Abstract
Pediatric low-grade gliomas (pLGG) account for more brain tumors in children than any other histologic subtype. While surgery, chemotherapy and radiation remain the mainstay of upfront treatment, recent advances in molecular interrogation of pLGG have shown a small number of recurring genetic mutations in these tumors that might be exploited therapeutically. Notable findings include abnormalities in the RAS/MAP kinase pathway such as NF-1 loss or BRAF activation and mTOR activation. Recent identification of activating re-arrangements in c-MYB and MYBL1 in pediatric diffuse astrocytoma also provide candidates for therapeutic intervention. Targeting these molecularly identified pathways may allow for improved outcomes for patients as pediatric oncology moves into the era of biology-driven medicine.
Background
Pediatric gliomas include multiple histologies including astrocytomas, ependymomas, and oligodendrogliomas. Pediatric low-grade gliomas (pLGG) are the most common pediatric brain tumors, and this review will focus on the emerging translational science related to these tumor types.
Pediatric astrocytic tumors are currently divided by the World Health Organization (WHO) into numerous subtypes (Table 1). pLGG include WHO grade I and II tumors with pilocytic astrocytomas predominating. Although most pLGG carry a favorable prognosis, a significant minority are more aggressive (1). Surgical resection can be curative, but this is often not feasible due to the tendency of these tumors to present in midline locations including the diencephalon and brain stem (2). Radiation therapy can be used in the adjuvant setting for recurrent or progressive tumors. Unfortunately, there can be significant long-term morbidity including impaired cognition, vasculopathy, endocrinopathies, and secondary tumors (3). Chemotherapy, which was presumed to be less efficacious in the treatment of LGG given the relatively slow growth rate of these tumors, was initially reluctantly used when surgical and radiation options had been exhausted. However, responses were seen in both the upfront and recurrence setting, generating interest in the use of chemotherapy to avoid, or delay, radiation therapy. Numerous regimens have been developed including carboplatin/vincristine (4, 5), TPCV (thioguanine/procarbazine/CCNU, vincristine; ref. 6), carboplatin alone (7), oral temozolomide (8), vinblastine (9), cisplatin/etoposide (10), and others. In general, regardless of regimen, a minority of children will have a measurable reduction in the size of the tumor, many will have stable disease, and the remainder will progress during or after completion of treatment. Progression-free survival (PFS) rate for most chemotherapeutic regimens tested is typically in the 30% to 40% range at 5 years. A recent large randomized Children’s Oncology Group trial showed that TPCV trended toward slightly better efficacy for patients with LGG than carboplatin/vincristine (CV; ref. 11).
Table 1.
WHO-defined astrocytic tumors
| Name | WHO Grade |
|---|---|
| Pilocytic astrocytoma | Grade 1 |
| SEGA | Grade 1 |
| PMA | Grade 2 |
| PXA | Grade 2 |
| Low-grade fibrillary astrocytoma | Grade 2 |
| Anaplastic astrocytoma | Grade 3 |
| Glioblastoma | Grade 4 |
| Gliomatosis cerebri | Grade 4 |
NOTE: Adapted from WHO Classification of Tumours of the Central Nervous System (IARC; WHO Classification of Tumors).
Diagnosis of a pLGG is generally based on imaging features and, when the tumor is biopsied or resected, histopathologic interpretation. Challenges abound in the classification of LGG in children with many tumors appearing to be a mix of histologic subtypes confounding classification. Molecular diagnostics have only recently begun to be incorporated into the initial evaluation of pLGG. One of the first applications of the knowledge gained from molecular profiling of tumors was the use of the mTOR inhibitor everolimus in the treatment of children with tuberous sclerosis (TSC) who frequently develop subependymal giant cell astrocytomas (SEGA). Most patients with TSC harbor a mutation in either the TSC1 (hamartin) or TSC2 (tuberin) gene; either mutation leads to overactivation of mTOR. Everolimus administration resulted in appreciable tumor reduction, and an associated decrease in the frequency of seizures is seen in these patients (12, 13).
