Abstract
Malignant gliomas, including glioblastoma and anaplastic astrocytomas, are characterized by their propensity to invade surrounding brain parenchyma, making curative resection difficult. These tumors typically recur within two centimeters of the resection cavity even after gross total removal. As a result, there has been an emphasis on developing therapeutics aimed at achieving local disease control. In this review, we will summarize the current developments in the delivery of local therapeutics, namely direct injection, convection-enhanced delivery and implantation of drug-loaded polymers, as well as the application of these therapeutics in future methods including microchip drug delivery and local gene therapy.
Malignant gliomas, including glioblastoma (GBM) and anaplastic astrocytoma (AA), are the most common primary brain tumor in adults [1]. The median survival for patients with malignant gliomas is less than two years, and some argue that it has not really improved over the past several years despite advances in surgical and medical therapy [2]. This minimal improvement in outcomes for patients with these tumors is due to several factors: these tumors have a propensity to migrate and invade surrounding normal brain parenchyma, making current local therapeutic strategies including surgical resection and radiation ineffective [3–6]; these tumors reside in the brain that is protected by the blood–brain barrier (BBB), making it difficult for systemic therapies to exert their tumoricidal effects [7] and they have the ability to resist current therapies because of their genetic instability and cellular heterogeneity, making it difficult to target and successfully treat all cells [3–6]. These collective obstacles strongly suggest that an effective drug-delivering strategy would need to be able to target these invading cells, bypass the BBB and achieve high levels in the brain while minimizing systemic toxicity in order to overcome tumor resistance.
There have been advances in the delivery of local therapeutics to the brain for brain tumors [8–11]. In this review, we will summarize the current developments in the delivery of local therapeutics, namely direct injection, convection-enhanced delivery (CED) and implantation of drug-impregnated polymers. These strategies may help improve future treatment modalities including drug-impregnated microchip implantation and local gene therapy [12,13]. These methods of delivering local therapeutics thus aim to overcome the barriers to effective brain tumor treatment.
Clinical outcomes & current therapies for patients with malignant gliomas
While different physicians have different philosophies, there are only three medical therapies specifically approved by the US FDA for use in treating GBM – temolzomide, carmustine wafers and bevacizumab. The median survival for patients with GBM ranges from 10 to 16 months [14–26], while the median progression free survival ranges from 6 to 12 months [15–17,27]. The median survival for patients with AA ranges from 18 to 60 months [22,23,28,29], while the median progression free survival ranges from 20 to 60 months [22,23,28,29]. Thus, while the median overall and progression free survival times for patients with malignant gliomas are relatively poor, individual survival is heterogeneous with some patients having short survival times and others having relatively long survival times. The clinical factors that have been consistently associated with improved survival are younger age, improved neurological function, increased extent of resection, use of carmustine wafers, temozolomide chemotherapy and radiation therapy [15–17,19–21,23,26,27,30–36]. More recently, tumor location near neurogenic niches has also been shown to be associated with survival, where tumors near the lateral ventricles are associated with worse survival [14,18,37]. Tumors with isocitrate dehydrogenase 1 and positive MGMT methylation are also associated with improved outcomes for patients with malignant gliomas [38,39].
Key terms.
- Malignant gliomas
Most common primary brain tumors in adults, and are classified by the World Health Organization as Grade III or IV based on cellular proliferation, cellular atypia, necrosis and vascular proliferation
- Convection-enhanced delivery
Delivery method whereby continuous injection of an agent under positive pressure of a fluid enhances agent distribution
- Drug-impregnated polymers
Polymers with drug impregnated into its construction. These polymers undergo sustained degradation with the continuous release of drugs
- Temozolomide
Orally administered alkylating agent that is most commonly used for malignant gliomas as well as melanoma, and works by interfering with DNA replication
- Drug-impregnated microchips
Microchips engineered to release drugs in a time dependent and/or external control mechanisms.
The majority of malignant gliomas recur in close proximity to the initial tumor bed [40,41]. In fact over 95% of tumor recurrences occur within 1–2 cm margin of the initial tumor bed, and, as a result, standard radiation treatment fields have shifted to treat the gross tumor volume and a 1–2 cm margin from the tumor bed [40,41]. Despite this concentration on the tumor margin for radiation therapy, malignant gliomas inevitably recur. This has placed an emphasis on developing local therapies aimed at targeting these tumor recurrences at the tumor margins.
Patients who present with radiographic imaging consistent with a malignant glioma are generally treated with extensive surgical resection, chemotherapy implants, followed by concurrent radiation and temozolomide chemotherapy [25,42–45]. Antiangiogenesis and therapeutic protocols are also used whenever appropriate [46]. The benefit of surgical resection for patients with malignant gliomas is a relatively new concept. In the 1920s, Walter Dandy performed hemispherectomies for patients with presumed GBM, and found that these tumors would recur in the contra-lateral hemisphere [47]. This perceived notion of surgical futility led to the widespread advocacy of biopsy for diagnosis followed by adjuvant therapy [48]. However, Laws et al. in 2003 analyzed the outcomes for patients with newly diagnosed malignant gliomas from a multi-institutional cohort and found that patients who underwent surgical resection for both GBM and AA had independently longer survival than patients who underwent needle biopsy [22]. The median survival for GBM patients who underwent surgical resection was 45.3 versus 21.0 weeks for patients who underwent needle biopsy, while the median survival for AA patients who underwent surgical resection was 87 versus 52.1 weeks for those who underwent needle biopsy [22]. More recently, we have shown that gross total resection (GTR) (>99%) is more beneficial than near total resection (NTR) (95–99%), and NTR is more beneficial than subtotal resection (STR) (<95%) for patients with both GBM and AA [23]. For patients with newly diagnosed GBM, the median survival times for patients with GTR, NTR and STR were 13, 11 and 8 months, respectively [23]. For patients with newly diagnosed AA, the median survival times for patients with GTR, NTR and STR were 58, 46 and 34 months, respectively [23]. Furthermore, Lacroix et al. in 2001 found that 98% resection was needed to achieve a meaningful difference for patients with GBM, regardless of being newly diagnosed or recurrent [20]. This threshold was updated to be 78% in 2007 by Sanai et al. [36], and more recently we found a lower threshold of 70% was needed to make a meaningful difference in patient outcomes [27]. The median survival for patients with >70% resection was 14.4 months as compared with 10.5 months for patients with lesser nonbiopsy, surgical resections [27]. All of these studies, however, concluded that more percent resection is associated with improved survival and recurrence outcomes [17,20,27,36].
Following surgery, adjuvant therapies include radiation and temozolomide chemotherapy [25]. Radiation therapy with external brain radiation therapy (EBRT) has been shown to be effective for patients with newly diagnosed GBM [40,49]. The median survival for patients who received EBRT was 9 months as compared with 5 months for patients who did not receive EBRT [40,49]. The standard radiation dose is 58 to 60 Gy, where previous studies have showed that 60 Gy was superior to 45 Gy, but there was no significant difference with 70 Gy dosing [33,50]. As a result, the current radiotherapy regimen is to use EBRT for a total of 58 to 60 Gy, which is given in 1.8 to 2.0 Gy fractions for 5 days per week for 30 total days [25]. In total, 40 Gy is administered to the tumor area and an additional boost of 20 Gy is given to the enhancing tumor plus a 2 cm tumor margin [51].
In addition to radiation therapy, concomitant temozolomide chemotherapy is typically given following surgical resection [25]. Temozolomide is an orally administered, second generation alkylating agent that functions by alkylating the DNA of dividing cells and thereby inhibiting DNA repair [25]. Alkylating agents, unlike other types of chemotherapeutic drugs, have an ability to cross the BBB, and therefore able to achieve cytotoxic concentrations in the CNS [25]. Temozolomide is similar to or slightly less lipid soluble than ethanol, and thus probably crosses the BBB by passive diffusion at a slightly slower rate than ethanol [25]. In 2005, Stuppet al. performed a randomized control trial for patients with newly diagnosed GBM [25]. Following surgical resection, patients who underwent temozolomide and radiation therapy had a significantly longer median survival than patients who received only radiation therapy (14.6 vs 12.1 months) [25]. However, some tumors have the ability to resist alkylating agents by over expressing the O6-methylguanine-DNA methyltransferase (MGMT) protein, which is a DNA repair protein that functions by removing the alkyl group from the O6 position of guanine [52]. Patients with promoter methylation have epigenetic silencing of the MGMT gene and therefore have longer progression free and overall survival times [38,53]. Among GBM patients who underwent temozolomide and radiation therapy, the median survival for patients with methylated tumors was 21.7 months as compared with 15.3 months for nonmethylated tumors [38].
