Abstract
Epithelial tissues are essential for barrier function, secretion, and regulation of fluid transport. Their function requires cell polarity and cell–cell adhesion, mediated through intercellular junctions. Conversely, disruption of adhesion and polarity is thought to drive cancer progression. The mammary gland is an important model for cell adhesion due to its postnatal hormonally regulated development; ducts undergo branching morphogenesis in response to steroid hormones during puberty. These hormonal signals induce a transition from simple to stratified architecture, initiated by asymmetric luminal cell divisions. Ductal elongation is accomplished by this multilayered, low-polarity epithelium, and polarity is reestablished as elongation ceases. The requirement for cell adhesion has been tested in 3D culture and in vivo, using gene deletion, knockdown, and misexpression in both developmental and homeostatic contexts. Attention has focused on E-cadherin, the major classical cadherin in luminal epithelial cells. Classic studies revealed a requirement for E-cadherin during lactation, and E-cadherin loss is widely posited to promote metastasis. However, recent findings demonstrated a broader requirement for E-cadherin during branching morphogenesis and homeostasis and also, surprisingly, in epithelial dissemination. These studies suggest that longstanding models of the role of adhesion in epithelial biology need to be revisited. Advances in inducible gene expression and knockdown, CRISPR/Cas9 technology, and fluorescent labeling of genetically modified cells offer the opportunity to test the roles of diverse adhesion systems and to develop a mechanistic understanding of how cell adhesion regulates development and cancer.
1. INTRODUCTION
Epithelium is one of the fundamental animal tissues and lines the cavities and surfaces of the body. Epithelial tissues were first defined anatomically based on their organization into layers of tightly connected cells with apicobasal polarity (Virchow, 1860). Despite this characteristic appearance, it has been challenging to develop a simple molecular definition that encompasses all epithelial cells. There is no single molecular marker expressed specifically in all epithelial cells, nor any master epithelial gene that is required for the genesis or function of all epithelial tissues (Davies & Garrod, 1997). However, despite differences in specific expression, adhesion proteins and their associated cell–cell junctions are a recognizable, shared feature of diverse epithelial organs (Nelson, 2003). Cell–cell adhesion is also required for the establishment and maintenance of distinct apical and basolateral membrane domains, which define epithelial cell polarity (Nelson, 2003). It is therefore reasonable to anticipate that adhesion proteins are important regulators of normal and pathologic epithelial biology.
Here, we review the role of cell–cell adhesion in mammary development and highlight the contributions of major adhesion molecules to specific developmental processes. We also briefly discuss the potential functions of adhesion proteins in breast cancer invasion and metastasis. We focus on E-cadherin, the major classical cadherin expressed in luminal epithelial cells, which is classified as a tumor and invasion suppressor in multiple epithelial cancers (van Roy & Berx, 2008). Recent data suggest novel roles for E-cadherin that challenge our traditional notions of adhesion proteins as limiting cell motility and migration. Supporting this concept, most breast tumors and their metastases are E-cadherin positive (Kowalski, Rubin, & Kleer, 2003), and studies have conflicted on the correlation between E-cadherin status, histologic grade, metastasis, and prognosis (Gamallo et al., 1993; Hunt, Douglas-Jones, Jasani, Morgan, & Pignatelli, 1997; Lipponen, Saarelainen, Ji, Aaltomaa, & Syrjanen, 1994; Oka et al., 1993; Siitonen et al., 1996; Tan et al., 1999). Finally, we revisit the definition of an epithelial cell and speculate on the limits of “epithelial” cell behaviors.