On The Horizon
Targeting the RAS/MAP kinase pathway
Nearly all pLGGs have alterations in the RAS/MAP kinase pathway (Fig. 1; refs. 14–16). Constitutive activation of this pathway can be due to loss of neurofibromin (NF-1), a RAS GTPase-activating protein (17, 18). In non–NF-1-associated LGG, the most common alteration is a fusion and tandem duplication of BRAF with KIAA1549, a protein of unknown function (19). This fusion deletes the regulatory domain of BRAF and deletes most of KIAA1549, leaving a rump of KIAA1549 and a constitutively active BRAF (14–16). A smaller percentage of LGG harbor the BRAFV600E mutation (20) that is a hallmark of cutaneous melanoma (21). Another subset harbors fibroblast growth factor receptor (FGFR) alterations leading to constitutive activation of the mitogen-activated protein (MAP) kinase and mTOR pathways (22). Interestingly, a group of predominately diffuse low-grade astrocytomas have activating alterations of the MYB or MYBL1 transcription factors (22, 23). These tumors also have MAP kinase activation equivalent to that observed in BRAF-driven tumors, suggesting that MAP kinase signaling may be a common driver pathway in pLGG. High-level activation of the MAP kinase pathway is associated with oncogene-induced senescence in many neoplasms, and the most aggressive pLGGs have deletion or silencing of the p16INK4a locus, allowing them to bypass this antitumor mechanism (24–26).
Figure 1.
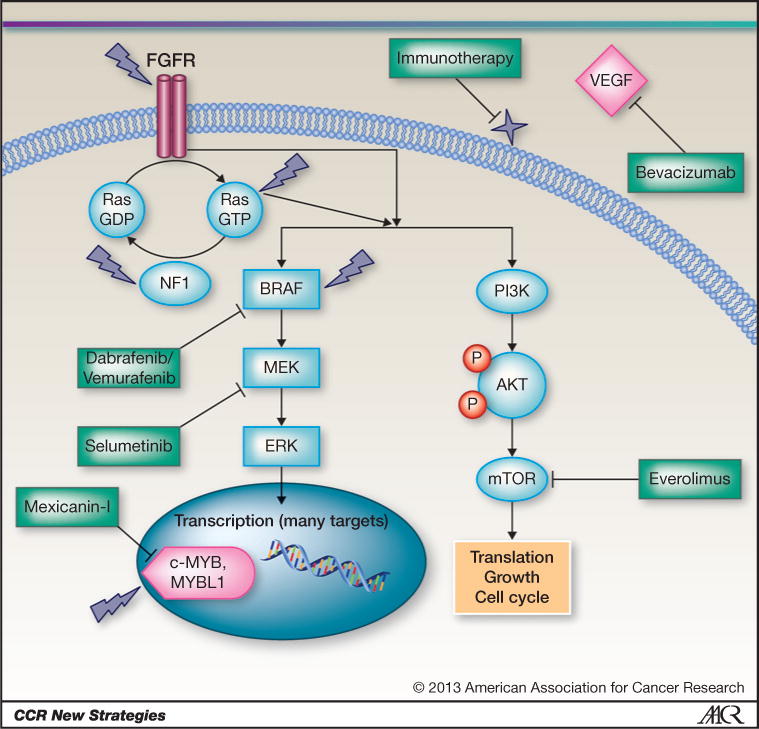
Overview of the major known mutations in pLGG, other promising targets, and potential therapeutics. Dark lightning strikes indicate genes known to be altered in LGGs, including FGF, NF-1, RAS, BRAF, and c-MYB/MYBL1. Many of the alterations in pLGG affect the MAP kinase pathway, leading to constitutive activation of MEK and ERK. Other alterations less frequently found in LGGs are RASSF1 and CRAF. Dabrafenib and vemurafenib are specific inhibitors of only the BRAFV600E alteration. These inhibitors should not be used in non–BRAFV600E-containing tumors because they can cause paradoxical activation of other forms of BRAF. MAP kinase pathway blocking alternatives include the MEK inhibitor selumetinib. The transcription factors c-MYB and MYBL1, which are rearranged in a subset of pLGG, can be targeted with the newly identified compound mexicanin-I, a sesquiterpene lactone isolated from the flowering plant Helenium mexicanum. Everolimus is a TORC1 inhibitor that has shown activity in subependymal giant cell astrocytoma and is currently being investigated in pLGGs. Many pLGGs avidly enhance on MRI after gadolinium contrast and show evidence of high VEGF expression. Immunotherapy appears to be particularly promising for pLGGs.