Blood–brain barrier
The BBB, as well as the blood-cerebrospinal fluid (CSF) barrier, is a specialized structure that surrounds most of the CNS [54,55]. It consists of CNS blood vessels and capillary endothelial cells that form tight junctions, also known as zona occludens [54,55]. These junctions, in addition to the low endocytic activity of these endothelial cells, limit the transcellular transport of various molecules into the CNS [54,55]. This barrier is essentially impervious to hydrophobic molecules and molecules larger than 200 kilodaltons, which includes most chemotherapeutics [54,55]. In patients with primary brain tumors, the BBB is only marginally compromised by the tumor and typically remains intact at the tumor periphery [54,55]. Additionally, tumor cells can migrate within the brain parenchyma away from compromised BBB [54,55]. As a result, the relatively intact integrity of the BBB in tumor-infiltrated regions severely limits the efficacy of systemically-administered chemotherapeutic drugs [54,55]. In addition to the local delivery techniques that will be discussed in this review, there is growing interest in other techniques used to increase the permeability of the BBB and include high-intensity focused-ultrasound, osmotic drugs like mannitol, and biochemical molecules such as bradykinins, among others [56]. This review will focus primarily on local delivery techniques of direct injection, CED, implantable drug-impregnated polymers, drug-impregnated microchips and local gene therapy.
Direct injection of chemotherapeutics
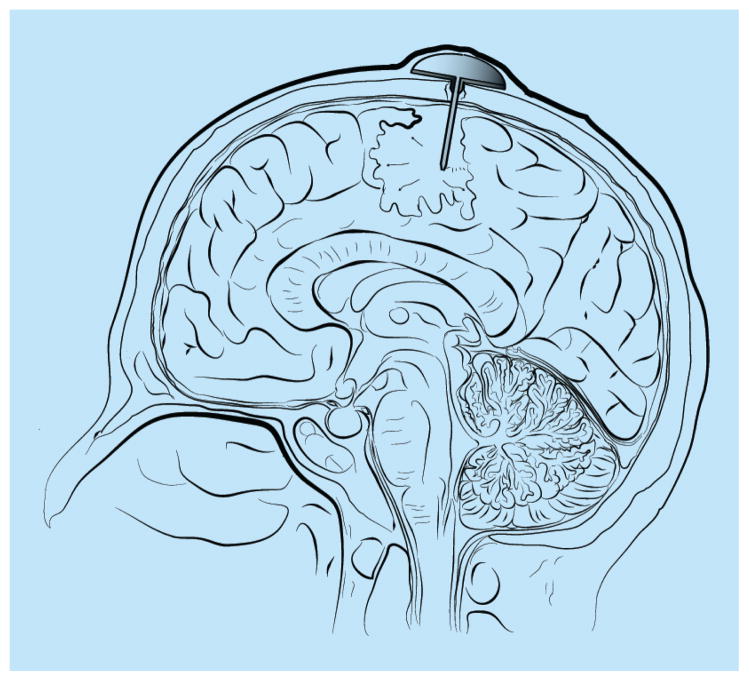
Direct injection of chemotherapeutics is the earliest method of local drug delivery (Figure 1 & Table 1). This method involves injection of chemotherapeutics into the tumor resection cavity, surrounding brain parenchyma, and/or the ventricle. This can be done via either repeated needle-based injections and/or catheter implants that are connected to a reservoir (i.e., Ommaya reservoir) for continued injection of chemotherapeutics including drugs, radioactive compounds, viruses, antibodies and lymphocytes, among others [57–66]. The distribution of chemotherapeutic drugs using this method relies on a concentration gradient and permeability of the agent into the tumor tissue and surrounding brain parenchyma.
Figure 1. Ommaya reservoir with a catheter placed in the intratumoral cavity following surgical resection, which is an example of local drug delivery.
Chemotherapeutic drugs can be placed in the reservoir and the drug will diffuse through the catheter into the surrounding brain parenchyma based on a concentration gradient.
Table 1.
Advantages and disadvantages of different local therapeutic delivery methods.
| Delivery methods | Advantages | Disadvantages |
|---|---|---|
| Direct injection |
|
|
| Convection-enhanced delivery |
|
|
| Drug-impregnated polymers |
|
|
| Drug-impregnated microchips |
|
|
| Local gene therapy |
|
|
The potential advantage of this approach is that it is simplistic and can be easily repeated (Table 1). A large volume of chemotherapeutics can be delivered with minimal systemic toxicity, and the reservoir can also be refilled for continued delivery of chemotherapeutics [67]. However, there are several limitations to the direct injection approach (Table 1). Direct injection into the ventricle and/or brain parenchyma requires repeated surgical procedures, which is associated with increased risk of intracranial hemorrhage, infection and malpositioned catheter, among others [58,62,68]. More importantly, this method is known to have poor drug distribution into the tumor tissue and the surrounding brain parenchyma [59,69]. Because it relies on a concentration gradient, the depth of distribution is often limited to approximately 3–5 mm, with an exponential decay in concentration from the injection site [59,69]. Thus, there is a high and often toxic concentration of drugs around the injection site and little drug presence in the surrounding areas [59]. Finally, this method is based on a bolus-based approach, making it difficult to predict drug concentration and distribution [59].
This method has been used to deliver intermittent bolus injections of both chemotherapeutic[67,70–74] and biological agents (Table 2) [57–66]. There are anecdotal case reports that have shown successful outcomes with this method, but to date there have been no successful large-scale clinical trials proving their efficacy [75–77]. Gasper et al. in 1999 placed permanent catheters containing 125I seeds into 59 patients with recurrent malignant astrocytomas (37 GBM, 22 anaplastic gliomas) from 1989 to 1997, which allowed a radiation dose of 0.05 Gy/h to the periphery of the contrast-enhancing tumor [58]. The median survival for the patients in this series was 1.34 years (0.9 years for GBM, 2.04 years for anaplastic gliomas), and 40% of patients required more surgery for tumor progression, 5% had skull infections and 13% had radiation necrosis [58]. Similarly, Riva and colleagues performed a Phase I study where they directly injected 131I radio-conjugated antibodies against the GBM-stromal antigen, tenascin, into patients with malignant gliomas, and found only a 17.8% response rate for bulky tumors but a 66% response for small tumors [57]. Torres et al. in 2008 performed a Phase I study, whereby they placed intracavitary catheters attached to Ommaya reservoirs into 9 patients with recurrent malignant astrocytomas (8 GBM, 1 AA) loaded with different concentrations of the 188Re-labeled humanized monoclonal antibody nimouzumab against the epidermal growth factor receptor [59]. They found that 85% of the antibody was retained in the surgical cavity after injection, and no survival analyses were conducted [59]. Other studies have evaluated the efficacy of direct injection of viral agents [62] and autologous lymphocytes with monoclonal antibodies [64,65,78]. In 2003, Prados et al. evaluated the efficacy of direct injection of herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration on 30 patients and found adverse effects in 16 patients [62]. Moreover, these studies using biological agents found that gene expression in the injected tissue was only present at distances of only a few millimeters from the resection cavity [69,79]. While local delivery initially seemed to have promising results, its use in clinical trials has dramatically decreased.
Table 2.