2. CELL–CELL ADHESION IN MAMMARY DEVELOPMENT: THE MAJOR PLAYERS
2.1 From simple to stratified: Transitions in adhesion during morphogenesis
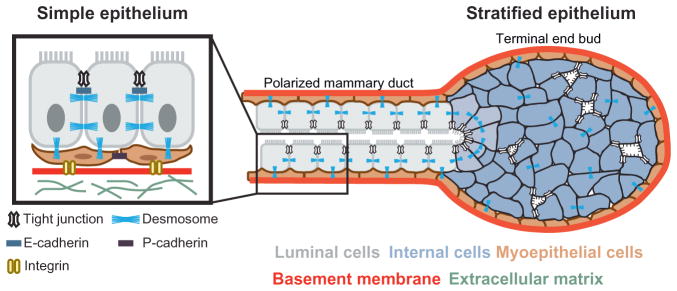
The mammary gland has long served as a valuable model system for studying cell adhesion in epithelial morphogenesis and tumor biology (Boussadia, Kutsch, Hierholzer, Delmas, & Kemler, 2002; Daniel, Strickland, & Friedmann, 1995; Gjorevski & Nelson, 2011; Knudsen & Wheelock, 2005; McNally & Martin, 2011; Nanba, Nakanishi, & Hieda, 2001; Runswick, O’Hare, Jones, Streuli, & Garrod, 2001). Mature mammary ducts exhibit simple epithelial architecture, with a bilayer of inner luminal and outer myoepithelial cells, each expressing distinct adhesion proteins (Daniel et al., 1995; Fig. 1). Luminal epithelial cells connect to each other through tight junctions, desmosomes, gap junctions, and adherens junctions (Pitelka, Hamamoto, Duafala, & Nemanic, 1973). Luminal and myoepithelial cells link through both desmosomes and gap junctions, and myoepithelial cells bind to the basement membrane through hemidesmosomes (Pitelka et al., 1973). However, these simple ducts arise from a multilayered, relatively unpolarized embryonic mammary placode (Hogg, Harrison, & Tickle, 1983; Nanba et al., 2001), and there are characteristic variations in the quantity and organization of intercellular junctions during periods of active morphogenesis (Ewald, Brenot, Duong, Chan, & Werb, 2008; Ewald et al., 2012; Pitelka et al., 1973).
Figure 1.
Normal transitions in adhesion during epithelial branching morphogenesis. Mammary epithelium initiates branching morphogenesis postnatally. Tube elongation is accomplished by a stratified terminal end bud, which contains many internal luminal cells that lack apicobasal polarity and display reduced numbers of intercellular junctions. The epithelium at the rear polarizes to a bilayered, simple ductal architecture consisting of an inner layer of luminal cells and a basal layer of myoepithelial cells. Epithelial cells in the ducts are connected by many cell–cell junctions. Schematic adapted from original by Robert Huebner, with permission.
From birth through puberty, mammary ducts are essentially quiescent and retain their polarized, bilayered organization (Hogg et al., 1983; Huebner, Lechler, & Ewald, 2014). The majority of branching morphogenesis occurs during puberty, regulated by steroid hormone and receptor tyrosine kinase signaling (Hennighausen & Robinson, 2005; Sternlicht, 2006; Sternlicht, Kouros-Mehr, Lu, & Werb, 2006). These signals induce a transition from simple to stratified architecture and lead to the formation of specialized terminal end buds (TEBs) at the tips of each duct (Hinck & Silberstein, 2005; Huebner et al., 2014; Silberstein & Daniel, 1982; Fig. 1). Recent studies revealed that this transition is initiated by asymmetric cell divisions within the luminal epithelial layer and that the products of these cell divisions lack apicobasal polarity and have few intercellular junctions (Ewald et al., 2012; Huebner et al., 2014). Mammary tubulogenesis is accomplished by these migratory, proliferative, unpolarized cells (Ewald et al., 2008, 2012; Huebner et al., 2014; reviewed in Huebner & Ewald, 2014).
2.2 Adherens junctions
A major contributor to epithelial integrity is the adherens junction, a supra-molecular structure that mediates homophilic intercellular interactions (Takeichi, 2014). The most frequently studied molecular components of the adherens junction are the adhesion receptor E-cadherin and the associated cytosolic proteins α-catenin, β-catenin, and p120 catenin (Takeichi, 2014). However, the mature adherens junction involves additional trans-membrane adhesion receptors and cytosolic proteins, such as nectin and afadin, and the core adhesive elements can exist on the membrane in both junctional and nonjunctional configurations (Niessen & Gottardi, 2008). Additionally, the connection of the adherens junction to the actin cytoskeleton is dependent on both cell contact and mechanical force (Buckley et al., 2014; Yamada & Nelson, 2007). In the mammary epithelial bilayer, cadherin isoforms are expressed in a cell type-specific manner: luminal cells express E-cadherin, and myoepithelial cells express P-cadherin (Daniel et al., 1995). Here, we discuss the roles of these two cadherins and the catenins in mammary epithelial development.