The presence of characteristic mutations provides a rational target for therapy. For example, the finding of BRAFV600E in a subset of pLGGs led to the hypothesis that some of the pharmaceuticals designed to inhibit this mutation in melanoma might be active against these tumors (27, 28). Dabrafenib has improved brain penetration and has shown activity against melanoma brain metastases (29, 30). A multinational pediatric phase I/II study of dabrafenib in patients with known BRAFV600E tumors is currently underway (NCT01677741). Recent studies have shown that current BRAF inhibitors are designed specifically for the BRAFV600E mutation and can cause paradoxical activation of other BRAF-activating mutations (such as the BRAF/KIAA1549 fusion protein; ref. 31). Adding further complexity, there are several different BRAF/KIAA1549 fusions, and several BRAF translocations identified that do not involve KIAA1549 (32).
The advent of improved MAP kinase pathway inhibitors (MEK inhibitors) has led to successful preclinical testing in murine models of pilocytic astrocytomas and initiation of a trial of one such inhibitor, selumetinib, in patients with LGG (NCT01089101; ref. 33). Downstream inhibition of the RAS/BRAF/MEK pathway is attractive because it is possible that this class of drug could be used regardless of the upstream mutation leading to pathway activation. However, RAS and BRAF are capable of signaling through other downstream effectors (such as the mTOR pathway), and MEK inhibitors may not target these other pathways effectively (34–36). In preclinical testing, one pilocytic astrocytoma xenograft harboring the BRAFV600E mutation responded to MEK inhibition, whereas another that did not have the mutation was resistant (33).
mTOR pathway inhibition
Several recent studies have shown that the most aggressive and refractory pLGGs have increased activation of the mTOR pathway (37, 38). The success of the rapalog everolimus in treating SEGAs associated with tuberous sclerosis proved that this drug can shrink mTOR-driven tumors (12, 13), suggesting that it might have activity in other pLGG. A trial of everolimus in refractory/recurrent pLGG has completed enrollment (NCT00782626), and a second phase II trial targeting larger numbers of patients will soon be opening (NCT01734512). A separate study of everolimus in patients with NF-1 and LGG is also open to enrollment (NCT01158651). A series of 19 children treated with rapamycin in combination with erlotinib showed prolonged stable disease in 2 children with NF-1 (39). It will be important to determine whether increased TORC1 or TORC2 expression correlates with response to mTOR inhibitors, as these rapalogs are likely to primarily inhibit TORC1 (40). New dual TORC1/TORC2 targeting agents such as TORC kinase inhibitors may also be promising drugs for treatment of aggressive LGG.
MYB and MYBL1 inhibition
In low-grade fibrillary astrocytomas (WHO grade II) and a percentage of pilocytic astrocytomas, genomic rearrangements remove the regulatory domain of c-MYB and the closely related MYBL1 transcription factors (22, 23). These rearrangements leave the transactivating domain constitutively active (22, 23). The overactivation of c-MYB is a known oncogene in leukemia (41). Recently, new inhibitors of c-MYB have been developed, suggesting that these may be eventually be deployed in MYB-rearranged pLGG (42).