Summary of completed clinical trials for malignant gliomas using direct injection via intratumoral cavity catheters attached to subcutaneous reservoirs.
| Study (year) | Clinical Phase | Histology | Patients (n) | Chemotherapeutic agent | In combination with | Response/survival results | Ref. |
|---|---|---|---|---|---|---|---|
| Nakagawa (1995) | I | MG | Nine | Lymphocytes | RT | Two complete responses Three partial responses Four no change One progression |
[78] |
| Boiardi (1996) | I/II | rMG | 12 | Mitoxantrone | Procarbazine, vincristine, CCNU | Two responses Three no change Three progression |
[80] |
| Bigner (1998) | I | rGBM | 34 | 131I-Mab (antitenascin) | RT | Median survival – 56 weeks | [66] |
| Boiardi (1999) | I | GBM | 10 | Bleomycin, mitoxantrone | Carmustine, cisplatin | Median survival – 23.1 months | [74] |
| Gaspar (1999) | I | rMG | 59 | 125I | RT | Median survival – 1.3 year | [58] |
| Quattrocchi (1999) | I | rMG | Six | IL-2 + lymphocytes | – | Three partial responses Two no change One progression |
[65] |
| Riva (1999) | I/II | MG, rMG | 111 | 131I-Mab (antitenascin) | RT | 24 complete response | [57] |
| Nine partial response | |||||||
| Ten no change | |||||||
| Boiardi (2001) | II | rMG | 99 | Mitoxantrone | – | Median survival – 26–27 months | [73] |
| Jung (2001) | I | MG | 11 | anti-EGFR Mab + lymphocytes | – | Two positive responses Five no response |
[64] |
| Paganelli (2001) | I | rMG | 24 | 90Y-biotin Mab (antitenascin) | RT | 25% positive response 50% stabilization 25% progression |
[61] |
| Patchell (2002) | I | rGBM | 90 | Bleomycin | – | Median survival – 6–8 months | [67] |
| Voulgaris (2002) | I | rMG | 10 | Doxorubicin | – | One complete response Four partial responses One no change |
[72] |
| Boiardi (2003) | I | rMG | 58 | Mitoxantrone, doxorubicin | RT | Median survival – 11–13 months | [68] |
| Goetz (2003) | I | MG | 37 | 131I, 90Y | RT | Median survival – 17 months | [63] |
| Prados (2003) | II | rGBM | 30 | HSV-TK + ganciclovir | – | Median survival – 8.4 months | [62] |
| Bartolomei (2004) | II | rGBM, GBM | 73 | 90Y-biotin (antitenascin) | RT + TMZ | 75% Stabilization 25% Progression |
[60] |
| Ferroli (2006) | I | rMG | 22 | Mitoxantrone | Mitoxantrone surgifoam | Not assessed | [70] |
| Oshiro (2006) | I | MG | Seven | TNF-a | RT + raniumstine | Four partial responses One no change Two progressions |
[76] |
| Torres (2008) | I | rMG | Nine | 188Re-Mab (anti- EGFR) | RT | Not assessed | [59] |
| Boiardi (2008) | II | rGBM | 276 | Mitoxantrone | RT + TMZ | Median survival – 11 months | [71] |
+: Increased survival; -: Decreased survival; EGFR: Epidermal growth factor receptor; GBM: Glioblastoma; HSV TK: Herpes simplex virus thymidine kinase; Mab: Monoclonal antibody; MG: Malignant glioma; ND: No difference; r: Recurrent; RT: Radiation therapy; TMZ: Temozolomide; TNF-a: Tumor necrosis factor – α.
Convection-enhanced delivery
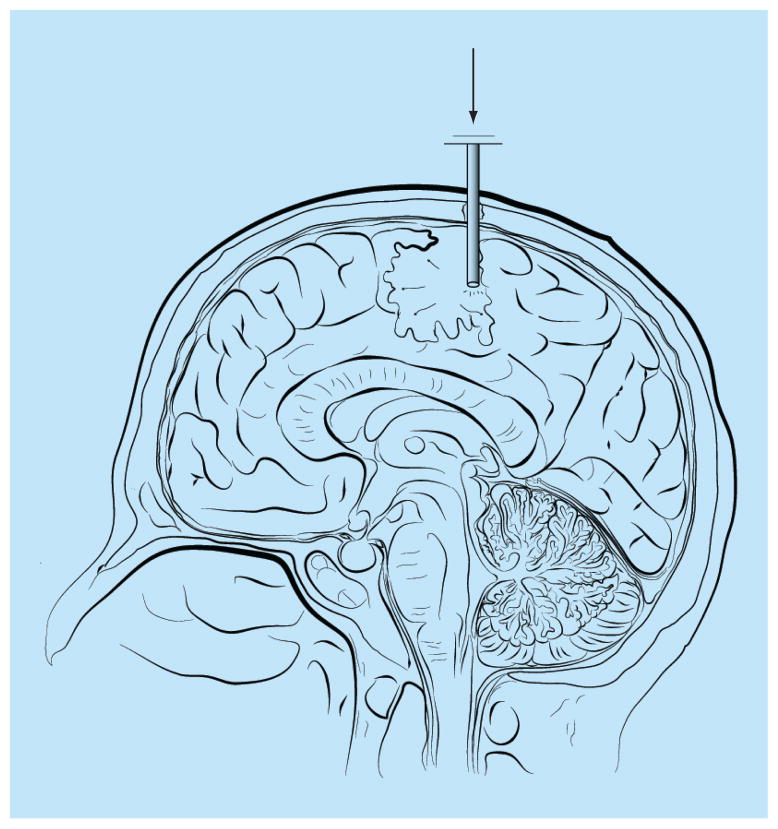
CED uses an external source to create a pressure gradient to facilitate local drug distribution [67]. It was designed to attempt to overcome the inadequacy of the limited chemotherapeutic distribution associated with the direct injection method (Figure 2 & Table 1). Similar to the direct injection method, CED relies on a concentration gradient for diffusion [67], but also incorporates a pressure gradient to increase chemotherapeutic distribution by displacement of extracellular fluid with infused fluid. In CED, a microcatheter is inserted into the tumor cavity or tumor border, and the catheter is connected to a motor-driven pumping device. This device creates a pressure gradient from the motor source by infusing chemotherapeutics at an infusion rate that typically ranges from 0.5 to 10 μl/min. CED typically distributes chemotherapeutics in an elliptical to spherical distribution up to 3 cm from the catheter source, where there is a linear relationship between the infused volume and the volume of distribution [81,82]. Therefore, with CED, the chemotherapeutic distribution relies on the concentration, rate and duration of infusion.
Figure 2. Convection-enhanced delivery with a catheter placed in the intratumoral cavity following surgical resection.
Chemotherapeutic drugs can be placed in the reservoir and the drug will move through the catheter into the surrounding brain parenchyma based on a pressure and, to a lesser extent, a concentration gradient.
The advantage of CED is that it has a wider distribution of chemotherapeutics than the direct injection method. In experimental models, radio-labeled albumin was injected into brain tissue via direct injection and CED (Table 1). Direct injection had a distribution distance of 2 mm from the catheter site, while CED had a 1.5 cm or almost eightfold increase in the distribution of albumin [81,82]. A disadvantage of this modality is that the reservoir, as with direct injection, needs to be continually refilled, which is especially critical with CED because distribution varies with the injected volume (Table 1). Additionally, the chemotherapeutic distribution varies not only with the chemotherapeutic agent, but other features of the delivery device. The factors that affect distribution of the chemotherapeutic agent include molecular size, surface characteristics and half-life, where larger size, increased binding to extracellular matrix components or surface receptors and shorter half-life are all independently associated with decreased distribution [10]. In addition, the CED device can also affect chemotherapeutic distribution, namely the infusion characteristics and the catheter dimensions. Lower infusion rate, decreased infused volume and larger bore catheter (as a result of increased back flow) are all independently associated with decreased distribution [10]. Backflow of infusate in the catheter is not trivial because backflow can cause the chemotherapeutic agent to egress along the catheter track, enter the subarachnoid space and widely distribute in the CNS, which not only decreases the ability to predict chemotherapeutic distribution but can lead to widespread neurotoxicity [83]. This leakage is inevitable and some studies have showed this leakage rate can be as high as 18.5% [84–86].
CED has been used in clinical studies to deliver both chemotherapeutic[85,87–89] and biological agents, namely immunotoxins (Table 3) [10,83,90–95]. Patel and colleagues performed a Phase I/II trial with 131I-labeled monoclonal antibody on 51 patients with newly diagnosed and recurrent malignant gliomas [91]. Significant cerebral edema occurred in 16%, hemiparesis in 14% and headaches in 14% [91]. In 11 patients with an evaluable radiographic response, 1 had a partial response, 6 had stable disease and 4 had disease progression [91]. Bruce et al. in 2011 performed a Phase I trial evaluating the safety profile of CED of topotecan in 16 patients with recurrent malignant gliomas [87]. Early response was seen in 4 (25%), pseudoprogression in 2 (44%) and progressive disease in 5 (31%), in addition to dose limiting toxicities in 2 (13%) [87]. Bogdahn and colleagues performed a Phase II trial with CED of a TGFB-2 inhibitor (trabedersen), whereby they randomized patients with recurrent malignant gliomas to low dose trabedersen, high dose trabedersen or standard chemotherapy consisting of temozolomide or procarbazine, lomustine, vincristine [92]. Despite some potentially promising findings with CED of trabedersen for patients with recurrent AA, there were no significant differences in overall and progression free survival for patients with recurrent GBM [92]. More recently, in a multi-institutional, Phase III trial (PRECISE study), 256 patients were randomized to either CED with Cintredekin besudotox (IL-13 pseudotoxin) or carmustine wafers for recurrent GBM [93]. There were no significant differences in survival between the groups, but the incidence of pulmonary embolism was higher in the CED group [93]. Thus, while CED offers promise, there has yet to be a clinical trial showing its superiority over current treatment methods.
