT, reflects the evolving technology and growing ability to limit radiation dose to adjacent normal structures. Similarly, advances in diagnostic radiology have aided the detection and follow-up of these tumors. The low rates of radiation complications overall may be due to the modern RT methods that most patients received, although these observations may reflect the limited nature of this retrospective case series and the small sample size. Recently, particle therapy has emerged as an attractive option for spinal tumors, because the ability to spare normal tissues could potentially decrease the developmental sequelae of RT for long-term survivors of low-grade astrocytomas. Carefully designed studies with long-term clinical outcomes are required to evaluate this technology.
Conclusion
Primary pediatric spinal cord astrocytomas vary widely in presentation and clinical course. Because of the small patient population, attempts to characterize the efficacy of treatments are difficult. Histopathologic grade remains a major prognostic factor. Patients with low-grade astrocytomas tend to have excellent disease control and long-term survival. Our experience suggests that the judicious application of RT in this sensitive patient population may enhance tumor control for low-grade astrocytomas with an acceptably low risk of long-term sequelae. However, possible late effects in the pediatric population must be weighed against the benefit of giving RT in children expected to experience long-term survival. By contrast, pediatric patients with high-grade spinal astrocytomas continue to demonstrate poor outcomes despite multimodality therapy, highlighting the need to develop novel interventions for these patients.
Summary.
Primary pediatric spinal cord astrocytomas vary widely in presentation and clinical course. This study reports the outcomes in pediatric patients with spinal cord astrocytomas treated at a tertiary care center. Patients with low-grade tumors experienced excellent disease control and long-term survival compared to those with high-grade tumors. This report suggests that the judicious application of radiation therapy in this sensitive patient population may enhance tumor control with an acceptably low risk of long-term sequelae.
Footnotes
Conflict of interest: none.
References
- 1.Lober R, Sharma S, Bell B, et al. Pediatric primary intramedullary spinal cord glioblastoma. Rare Tumors. 2010;2:e48. doi: 10.4081/rt.2010.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binning M, Klimo P, Jr, Gluf W, et al. Spinal tumors in children. Neurosurg Clin N Am. 2007;18:631–658. doi: 10.1016/j.nec.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Auguste KI, Gupta N. Pediatric intramedullary spinal cord tumors. Neurosurg Clin N Am. 2006;17:51–61. doi: 10.1016/j.nec.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Epstein FJ, Rezai AR, et al. Nonneoplastic intramedullary spinal cord lesions mimicking tumors. Neurosurgery. 1998;43:788–794. doi: 10.1097/00006123-199810000-00034. discussion 794–795. [DOI] [PubMed] [Google Scholar]
- 5.DeSousa AL, Kalsbeck JE, Mealey J, Jr, et al. Intraspinal tumors in children: a review of 81 cases. J Neurosurg. 1979;51:437–445. doi: 10.3171/jns.1979.51.4.0437. [DOI] [PubMed] [Google Scholar]
- 6.Houten JK, Cooper PR. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol. 2000;47:219–224. doi: 10.1023/a:1006466422143. [DOI] [PubMed] [Google Scholar]
- 7.Scheinemann K, Bartels U, Huang A, et al. Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr. 2009;4:254–261. doi: 10.3171/2009.4.PEDS08411. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan C, Jenkin RD, Doherty MA, et al. Spinal cord tumors in children: long-term results of combined surgical and radiation treatment. J Neurosurg. 1994;81:507–512. doi: 10.3171/jns.1994.81.4.0507. [DOI] [PubMed] [Google Scholar]
- 9.Hassall TE, Mitchell AE, Ashley DM. Carboplatin chemotherapy for progressive intramedullary spinal cord low-grade gliomas in children: three case studies and a review of the literature. Neuro Oncol. 2001;3:251–257. doi: 10.1093/neuonc/3.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Common Terminology Criteria for Adverse Events v3.0 (CTCAE) Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. 2003 Mar 31; http://ctep.cancer.gov, Publish Date: August 9, 2006.
- 11.Cox D. Regression models and life-tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 12.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 13.Akaike H. Likelihood and the Bayes Procedure. Valencia: University Press; 1980. [Google Scholar]
- 14.Stata Statistical Software. Vol. 9. StataCorp; 2005. [Google Scholar]
- 15.SPSS Statistics. Vol. 20. IBM; [Google Scholar]
- 16.Houten JK, Weiner HL. Pediatric intramedullary spinal cord tumors: special considerations. J Neurooncol. 2000;47:225–230. doi: 10.1023/a:1006418506213. [DOI] [PubMed] [Google Scholar]
- 17.Nadkarni TD, Rekate HL. Pediatric intramedullary spinal cord tumors. Critical review of the literature. Childs Nerv Syst. 1999;15:17–28. doi: 10.1007/s003810050321. [DOI] [PubMed] [Google Scholar]
- 18.Garces-Ambrossi GL, McGirt MJ, Mehta VA, et al. Factors associated with progression-free survival and long-term neurological outcome after resection of intramedullary spinal cord tumors: analysis of 101 consecutive cases. J Neurosurg Spine. 2009;11:591–599. doi: 10.3171/2009.4.SPINE08159. [DOI] [PubMed] [Google Scholar]
- 19.McGirt MJ, Chaichana KL, Atiba A, et al. Resection of intramedullary spinal cord tumors in children: assessment of long-term motor and sensory deficits. J Neurosurg Pediatr. 2008;1:63–67. doi: 10.3171/PED-08/01/063. [DOI] [PubMed] [Google Scholar]
- 20.Tendulkar RD, Pai Panandiker AS, Wu S, et al. Irradiation of pediatric high-grade spinal cord tumors. Int J Radiat Oncol Biol Phys. 2010;78:1451–1456. doi: 10.1016/j.ijrobp.2009.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ononiwu C, Mehta V, Bettegowda C, et al. Pediatric spinal glioblastoma multiforme: current treatment strategies and possible predictors of survival. Childs Nerv Syst. 2012;28:715–720. doi: 10.1007/s00381-012-1705-0. [DOI] [PubMed] [Google Scholar]
- 22.Adams H, Avendano J, Raza SM, et al. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: a populationbased analysis from 1973 to 2007. Spine (Phila Pa 1976) 2012;37:E727–E735. doi: 10.1097/BRS.0b013e31824584c0. [DOI] [PMC free article] [PubMed] [Google Scholar]