Anti-angiogenic therapy
The intense expression of vascular growth factors in pilocytic astrocytoma suggests that these tumors may be dependent on neovascularization for their continued growth (43). Targeting these abnormal blood vessels with the VEGF inhibitor bevacizumab in conjunction with irinotecan led to radiologic and clinical responses in small numbers of children (44). Bevacizumab monotherapy has also been shown to lead to tumor regression and disease stability in patients with LGG (45).
Immunomodulatory therapy
The presence of characteristic mutations in pLGG that are not present in other cells in the body raises the possibility of targeting these abnormal peptides. One way that the immune system can be vectored to pLGG is with the immunomodulatory drug lenalidomide. In addition to its ability to alter the immune milieu, lenalidomide has anti-angiogenic and direct antitumor effects (46). A phase I study of lenalidomide in patients with refractory/recurrent pediatric brain tumors found that patients with LGG were most likely to show cessation of tumor progression (46). A phase II study comparing 2 dose levels of lenalidomide to determine whether there is improved response with higher doses (NCT01553149) is ongoing.
A trial is underway investigating if vaccination with glioma-associated antigens along with concurrent administration of the immunostimulant poly-ICLC (polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose) can activate the immune system to attack refractory/recurrent LGG (NCT01130077). Poly ICLC is an immunomodulatory agent that promotes infiltration of T cells into tumors (47, 48). A phase II study of poly-ICLC alone in LGG is currently underway as well (NCT01188096).
Rational for combination therapy in pLGGs
Combination chemotherapy has been adapted to pLGGs not amenable to surgical resection (49). Activity of combination chemotherapy in LGGs remains controversial because a number of single-agent therapies (see above) have shown results similar in outcome to combination therapy. However, there have not been controlled randomized trials comparing single versus multiagent therapy in pLGGs. When considering why pLGGs may be effectively treated by single-agent therapy, it is important to recall the limited malignant capacity of these tumors. Radiation therapy for most pLGGs could be considered optimal single-agent treatment based on its ability to stabilize tumor growth. The predominance of mutations along the single RAS/RAF/MEK pathway in the absence of other concurrent oncogenic lesions may account for the activity of low-dose treatment regimens in pLGGs. Two features differentiate pLGGs from most other tumors, both for adults and pediatric patients. The first is the anecdotal observation by many practitioners that patients that have been previously responsive to a particular LGG therapy can reuse the same treatment again, often with good effect. This contrasts with most treatments, where once a tumor has seen a set of agents in a treatment and then recurred or progressed, further therapy with those same agents is ineffective. The second feature of pLGGs that differentiate them from most other tumors, including adult LGGs, is the overall excellent long-term survival of these patients. Even in the context of repeated recurrences through childhood, the majority of pediatric patients will eventually have cessation of tumor growth without the need for further therapy rather than slow transformation to progressively more malignant gliomas, as is routinely observed in adults. This effect has been especially well-identified in LGGs in patients with NF-1 and can occasionally even result in spontaneous tumor regression. A similar slowing of growth and eventual growth arrest appears to occur in sporadic pLGGs as these patients enter adulthood.
While clinical trials with a host of targeted agents for pLGGs have only recently started, it is reasonable to begin to consider how combination therapies for these kinds of agents could be developed (50). For pLGGs where the mutational heterogeneity is limited, targeting multiple pathways might be less important. If pLGGs, which are largely characterized by activation of a single pathway (RAS, RAF, TSC, FGFR1, or MYB/MYBL1), then perhaps 2 inhibitors that target the same pathway would better ensure that any signal that gets through the first blockage (say a BRAFV600E inhibitor) could be eliminated with a MEK, ERK, or mTOR inhibitor further downstream. In melanoma trials of BRAFV600E-targeted inhibitors, the addition of downstream inhibition of MEK improved the activity over either drug alone (51). Early-phase trials in adults with solid tumors are investigating combination therapy with TORC1 inhibitors (everolimus or temsirolimus) and BRAFV600E inhibitors (NCT01596140). Alternatively, dual phosphoinositide 3-kinase (PI3K)/mTOR inhibitors such as BEZ235 are being combined with the MEK1/2 inhibitor MEK162 (NCT01337765). If such combinations are tolerable, the dual activation of BRAF/MEK/ERK and mTOR pathways in aggressive pLGG provides a strong rationale for moving these, or similar drugs, into clinical trials in children.