Table 3.
Summary of completed clinical trials using convection-enhanced delivery for patients with malignant gliomas.
| Study (year) | Clinical Phase | Histology | Patients (n) | Chemotherapeutic agent | In combination with | Response/survival results | Ref. |
|---|---|---|---|---|---|---|---|
| Sampson (2003) | I | rMG | 20 | TGF-a/pseudotoxin | – | Median survival – 23 week | [95] |
| Vogues (2003) | I/II | rGBM | Eight | HSV1-tk | Ganciclovir | Median survival – 28 week Partial response – two Focal response – six |
[10] |
| Idar (2004) | I/II | rMG | 15 | Paclitaxel | – | Complete response – five Partial response – six Progression – four |
[89] |
| Boiardi (2005) | I | rMG | 12 | Mitoxantrone | – | Median survival – 11 months | [94] |
| Patel (2005) | I | MG, rMG | 51 | 131I-Mab | – | Median survival – 37.9 week Partial response – one Stable – six Progression – four |
[91] |
| Kunwar (2007) | I | MG | 51 | IL-13/pseudotoxin | – | Median survival – 42.7 week | [83] |
| Tanner (2007) | I/II | rGBM | Eight | Paclitaxel | – | Not assessed | [85] |
| Carpentier (2010) | II | rGBM | 34 | Oligonucleotides | – | Median survival – 28 week Partial response – one Minor response – three |
[90] |
| Kunwar (2010) | III | rGBM | 183 | IL-13/pseudotoxin | – | Median survival – 9.1 months | [93] |
| Boghdan (2011) | II | rMG | 135 | TGFB-2 inhibitor | – | Median survival – 35.2–39.1 months | [92] |
| Bruce (2011) | I | rMG | 16 | Topotecan | – | Early response – 25% progression – 31% Pseudoprogression – 44% |
[87] |
GBM: Glioblastoma; Mab: Monoclonal antibody; MG: Malignant glioma; r: Recurrent; RT: Radiation therapy; TGF: Transforming growth factor; TMZ: Temozolomide.
Implantable drug-impregnated polymers
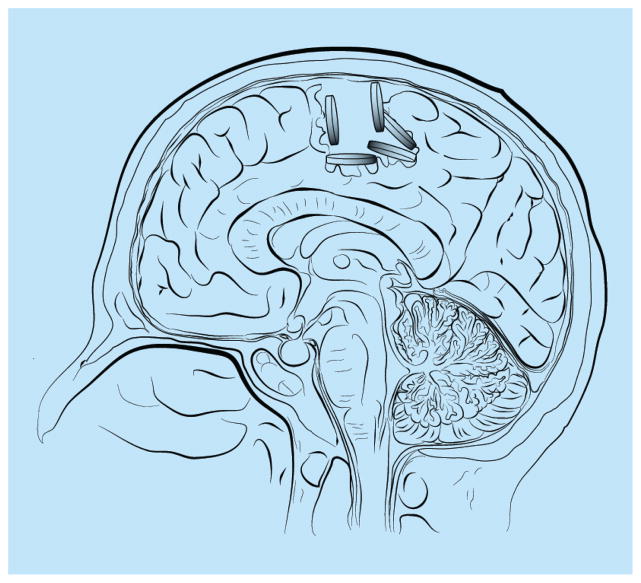
Implantable drug-impregnated polymers were designed to be implanted in the tumor resection cavity and deliver chemotherapeutic drugs to the surrounding brain tissue (Figure 3 & Table 1) [42,96,97]. As the polymer degrades, it allows for sustained release of the chemotherapeutic drug at the tumor site and surrounding peripheral tissue [42,96,97]. Unlike catheter-based technologies including Ommaya reservoirs and CED, polymer technology relies on a polymer matrix being able to incorporate drug, be biocompatible and degrade in a dependable manner with the sustained release of active drug. The currently used polymer for brain tumor treatment is composed of polyanhydride poly[1,3-bis (carboxyphenoxy) propane-co-sebacic-acid], and is designed to incorporate only one type of chemotherapeutic drug, carmustine [42,96,97]. In the laboratory setting, there are other polymeric designs that have been tested in the animal setting, but have not yet been used in humans [98]. These include the fatty acid dimersebacic acid copolymer, which is another type of polyanydride that has been used to delivery hydrophilic drugs including platinum-based drugs such as carboplatin [98]; poly(lactide-co-glycolide) polymers or microspheres that are designed to carry larger molecules such a 5-fluorouracil [99] and poly(lactide-co-glycolide) nanospheres that are covalently linked to a polyethylene glycol coating to reduce immune system detection and elimination [100], among others [101,102]. Polymer technology has several advantages (Table 1). First, this technology does not rely on catheter placement as seen in local drug delivery and CED [9,96–98,103]. As a result, it is not subject to the physical restraints of catheters including location (intra vs peritumoral), backflow and clogging [9,96–98,103]. Second, polymers allow a sustained release of drug through degradation of the polymer matrix, as opposed to a bolus or volume-dependent mechanism as seen in local delivery and CED [9,96–98,103]. Additionally, polymers can be manipulated at the time of surgery, which allows them to be placed on all edges of the tumor cavity as opposed to being dependent on catheter location [9,96–98,103]. Despite these advantages, there are some intrinsic disadvantages [9,96–98,103]. Sustained polymer drug release occurs until the polymer is degraded. The half-life of carmustine is <15 min, and carmustine polymer release at tumoricidal levels can be seen for at least 21 days in animal models [104]. Moreover, the use of an adequate number of polymers (preferably eight) requires a large surgical cavity, which is therefore not always possible with needle biopsies and eloquent tumor locations [9,96–98,103]. It also cannot be placed beyond the resection cavity, which limits its distribution in peritumoral areas [9,96–98,103]. Moreover, it is also a relative contra-indication to place these wafers when the ventricle has been opened as drug can be released into the cerebrospinal fluid leading to diffuse neural toxicity [9,96–98,103].
Figure 3. Drug-impregnated polymers placed in the intratumoral cavity following surgical resection.
Chemotherapeutic drugs are impregnated into the biodegradable polymers, which, as a result of degradation, release the drugs in a sustained manner into the surrounding brain parenchyma.
In comparison to local drug delivery and CED, the use of drug-impregnated polymers is the only local drug delivery technique to improve survival in a randomized control trial and has been FDA approved for both newly diagnosed and recurrent malignant gliomas (Table 4) [97]. The human use of carmustine wafers started in 1987 in a Phase I/II clinical trial to identify the best-tolerated carmustine or BCNU dose [96]. In this study of 21 patients, carmustine doses of 1.9, 3.8 and 6.4% per weight were given to 5, 5 and 11 patients with recurrent malignant gliomas, respectively [96]. There were no significant side effects in any of the dosing groups, and the median survival was 65, 64 and 32 week in the 1.9, 3.8 and 6.4% groups, respectively [96]. Based on these findings, a carmustine dose of 3.8% was chosen for a Phase III study, and it is unknown why the 6.4% group had lesser survival [96]. In the Phase III study, 222 patients from 27 institutions were randomized to receive carmustine wafers impregnated with either 3.8% carmustine (n = 110) or no carmustine (n = 112) [97]. The median survival for patients who received carmustine wafers was 31 weeks as compared with 23 weeks for the placebo group [97]. In addition to this survival benefit, there were no significant side effects attributable to carmustine wafers [97]. Following this study, carmustine wafers were FDA approved for recurrent malignant gliomas [46].
Table 4.