Early functional analyses of cell adhesion frequently relied on function perturbing antibodies. Classic work in kidney epithelial cells in culture revealed that antibody-based disruption of E-cadherin coordinately inhibits the formation of adherens junctions, tight junctions, and desmosomes (Gumbiner, Stevenson, & Grimaldi, 1988). These reagents can also be utilized in vivo, by implanting antibody-soaked beads in the mammary fat pad. This approach revealed that disruption of E-cadherin specifically affects the luminal epithelium, while disruption of P-cadherin specifically affects the myoepithelium (Daniel et al., 1995). Further supporting the importance of differential cadherin expression in mammary epithelial development, engineered combinations of human luminal and myoepithelial cells self-organize into bilayers in culture, and this organization is disrupted by addition of antibodies targeting E-cadherin or P-cadherin (Chanson et al., 2011). Below, we review the subsequent genetic analyses of mammary-specific deletion or misexpression of adhesion molecules and identify remaining gaps in our knowledge.
2.2.1 Early requirement for E-cadherin
E-cadherin is broadly expressed in epithelial cells from early embryonic stages through to mature organs. Accordingly, it was reasonable to expect that it was required for the formation of epithelial tissues. Consistent with that concept, E-cadherin null embryos initially compact due to residual maternal E-cadherin but fail to form a trophectoderm and blastocyst cavity and die at the time of implantation (Larue, Ohsugi, Hirchenhain, & Kemler, 1994; Riethmacher, Brinkmann, & Birchmeier, 1995). Electron microscopy analysis of E-cadherin null embryos has demonstrated that cell–cell contacts are structurally distorted and form irregular interdigitating membranes, yet they maintain desmosomes and tight junctions (Riethmacher et al., 1995). Interestingly, these interdigitating membranes are morphologically similar to those observed connecting normal mammary epithelial cells during periods of active morphogenesis, suggesting that ductal elongation may involve partial disassembly of adherens junctions (Ewald et al., 2012). These studies established an essential role for E-cadherin, but its very early requirement prevented analysis at postnatal stages.
2.2.2 Strategies for genetic analysis in the postnatal mammary gland
To overcome this challenge, investigators have relied on cell type-specific expression of Cre recombinase to conditionally delete genes. In the mammary gland, most studies have relied on the mouse mammary tumor virus (MMTV) long terminal repeat (Wagner et al., 2001) and whey acidic protein (WAP) (Wagner et al., 1997) promoters. These tools have been very productive and have enabled the analysis of mammary-specific requirements for many genes (McNally & Martin, 2011). However, several challenges have emerged that limit the ability of either line to generate “perfect” mammary-specific gene deletions. The first is that both promoters exhibit a degree of mosaicism within the epithelial compartment, resulting in a varying mixture of wild-type and recombined cells at different stages. The second is the varying timing of Cre activity; depending on the founder line and strain background, the MMTV promoter becomes active beginning in embryogenesis, whereas the WAP promoter becomes active during the second half of pregnancy (Wagner et al., 2001, 1997). However, both promoters are most active during late pregnancy and lactation, which has meant that effects of gene ablation on pubertal branching morphogenesis have been less frequently characterized. Importantly, differences in the timing of gene deletion in similarly targeted cell populations can result in divergent phenotypes. For example, conditional loss of p53 and E-cadherin in alveolar progenitor cells (via the MMTV promoter) induces invasive lobular carcinoma (ILC) (Derksen et al., 2011, 2006); however, loss of p53 and E-cadherin in mature alveolar cells (via the WAP promoter) does not result in tumor formation (Kotb, Hierholzer, & Kemler, 2011). Finally, recent studies from multiple investigators reported significant lactational defects in mice expressing the MMTV-Cre transgene from the A founder line (Robinson & Hennighausen, 2011; Yuan, Wang, Pao, Anderson, & Gu, 2011). Even accounting for these limitations, existing promoter-Cre transgenic lines have been essential in enabling an analysis of the role of cell adhesion in mammary development.