The challenges for the rare subtypes of pLGGs (e.g., PXA, PMA)
With the development of improved molecular diagnosis of pLGGs, what was once considered to be a limited number of different tumor types has developed into a continuum of tumors with shared molecular defects. Although it has been suggested that the presence of KIAA/BRAF fusions may correlate with improved event-free survival (52), another study has not associated BRAF molecular alteration with outcome or identified a mutational signature in all members of a specific tumor subtype (53). Thus, it remains to be determined whether classification needs to switch from a purely immunohistochemical one to a system that prioritizes these molecular aspects. Current chemotherapy approaches have been effective in the more common forms of pLGGs such as pilocytic astrocytomas, fibrillary astrocytomas, and astrocytoma not otherwise specified. Retrospective studies are now being conducted to assess what impact the mutational pattern in these tumors had in relation to response to therapy. Although the rare subtypes of pLGGs are less well-studied, their responses appear to approximate those mentioned above in reports where they were included in chemotherapy treatment protocols.
Deciding on how to approach rare subtypes of pLGGs such as ganglioglioma, pleomorphic xanthoastrocytoma (PXA), dysembryoplastic neuroepithelial tumor (DNT), angiocentric glioma, pilomyxoid astrocytoma (PMA), and others has become less of a diagnostic issue. Rather, as we move toward identification of the molecular pathways driving these rare subtypes, we recognize that their genomic changes show patterns of overlap between each other in some, but not all, cases. The presence of the BRAFV600E mutation for example is identified in approximately 20% of fibrillary grade II astrocytomas but not most pilocytic astrocytomas (20). It is very common in both ganglioglioma (20) and PXAs (54) and occasionally identified in pilomxyoid astrocytomas. Because all of these tumors are potential targets for BRAFV600E inhibitors, should treatment be based on the WHO classification of the tumor subtype or on the specific molecular defect? As mentioned above, a new clinical trial of a specific BRAFV600E inhibitor is now underway and combines all pLGGs with this mutation together into a single protocol. For many of the rarer subtypes of pLGGs, not all of the molecular defects have been identified. For example, while 60% of ganglioglioma and PXAs have the BRAFV600E mutation, we do not yet know what drives the remaining 40%. Similarly, for pilomxyoid astrocytomas, some have the BRAFV600E mutation, some have the KIAA-truncated fusion of BRAF and some have neither. It is likely that to optimize therapy in this group of patients, different therapies for these different molecular subgroups of this single entity may be needed.
Conclusion
This is an exciting time in the treatment of pLGGs. Our integration of the WHO classification with molecular genotypes offers us the opportunity to tailor therapy and ideally minimize toxicity in this patient population. Whether the application of these therapies will improve on the historic PFS seen with traditional chemotherapy and result in durable complete responses is unknown. Moreover, whether biology-driven targeted therapy should be integrated with, or replace, standard upfront therapies such as carboplatin-based regimens or TPCV remains to be tested. Improvements in preclinical models of pLGGs, and clinical trials with targeted therapy that will provide some of this information, are already underway.
Acknowledgments
Financial or Other Support:
This activity does not receive commercial support.
Grant Support
This study is supported by St. Baldrick’s Scholar (to E.H. Raabe), Pediatric Low-Grade Astrocytoma Foundation (to E.H. Raabe and M.W. Kieran), Andrysiak Low-Grade Glioma Scholar Award (to M.W. Kieran), and Solving Kid’s Cancer (K.J. Cohen).