Summary of completed clinical trials using drug-impregnated polymers for patients with malignant gliomas.
| Study (Year) | Clinical Phase | Histology | Patients (n) | In combination with | Response/survival results | Ref. |
|---|---|---|---|---|---|---|
| Brem (1991) | I/II | rMG | 21 | – | Median survival – 46 week | [96] |
| Brem (1995) | III | rMG | 110† | – | Median survival – 31 vs 23 week | [97] |
| Brem (1995) | I | MG | 22 | RT | Median survival – 42 week | [42] |
| Valtonen (1997) | III | MG | 16 | RT | Median survival – 58.1 week | [43] |
| Guruangan (2001) | I | rMG | 10 | RT + TMZ | Not assessed | [114] |
| Olivi (2003) | I | rMG | 44 | – | Median survival – 35.9 week | [103] |
| Westphal (2003) | III | MG | 120 | RT | Median survival – 13.9 months | [44,45] |
| Pan (2008) | Retro | GBM | 21 | RT + TMZ | Median survival – 17 months | [111] |
| Affronti (2009) | Retro | GBM | 36 | RT + TMZ | Median survival – 72.7 week | [123] |
| McGirt (2009) | Retro | GBM | 333 | RT + TMZ | Median survival – 20.7 months | [35] |
| Quinn (2009) | II | rGBM | 52 | O6-BG | Median survival – 50.3 week | [117] |
| Menei (2010) | Retro | MG/rMG | 163 | RT | MG median survival – 17 months rMG median survival – 7 months |
[105] |
| Chaichana (2011) | Retro | GBM | 45 | – | Median survival – 5.9 months | [32] |
| Salvati (2011) | Retro | MG | 32 | RT + TMZ | Not assessed | [112] |
| Lechapt-Zalcman (2012) | Prosp | GBM | 111 | RT + TMZ | Median survival – 17.5 months | [113] |
| McPherson (2012) | I | GBM | 18 | 125I + RT + TMZ | Not assessed | [116] |
| Miglierini (2012) | Retro | GBM | 24 | RT + TMZ | Median survival – 19.2 months | [110] |
| Noel (2012) | Retro | MG | 28 | RT + TMZ | Median survival – 20.8 months | [109] |
| Salmaggi (2013) | I/II | GBM | 35 | RT + TMZ | Median survival – 17.8 months | [115] |
Study also included 112 patients who did not receive carmustine wafers.
GBM: Glioblastoma; MG: Malignant glioma; O6-BG: O6-benzylguanine; Prosp: Prospective; r: Recurrent; Retro: Retrospective; RT: Radiation therapy; TMZ: Temozolomide.
Carmustine wafers were also studied for patients with newly diagnosed malignant gliomas. In a Phase I study in 1995, the use of carmustine wafers followed by radiation therapy was considered safe, where 22 patients with newly diagnosed malignant gliomas underwent carmustine wafer placement, followed by standard external beam radiation therapy [42]. There was no increase in side effects compared with historical controls [42]. Valtonen et al. then performed a randomized control trial whereby 100 patients with newly diagnosed malignant gliomas were randomized to receive either carmustine wafers or placebo [43]. Because they were unable to obtain enough of the drug, the study was stopped prematurely at 32 patients (16 per group) [43]. Nonetheless, the median survival for the treatment group was significantly longer than the placebo group (58.1 vs 39.9 weeks) [43]. This led to a larger, Phase III clinical trial where 240 patients were randomized to receive either carmustine wafers or placebo for a newly diagnosed malignant glioma [44,45]. The median survival was significantly longer in the treatment group as compared with the placebo controls (13.9 vs 11.6 months) [44,45]. This led to the approval of carmustine wafers for both recurrent and newly diagnosed malignant gliomas. Furthermore, this survival advantage was validated in retrospective, multi-institutional French and Japanese studies for both newly diagnosed and recurrent malignant gliomas [105–107].
The utility of carmustine wafers has also been evaluated in specialized settings [35,108,109]. In light of current typical adjuvant therapy (radiation and temozolomide chemotherapy), patients who received carmustine wafers in addition to typical adjuvant therapy had improved survival than patients only receiving standard adjuvant therapy (21.3 vs 12.4 months) [35,108]. Noel et al., however, in a smaller retrospective study with no internal controls, did not find a significant survival advantage in 28 patients who received triple therapy (carmustine, temozolomide, radiation) as compared with patients who underwent only typical adjuvant therapy (temozolomide, radiation) [109]. More importantly, the use of wafers was not associated with increased complications in this setting [35,108,110–113], and has been validated in Phase I/II studies for administration with temozolomide and radiation therapy for both newly diagnosed and recurrent malignant gliomas [114,115]. Additionally, carmustine wafers have been tried with radiation iodine seeds and O6-benzyguanine chemotherapy, without a significant increased risk of complications [116,117]. In a matched-pair analysis, the use of carmustine wafers is also effective in prolonging survival for older (age >65 years) patients with GBM (8.7 vs 5.5 months) [32]. Despite these studies, there is a concern that the use of carmustine wafers can lead to increased infection, cerebral edema and seizures, but large-scale studies do not confirm these findings [97]. Additionally, while 3.8% is the standard carmustine concentration, a dose-escalation clinical study showed that the maximum tolerated dose was 20% carmustine by weight (approximately five times the standard dose) without an increase in side effects [103]. At 28% carmustine concentration, three of four patients had severe brain edema and seizures [103]. Carmustine wafers are also being investigated for patients with anaplastic ependymomas and metastatic brain tumors [108]. Despite these promising results, newer technologies are being designed to overcome some of the limitations of drug-impregnated polymers including microchip drug delivery and local therapy.
Drug-impregnated microchip delivery
As seen with carmustine wafers, the release of chemo-therapeutics drugs is dependent on the degradation of the polymer [42,96,97]. This release is sustained and therefore is not pulsatile and cannot be controlled in a time-dependent fashion [42,96,97]. Drug-impregnated micro-chips can overcome some of the limitations of polymer technology (Table 1) [12,13,118,119]. Microchip technology for local chemotherapeutic delivery has existed since the 1990s [119]. Microchips are composed of pumps, valves and channels at the micrometer scale and are controlled by time-dependent biodegradation [118] or electrochemical dissolution [119]. Moreover, they can be remotely controlled [12] and can release single or multiple agents [118]. These chips have only been used in human patients to control release of parathyroid hormones, where eight females with postmenopausal osteoporosis had these chips implanted without any significant side effects and all had increased bone formation [12]. Scott et al. demonstrated that these micro-chips could also be used in a rodent gliosarcoma model to release temozolomide [13]. Additionally, they found that the temozolomide flow rates from the microchips were predictable and led to prolonged survival in these rodents as compared with animals that underwent oral temozolomide therapy [13]. As a result, microchip technology may be advantageous over drug-impregnated polymers as a mode of local therapy for patients with malignant gliomas. The potential disadvantages of this approach are the need for refilling these devices, possible electronic malfunction and conceivable alterations in magnetic fields. Clinical trials have yet to be done in patients with malignant gliomas.
Local gene therapy
Gene therapy involves the transfer of genetic material (i.e., DNA) to cells within the body [8,120]. This genetic material can be transferred to either somatic cells or germs cells, but only somatic cells have been approved for human therapy [8,120]. This is because somatic cells have an intrinsically lower risk because they are unable to transfer this genetic material to the next cell generation [8,120]. This genetic material is transferred to glioma cells by either biological (i.e., viral) or synthetic (i.e., nanoparticles) vectors [8,120,121]. The primary biological vector are viruses [8,120]. Viral vectors, including adenoviruses, retroviruses and herpes simplex viruses, are engineered to be nonreplicating and function by transferring genetic material with the ability to induce intracellular toxicity to tumor cells or destroy tumor cells while replicating [8,120]. Synthetic vectors, including nanoparticles and liposomes, carry and transfer genetic material to tumor cells that induce cell toxicity [120]. Regardless of the type of vector, they have relied on direct injection methods [8,120]. Direct injection involves either directly injecting the genetic material into the tumor cavity or ventricular system, while systemic injections involve systemic intravenous or intra-arterial injections [122]. As with chemotherapeutics, systemic injections still have difficulty in bypassing the BBB despite their smaller molecular size and, thus, direct injection has been the preferred injection method in the clinical setting [122].
The primary advantage of gene therapy is that it is more selective in tumor cell activity by targeting specific cell receptors and/or cellular mechanisms unique to tumor cells [8,120]. However, this theoretical advantage of local gene therapy has yet to be seen in clinical trials [8,120]. This has been attributable to several reasons. First, there has been an inability to have high gene expression among tumor cells [8,120]. This is believed to be due to the fact that the genetic material can only diffuse a few millimeters from the injection site, and many tumor cells can be several centimeters away [8,120]. Another reason for this lack of promising results is that the genetic transduction frequency is low [8,120]. This may also be due to the poor diffusion as well as the inability to target tumor cells efficiently [8,120]. There are currently clinical studies aimed at addressing these limitations including using CED and/or polymer based methods [8,120].
Future perspective
Local delivery appears to be a mainstay treatment option for patients with malignant gliomas. However, several barriers still remain. First, there needs to be a better understanding of the diffusion of macromolecules, namely chemotherapeutics, within the brain parenchyma. This is made difficult by the fact that the brain parenchyma is heterogeneous as a result of tumor invasion, gliosis associated with prior treatments including surgery and radiation therapy and location to neural structures including the subarachnoid space and ventricles [4–6]. There is also a lack of imaging techniques to track chemotherapeutic molecules in vivo, making it difficult to quantify the distribution and calculate treatment efficacy [4–6]. Second, the preferred method would have to incorporate several different strategies of local therapeutic delivery. The best method would have to entail controlled release of chemotherapeutic drugs, delivery that is enhanced by an exogenous force to facilitate wider diffusion, and be able to be tracked in vivo [4–6]. Furthermore, an ideal method would need to specifically target tumor cells and avoid collateral damage to nontumor cells [4–6]. Damage to nontumor cells can lead to memory impairment, functional decline and potentially poor quality of life [4–6]. As a result, there is an increased impetus to develop targeted tumor therapy based on cell surface markers, proliferation status and even stem cell based therapy, among others [4–6]. Moreover, further studies are needed to compare the rate of drug transfer in biological versus synthetic vectors.
Conclusion
Patients with malignant gliomas have tumors with individual tumor cells possessing the capability to migrate long distances within the brain. This ability to migrate from the tumor bulk explains the ineffectiveness of focal based therapies including surgical resection and radiation therapy. Moreover, the blood–brain barrier hinders the effectiveness of systemic therapies. The delivery of local therapeutics aims to overcome these limitations and is constantly evolving. Convection enhanced delivery and drug-impregnated polymers evolved from direct injection methods. Furthermore, new methods such as micro-chips and gene delivery are incorporating these delivery strategies in order to improve the outcomes for patients with malignant gliomas. While primarily still in the early clinical phases, these new technologies have the chance to improve the outcomes for patients who harbor these debilitating tumors, but should also be compared with emerging technologies in disrupting the BBB including focal ultrasound and biochemical systemic therapies.
Executive summary.
Clinical outcomes & current therapies for patients with malignant gliomas
Current management of malignant gliomas includes extensive surgical resection, radiation therapy and chemotherapy.
Blood–brain barrier
The use of local therapy aims to overcome the drawbacks of systemic medial therapies by bypassing the blood–brain barrier, achieving high drug levels at the tumor site and limit systemic side effects.
Direct injection
Direct injection involves injection of chemotherapeutics directly into the surgical cavity, and is impeded by poor drug distribution, requires refilling and is dependent on catheter placement.
Convection-enhanced delivery
Convection-enhanced delivery utilizes a pressure gradient to drive diffusion of chemotherapeutics into the brain parenchyma and has better distribution than direct injection, but is impeded by need for refilling, subject to reflux and sometimes associated with CNS toxicity.
Drug-impregnated polymers
Drug-impregnated polymers are placed in the surgical cavity, undergo sustained degradation and release of chemotherapeutics, but are impeded by lack of refill ability, require large surgical cavities, limited distribution.
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Dubrow R, Darefsky AS. Demographic variation in incidence of adult glioma by subtype, United States, 1992–2007. BMC Cancer. 2011;11:325. doi: 10.1186/1471-2407-11-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg. 2007;21(5):496–500. doi: 10.1080/02688690701449251. [DOI] [PubMed] [Google Scholar]
- 3.Chaichana K, Zamora-Berridi G, Camara-Quintana J, Quinones-Hinojosa A. Neurosphere assays: growth factors and hormone differences in tumor and nontumor studies. Stem Cells. 2006;24(12):2851–2857. doi: 10.1634/stemcells.2006-0399. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL, Capilla-Gonzalez V, Gonzalez-Perez O, et al. Preservation of glial cytoarchitecture from ex vivo human tumor and non-tumor cerebral cortical explants: a human model to study neurological diseases. J Neurosci Methods. 2007;164(2):261–270. doi: 10.1016/j.jneumeth.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaichana KL, Guerrero-Cazares H, Capilla-Gonzalez V, et al. Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J Neurosci Methods. 2009;180(1):116–125. doi: 10.1016/j.jneumeth.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205(2):313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 7.De Bock M, Vandenbroucke RE, Decrock E, Culot M, Cecchelli R, Leybaert L. A new angle on blood-CNS interfaces: a role for connexins? FEBS Lett. 2014;588(8):1259–1270. doi: 10.1016/j.febslet.2014.02.060. [DOI] [PubMed] [Google Scholar]
- 8.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3(6):499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 9•.Raza SM, Pradilla G, Legnani FG, et al. Local delivery of antineoplastic agents by controlled-release polymers for the treatment of malignant brain tumours. Expert Opin Biol Ther. 2005;5(4):477–494. doi: 10.1517/14712598.5.4.477. Details the history of controlled-release polymers for the treatment of malignant astrocytomas. [DOI] [PubMed] [Google Scholar]
- 10.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54(4):479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 11.Walter KA, Tamargo RJ, Olivi A, Burger PC, Brem H. Intratumoral chemotherapy. Neurosurgery. 1995;37(6):1128–1145. [PubMed] [Google Scholar]
- 12.Farra R, Sheppard NF, Jr, McCabe L, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4(122):122ra21. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 13.Scott AW, Tyler BM, Masi BC, et al. Intracranial microcapsule drug delivery device for the treatment of an experimental gliosarcoma model. Biomaterials. 2011;32(10):2532–2539. doi: 10.1016/j.biomaterials.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 14•.Chaichana K, Parker S, Olivi A, Quinones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. 2010;112(5):997–1004. doi: 10.3171/2009.9.JNS09805. Assigns preoperative risk of survival for patients with glioblastoma. [DOI] [PubMed] [Google Scholar]
- 15.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82(1–2):e257–e265. doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Chaichana KL, Chaichana KK, Olivi A, et al. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J Neurosurg. 2011;114(3):587–594. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18(1):239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89(2):219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 19.Chaichana KL, Parker SL, Mukherjee D, Cheng JS, Gokaslan ZL, McGirt MJ. Assessment of the extent of surgical resection as a predictor of survival in patients with primary osseous spinal neoplasms. Clin Neurosurg. 2011;58:117–121. doi: 10.1227/neu.0b013e318226fff7. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 21.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6(3):227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 23•.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. Study identifies the role of extent of resection with survival for patients with malignant gliomas. [DOI] [PubMed] [Google Scholar]
- 24•.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463–469. doi: 10.1227/01.NEU.0000349763.42238.E9. discussion 469–470. Identifies the importance of surgical resection while avoiding iatrogenic deficits. [DOI] [PubMed] [Google Scholar]
- 25.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 26.Chaichana KL, Zadnik P, Weingart JD, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013;118(4):812–820. doi: 10.3171/2012.9.JNS1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. doi: 10.1093/neuonc/not137. Established minimum percent resection thresholds and maximum residual volume thresholds associated with survival for patients with glioblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaichana KL, Kosztowski T, Niranjan A, et al. Prognostic significance of contrast-enhancing anaplastic astrocytomas in adults. J Neurosurg. 2010;113(2):286–292. doi: 10.3171/2010.2.JNS091010. [DOI] [PubMed] [Google Scholar]