Footnotes
Disclosure of Potential Conflicts of Interest
M.W. Kieran is a consultant/advisory board member of Boehringer-Ingelheim, Incyte, Merck, Novartis, and Sanofi. No potential conflicts of interest were disclosed by the other authors.
CME Staff Planners’ Disclosures
The members of the planning committee have no real or apparent conflict of interest to disclose.
Learning Objective(s)
Upon completion of this activity, the participant should have a better understanding of the molecular pathways that are active in pediatric low-grade gliomas and the biologic rationale underlying novel therapeutic strategies for children with these tumors.
Authors’ Contributions
Conception and design: E.H. Raabe, M.W. Kieran, K.J. Cohen
Development of methodology: E.H. Raabe
Writing, review, and/or revision of the manuscript: E.H. Raabe, M.W. Kieran, K.J. Cohen
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M.W. Kieran
References
- 1.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terashima K, Chow K, Jones J, Ahern C, Jo E, Ellezam B, et al. Long-term outcome of centrally located low-grade glioma in children. Cancer. 2013;119:2630–8. doi: 10.1002/cncr.28110. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–7. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–54. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Lange B, Ater J, Nicholson HS, Allen J, Walker R, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–6. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]
- 6.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–41. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 7.Gururangan S, Cavazos CM, Ashley D, Herndon JE, II, Bruggers CS, Moghrabi A, et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20:2951–8. doi: 10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, Fort D, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110:1542–50. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 9.Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358–63. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 10.Massimino M, Spreafico F, Riva D, Biassoni V, Poggi G, Solero C, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100:65–71. doi: 10.1007/s11060-010-0136-6. [DOI] [PubMed] [Google Scholar]
- 11.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2641–7. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–32. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 13.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 14.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–87. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 15.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–7. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–49. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balestri P, Calistri L, Vivarelli R, Bartalini G, Mancini L, Berardi A, et al. Central nervous system imaging in reevaluation of patients with neurofibromatosis type 1. Childs Nerv Syst. 1993;9:448–51. doi: 10.1007/BF00393546. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Gutmann DH. The molecular and cell biology of pediatric low-grade gliomas. Oncogene. 2013 Apr 29; doi: 10.1038/onc.2013.148. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Jones DT, Gronych J, Lichter P, Witt O, Pfister SM. MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci. 2012;69:1799–811. doi: 10.1007/s00018-011-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacConaill LE, Campbell CD, Kehoe SM, Bass AJ, Hatton C, Niu L, et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS One. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–4. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–12. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013;110:8188–93. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horbinski C, Nikiforova MN, Hagenkord JM, Hamilton RL, Pollack IF. Interplay among BRAF, p16, p53, and MIB1 in pediatric low-grade gliomas. Neuro Oncol. 2012;14:777–89. doi: 10.1093/neuonc/nos077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob K, Quang-Khuong DA, Jones DT, Witt H, Lambert S, Albrecht S, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17:4650–60. doi: 10.1158/1078-0432.CCR-11-0127. [DOI] [PubMed] [Google Scholar]