- 29.Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther. 2006;6(7):1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- 30••.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. The first randomized-control trial to establish the efficacy of drug-impregnanted polymers for recurrent glioblastoma. [DOI] [PubMed] [Google Scholar]
- 31.Chaichana KL, Halthore AN, Parker SL, et al. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114(3):604–612. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaichana KL, Zaidi H, Pendleton C, et al. The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res. 2011;33(7):759–764. doi: 10.1179/1743132811Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint radiation therapy oncology group and eastern cooperative oncology group study. Cancer. 1983;52(6):997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Chang SM, Parney IF, McDermott M, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the glioma outcome project. J Neurosurg. 2003;98(6):1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 35.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 37.Chaichana KL, Pendleton C, Chambless L, et al. Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. J Clin Neurosci. 2013;20(10):1422–1426. doi: 10.1016/j.jocn.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 39.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 41.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 42.Brem H, Ewend MG, Piantadosi S, Greenhoot J, Burger PC, Sisti M. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neurooncol. 1995;26(2):111–123. doi: 10.1007/BF01060217. [DOI] [PubMed] [Google Scholar]
- 43.Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41(1):44–48. doi: 10.1097/00006123-199707000-00011. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 44••.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. Established the importance of drug-impregnated polymers for primary glioblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. discussion 275. [DOI] [PubMed] [Google Scholar]
- 46.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3(5):721–728. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 47.Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA. 1928;90(11):823–825. [Google Scholar]
- 48.Metcalfe SE, Grant R. Biopsy versus resection for malignant glioma. Cochrane Database Syst Rev. 2001;3:CD002034. doi: 10.1002/14651858.CD002034. [DOI] [PubMed] [Google Scholar]
- 49.Andersen AP. Postoperative irradiation of glioblastomas. Results in a randomized series. Acta Radiol Oncol Radiat Phys Biol. 1978;17(6):475–484. doi: 10.3109/02841867809128178. [DOI] [PubMed] [Google Scholar]
- 50.Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64(4):769–774. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garden AS, Maor MH, Yung WK, et al. Outcome and patterns of failure following limited-volume irradiation for malignant astrocytomas. Radiother Oncol. 1991;20(2):99–110. doi: 10.1016/0167-8140(91)90143-5. [DOI] [PubMed] [Google Scholar]
- 52.Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014;16(9):1263–73. doi: 10.1093/neuonc/nou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 54.Groothuis DR, Fischer JM, Lapin G, Bigner DD, Vick NA. Permeability of different experimental brain tumor models to horseradish peroxidase. J Neuropathol Exp Neurol. 1982;41(2):164–185. doi: 10.1097/00005072-198203000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Neuwelt EA, Barnett PA, Bigner DD, Frenkel EP. Effects of adrenal cortical steroids and osmotic blood-brain barrier opening on methotrexate delivery to gliomas in the rodent: the factor of the blood-brain barrier. Proc Natl Acad Sci USA. 1982;79(14):4420–4423. doi: 10.1073/pnas.79.14.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu HL, Fan CH, Ting CY, Yeh CK. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4(4):432–444. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riva P, Franceschi G, Frattarelli M, et al. 131I radioconjugated antibodies for the locoregional radioimmunotherapy of high-grade malignant glioma–phase I and II study. Acta Oncol. 1999;38(3):351–359. doi: 10.1080/028418699431438. [DOI] [PubMed] [Google Scholar]
- 58.Gaspar LE, Zamorano LJ, Shamsa F, Fontanesi J, Ezzell GE, Yakar DA. Permanent 125iodine implants for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;43(5):977–982. doi: 10.1016/s0360-3016(98)00494-5. [DOI] [PubMed] [Google Scholar]
- 59.Torres LA, Coca MA, Batista JF, et al. Biodistribution and internal dosimetry of the 188Re-labelled humanized monoclonal antibody anti-epidemal growth factor receptor, nimotuzumab, in the locoregional treatment of malignant gliomas. Nucl Med Commun. 2008;29(1):66–75. doi: 10.1097/MNM.0b013e3282f1bbce. [DOI] [PubMed] [Google Scholar]
- 60.Bartolomei M, Mazzetta C, Handkiewicz-Junak D, et al. Combined treatment of glioblastoma patients with locoregional pre-targeted 90Y-biotin radioimmunotherapy and temozolomide. Q J Nucl Med Mol Imaging. 2004;48(3):220–228. [PubMed] [Google Scholar]
- 61.Paganelli G, Bartolomei M, Ferrari M, et al. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001;16(3):227–235. doi: 10.1089/10849780152389410. [DOI] [PubMed] [Google Scholar]
- 62.Prados MD, McDermott M, Chang SM, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J Neurooncol. 2003;65(3):269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 63.Goetz C, Riva P, Poepperl G, et al. Locoregional radioimmunotherapy in selected patients with malignant glioma: experiences, side effects and survival times. J Neurooncol. 2003;62(3):321–328. doi: 10.1023/a:1023309927635. [DOI] [PubMed] [Google Scholar]
- 64.Jung G, Brandl M, Eisner W, et al. Local immunotherapy of glioma patients with a combination of 2 bispecific antibody fragments and resting autologous lymphocytes: evidence for in situ t-cell activation and therapeutic efficacy. Int J Cancer. 2001;91(2):225–230. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1038>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]
- 65.Quattrocchi KB, Miller CH, Cush S, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45(2):141–157. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 66.Bigner DD, Brown MT, Friedman AH, et al. Iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with recurrent malignant gliomas: phase I trial results. J Clin Oncol. 1998;16(6):2202–2212. doi: 10.1200/JCO.1998.16.6.2202. [DOI] [PubMed] [Google Scholar]
- 67.Patchell RA, Regine WF, Ashton P, et al. A phase I trial of continuously infused intratumoral bleomycin for the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2002;60(1):37–42. doi: 10.1023/a:1020291229317. [DOI] [PubMed] [Google Scholar]
- 68.Boiardi A, Eoli M, Salmaggi A, et al. New approach in delivering chemotherapy: locoregional treatment for recurrent glioblastoma (rGBM) J Exp Clin Cancer Res. 2003;22(4 Suppl):123–127. [PubMed] [Google Scholar]
- 69.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21(13):2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 70.Ferroli P, Broggi M, Franzini A, et al. Surgifoam and mitoxantrone in the glioblastoma multiforme postresection cavity: the first step of locoregional chemotherapy through an ad hoc-placed catheter: technical note. Neurosurgery. 2006;59(2):E433–E434. doi: 10.1227/01.NEU.0000223499.81032.85. discussion E433–E434. [DOI] [PubMed] [Google Scholar]
- 71.Boiardi A, Silvani A, Eoli M, et al. Treatment of recurrent glioblastoma: can local delivery of mitoxantrone improve survival? J Neurooncol. 2008;88(1):105–113. doi: 10.1007/s11060-008-9540-6. [DOI] [PubMed] [Google Scholar]
- 72.Voulgaris S, Partheni M, Karamouzis M, Dimopoulos P, Papadakis N, Kalofonos HP. Intratumoral doxorubicin in patients with malignant brain gliomas. Am J Clin Oncol. 2002;25(1):60–64. doi: 10.1097/00000421-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 73.Boiardi A, Eoli M, Salmaggi A, et al. Efficacy of intratumoral delivery of mitoxantrone in recurrent malignant glial tumours. J Neurooncol. 2001;54(1):39–47. doi: 10.1023/a:1012510513780. [DOI] [PubMed] [Google Scholar]
- 74.Boiardi A, Silvani A, Pozzi A, Fariselli L, Broggi G, Salmaggi A. Interstitial chemotherapy plus systemic chemotherapy for glioblastoma patients: improved survival in sequential studies. J Neurooncol. 1999;41(2):151–157. doi: 10.1023/a:1006119505170. [DOI] [PubMed] [Google Scholar]
- 75.Boiardi A, Eoli M, Pozzi A, Salmaggi A, Broggi G, Silvani A. Locally delivered chemotherapy and repeated surgery can improve survival in glioblastoma patients. Ital J Neurol Sci. 1999;20(1):43–48. doi: 10.1007/s100720050009. [DOI] [PubMed] [Google Scholar]
- 76.Oshiro S, Tsugu H, Komatsu F, et al. Evaluation of intratumoral administration of tumor necrosis factor-alpha in patients with malignant glioma. Anticancer Res. 2006;26(6A):4027–4032. [PubMed] [Google Scholar]
- 77.Koch D, Hundsberger T, Boor S, Kaina B. Local intracerebral administration of O(6)-benzylguanine combined with systemic chemotherapy with temozolomide of a patient suffering from a recurrent glioblastoma. J Neurooncol. 2007;82(1):85–89. doi: 10.1007/s11060-006-9244-8. [DOI] [PubMed] [Google Scholar]
- 78.Nakagawa K, Kamezaki T, Shibata Y, Tsunoda T, Meguro K, Nose T. Effect of lymphokine-activated killer cells with or without radiation therapy against malignant brain tumors. Neurol Med Chir (Tokyo) 1995;35(1):22–27. doi: 10.2176/nmc.35.22. [DOI] [PubMed] [Google Scholar]
- 79.Hadaczek P, Mirek H, Berger MS, Bankiewicz K. Limited efficacy of gene transfer in herpes simplex virus-thymidine kinase/ganciclovir gene therapy for brain tumors. J Neurosurg. 2005;102(2):328–335. doi: 10.3171/jns.2005.102.2.0328. [DOI] [PubMed] [Google Scholar]
- 80.Boiardi A, Salmaggi A, Pozzi A, Broggi G, Silvani A. Interstitial chemotherapy with mitoxantrone in recurrent malignant glioma: preliminary data. J Neurooncol. 1996;27(2):157–162. doi: 10.1007/BF00177479. [DOI] [PubMed] [Google Scholar]
- 81.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82(6):1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 83.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25(7):837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 84.Sampson JH, Akabani G, Friedman AH, et al. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus. 2006;20(4):E14. doi: 10.3171/foc.2006.20.4.9. [DOI] [PubMed] [Google Scholar]
- 85.Tanner PG, Holtmannspotter M, Tonn JC, Goldbrunner R. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery. 2007;61(4):E880–E882. doi: 10.1227/01.NEU.0000298922.77921.F2. discussion E882. [DOI] [PubMed] [Google Scholar]
- 86.Varenika V, Dickinson P, Bringas J, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J Neurosurg. 2008;109(5):874–880. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruce JN, Fine RL, Canoll P, et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery. 2011;69(6):1272–1279. doi: 10.1227/NEU.0b013e3182233e24. discussion 1279–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saito R, Sonoda Y, Kumabe T, Nagamatsu K, Watanabe M, Tominaga T. Regression of recurrent glioblastoma infiltrating the brainstem after convection-enhanced delivery of nimustine hydrochloride. J Neurosurg Pediatr. 2011;7(5):522–526. doi: 10.3171/2011.2.PEDS10407. [DOI] [PubMed] [Google Scholar]
- 89.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100(3):472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 90.Carpentier A, Metellus P, Ursu R, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 2010;12(4):401–408. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel SJ, Shapiro WR, Laske DW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56(6):1243–1252. doi: 10.1227/01.neu.0000159649.71890.30. discussion 1252–1253. [DOI] [PubMed] [Google Scholar]
- 92.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boiardi A, Eoli M, Salmaggi A, et al. Local drug delivery in recurrent malignant gliomas. Neurol Sci. 2005;26(Suppl 1):S37–S39. doi: 10.1007/s10072-005-0403-z. [DOI] [PubMed] [Google Scholar]
- 95.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65(1):27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 96.Brem H, Mahaley MS, Jr, Vick NA, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74(3):441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 97.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 98.Olivi A, Ewend MG, Utsuki T, et al. Interstitial delivery of carboplatin via biodegradable polymers is effective against experimental glioma in the rat. Cancer Chemother Pharmacol. 1996;39(1–2):90–96. doi: 10.1007/s002800050542. [DOI] [PubMed] [Google Scholar]
- 99.Menei P, Capelle L, Guyotat J, et al. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: a randomized phase II trial. Neurosurgery. 2005;56(2):242–248. doi: 10.1227/01.neu.0000144982.82068.a2. discussion 242–248. [DOI] [PubMed] [Google Scholar]
- 100.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 101.Gabizon A, Isacson R, Libson E, et al. Clinical studies of liposome-encapsulated doxorubicin. Acta Oncol. 1994;33(7):779–786. doi: 10.3109/02841869409083948. [DOI] [PubMed] [Google Scholar]
- 102.Rhines LD, Sampath P, DiMeco F, et al. Local immunotherapy with interleukin-2 delivered from biodegradable polymer microspheres combined with interstitial chemotherapy: a novel treatment for experimental malignant glioma. Neurosurgery. 2003;52(4):872–879. doi: 10.1227/01.neu.0000053211.39087.d1. discussion 879–880. [DOI] [PubMed] [Google Scholar]
- 103••.Olivi A, Grossman SA, Tatter S, et al. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: a new approaches to brain tumor therapy CNS consortium trial. J Clin Oncol. 2003;21(9):1845–1849. doi: 10.1200/JCO.2003.09.041. Established that higher percentage of drug could safely be used in drug-impregnated polymers. [DOI] [PubMed] [Google Scholar]
- 104.Fung LK, Ewend MG, Sills A, et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58(4):672–684. [PubMed] [Google Scholar]
- 105.Menei P, Metellus P, Parot-Schinkel E, et al. Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol. 2010;17(7):1740–1746. doi: 10.1245/s10434-010-1081-5. [DOI] [PubMed] [Google Scholar]
- 106.Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev. 2011;3:CD007294. doi: 10.1002/14651858.CD007294.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aoki T, Nishikawa R, Sugiyama K, et al. A multicenter phase I/II study of the BCNU implant (Gliadel((R)) wafer) for Japanese patients with malignant gliomas. Neurol Med Chir (Tokyo) 2014;54(4):290–301. doi: 10.2176/nmc.oa2013-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McGirt MJ, Brem H. Carmustine wafers (Gliadel) plus concomitant temozolomide therapy after resection of malignant astrocytoma: growing evidence for safety and efficacy. Ann Surg Oncol. 2010;17(7):1729–1731. doi: 10.1245/s10434-010-1092-2. [DOI] [PubMed] [Google Scholar]
- 109.Noel G, Schott R, Froelich S, et al. Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys. 2012;82(2):749–755. doi: 10.1016/j.ijrobp.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 110.Miglierini P, Bouchekoua M, Rousseau B, Hieu PD, Malhaire JP, Pradier O. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg. 2012;114(9):1222–1225. doi: 10.1016/j.clineuro.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 111.Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol. 2008;88(3):353–357. doi: 10.1007/s11060-008-9576-7. [DOI] [PubMed] [Google Scholar]
- 112.Salvati M, D’Elia A, Frati A, Brogna C, Santoro A, Delfini R. Safety and feasibility of the adjunct of local chemotherapy with biodegradable carmustine (BCNU) wafers to the standard multimodal approach to high grade gliomas at first diagnosis. J Neurosurg Sci. 2011;55(1):1–6. [PubMed] [Google Scholar]
- 113.Lechapt-Zalcman E, Levallet G, Dugue AE, et al. O(6) -methylguanine-DNA methyltransferase (MGMT) promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide. Cancer. 2012;118(18):4545–4554. doi: 10.1002/cncr.27441. [DOI] [PubMed] [Google Scholar]
- 114.Gururangan S, Cokgor L, Rich JN, et al. Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro Oncol. 2001;3(4):246–250. doi: 10.1093/neuonc/3.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salmaggi A, Milanesi I, Silvani A, et al. Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: preliminary results. J Neurosurg. 2013;118(4):821–829. doi: 10.3171/2012.12.JNS111893. [DOI] [PubMed] [Google Scholar]
- 116.McPherson CM, Gerena-Lewis M, Breneman JC, Warnick RE. Results of phase I study of a multi-modality treatment for newly diagnosed glioblastoma multiforme using local implantation of concurrent BCNU wafers and permanent I-125 seeds followed by fractionated radiation and temozolomide chemotherapy. J Neurooncol. 2012;108(3):521–525. doi: 10.1007/s11060-012-0854-z. [DOI] [PubMed] [Google Scholar]
- 117.Quinn JA, Jiang SX, Carter J, et al. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15(3):1064–1068. doi: 10.1158/1078-0432.CCR-08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Richards Grayson AC, Choi IS, Tyler BM, et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2(11):767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 119.Santini JT, Jr, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 120.Tyler MA, Sonabend AM, Ulasov IV, Lesniak MS. Vector therapies for malignant glioma: shifting the clinical paradigm. Expert Opin Drug Deliv. 2008;5(4):445–458. doi: 10.1517/17425247.5.4.445. [DOI] [PubMed] [Google Scholar]
- 121.Tzeng SY, Guerrero-Cazares H, Martinez EE, Sunshine JC, Quinones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on poly(beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wirth T, Samaranayake H, Pikkarainen J, Maatta AM, Yla-Herttuala S. Clinical trials for glioblastoma multiforme using adenoviral vectors. Curr Opin Mol Ther. 2009;11(5):485–492. [PubMed] [Google Scholar]
- 123.Affronti ML, Heery CR, Herndon JE, 2nd, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115(15):3501–3511. doi: 10.1002/cncr.24398. [DOI] [PubMed] [Google Scholar]