- 26.Raabe EH, Lim KS, Kim JM, Meeker A, Mao XG, Nikkhah G, et al. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 2011;17:3590–9. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huillard E, Hashizume R, Phillips JJ, Griveau A, Ihrie RA, Aoki Y, et al. Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc Natl Acad Sci U S A. 2012;109:8710–5. doi: 10.1073/pnas.1117255109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 29.Mittapalli RK, Vaidhyanathan S, Dudek AZ, Elmquist WF. Mechanisms limiting distribution of the threonine-protein kinase B-RaF (V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013;344:655–64. doi: 10.1124/jpet.112.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 31.Sievert AJ, Lang SS, Boucher KL, Madsen PJ, Slaunwhite E, Choudhari N, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A. 2013;110:5957–62. doi: 10.1073/pnas.1219232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horbinski C. To BRAF or not to BRAF: is that even a question anymore? J Neuropathol Exp Neurol. 2013;72:2–7. doi: 10.1097/NEN.0b013e318279f3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Neale G, Keir ST, et al. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55:668–77. doi: 10.1002/pbc.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chetram MA, Odero-Marah V, Hinton CV. Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells. Mol Cancer Res. 2011;9:90–102. doi: 10.1158/1541-7786.MCR-10-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Montalto G, Cervello M, et al. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3:954–87. doi: 10.18632/oncotarget.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirza AM, Gysin S, Malek N, Nakayama K, Roberts JM, McMahon M. Cooperative regulation of the cell division cycle by the protein kinases RAF and AKT. Mol Cell Biol. 2004;24:10868–81. doi: 10.1128/MCB.24.24.10868-10881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller S, Phillips J, Onar-Thomas A, Romero E, Zheng S, Wiencke JK, et al. PTEN promoter methylation and activation of the PI3K/Akt/mTOR pathway in pediatric gliomas and influence on clinical outcome. Neuro Oncol. 2012;14:1146–52. doi: 10.1093/neuonc/nos140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121:407–20. doi: 10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yalon M, Rood B, MacDonald TJ, McCowage G, Kane R, Constantini S, et al. A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG) Pediatr Blood Cancer. 2013;60:71–6. doi: 10.1002/pbc.24142. [DOI] [PubMed] [Google Scholar]
- 40.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 41.Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes Dev. 2011;25:1628–40. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bujnicki T, Wilczek C, Schomburg C, Feldmann F, Schlenke P, Muller-Tidow C, et al. Inhibition of Myb-dependent gene expression by the sesquiterpene lactone mexicanin-I. Leukemia. 2012;26:615–22. doi: 10.1038/leu.2011.275. [DOI] [PubMed] [Google Scholar]
- 43.Bartels U, Hawkins C, Jing M, Ho M, Dirks P, Rutka J, et al. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg. 2006;104:314–20. doi: 10.3171/ped.2006.104.5.314. [DOI] [PubMed] [Google Scholar]
- 44.Packer RJ, Jakacki R, Horn M, Rood B, Vezina G, MacDonald T, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52:791–5. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 45.Hwang EI, Jakacki RI, Fisher MJ, Kilburn LB, Horn M, Vezina G, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer. 2013;60:776–82. doi: 10.1002/pbc.24297. [DOI] [PubMed] [Google Scholar]
- 46.Warren KE, Goldman S, Pollack IF, Fangusaro J, Schaiquevich P, Stewart CF, et al. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol. 2011;29:324–9. doi: 10.1200/JCO.2010.31.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X, Fallert-Junecko BA, Fujita M, Ueda R, Kohanbash G, Kastenhuber ER, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunol Immunother. 2010;59:1401–9. doi: 10.1007/s00262-010-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker DA, Liu J, Kieran M, Jabado N, Picton S, Packer R, et al. A multidisciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013;15:462–8. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31:1592–605. doi: 10.1200/JCO.2011.37.6418. [DOI] [PubMed] [Google Scholar]
- 51.Lemech C, Infante J, Arkenau HT. Combination molecularly targeted drug therapy in metastatic melanoma: progress to date. Drugs. 2013;73:767–77. doi: 10.1007/s40265-013-0049-8. [DOI] [PubMed] [Google Scholar]
- 52.Hawkins C, Walker E, Mohamed N, Zhang C, Jacob K, Shirinian M, et al. BRAF-KIAA1549 fusion predicts better clinical outcome in pediatric low-grade astrocytoma. Clin Cancer Res. 2011;17:4790–8. doi: 10.1158/1078-0432.CCR-11-0034. [DOI] [PubMed] [Google Scholar]
- 53.Horbinski C, Hamilton RL, Nikiforov Y, Pollack IF. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119:641–9. doi: 10.1007/s00401-009-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR, et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6:e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]