2.2.3 Postnatal analysis of E-cadherin function in the mammary gland
An early application of this approach was expression of a truncated form of E-cadherin under the MMTV promoter to test the specific contribution of E-cadherin’s cytoplasmic domain to mammary development (Delmas et al., 1999). In the virgin and pregnant gland, overexpression of the cytoplasmic domain induces precocious alveolar formation and differentiation but no histologic adhesion defects. In contrast, in the lactating gland, the cytoplasmic domain exerts a dominant-negative effect on cell–cell adhesion, cell polarity, and the integrity of the basement membrane (Delmas et al., 1999). Importantly, transgene activation is highest during lactation, and variation in protein levels of E-cadherin’s cytoplasmic domain may account for the discrepancy in effects on cell adhesion and morphology at different stages of development.
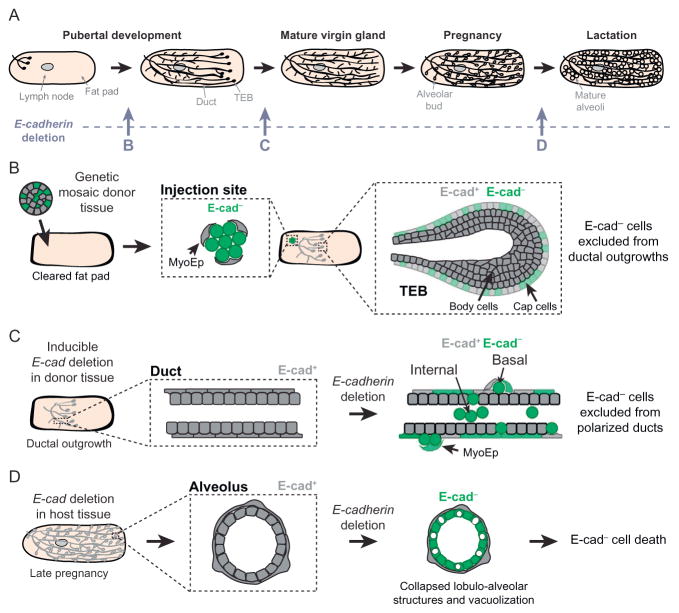
Conditional gene deletion was next used to test the consequences of E-cadherin loss in the pregnant and lactating mammary gland (Fig. 2A and D; Boussadia et al., 2002). MMTV-Cre-mediated recombination in E-cadherin fl/fl mice induces E-cadherin deletion in differentiating alveolar epithelium, which impairs terminal differentiation during late pregnancy. The gland develops normally until about 16–18 days of pregnancy, after which there is significantly reduced milk protein production and massive apoptosis at parturition, similar to an involuting gland (Boussadia et al., 2002). E-cadherin thus plays an essential role in the survival and function of alveolar epithelial cells. However, E-cadherin loss did not result in mammary tumor formation (Boussadia et al., 2002). In two subsequent studies, conditional E-cadherin deletion was driven by K14-Cre expression, which has low, stochastic activity in the mammary epithelium, and by WAP-Cre expression, which has patchy activity in the virgin gland in addition to high activity in the lactating gland (Derksen et al., 2011, 2006). Neither mouse model yields observable developmental defects in virgin, pregnant, or parous mice or results in mammary tumors (Derksen et al., 2011, 2006). Importantly, no E-cadherin− ducts were observed, which was inferred to result from rapid elimination of E-cadherin− cells by apoptosis (Derksen et al., 2011, 2006).
Figure 2.
E-cadherin is required at multiple stages of mammary epithelial development. (A) Mammary pubertal branching morphogenesis initiates at approximately 3 weeks postnatal. A stratified terminal end bud (TEB) leads the elongation front, and a full ductal network forms over 7 weeks. During pregnancy, lactogenic hormones induce the ductal epithelium to form specialized structures called alveoli that mature to secrete milk during lactation. Different genetic approaches have been used to test the consequences of E-cadherin loss during distinct stages of development. (B) E-cadherin is required for branching morphogenesis (Shamir et al., 2014). E-cadherin− cells (green) within transplanted genetic mosaic epithelium are excluded from a ductal network elaborated exclusively by E-cadherin+ cells. (C) E-cadherin is required for maintenance of epithelial architecture (Shamir et al., 2014). E-cadherin− cells (green) are extruded apically and basally from mature E-cadherin+ epithelial ducts. (D) E-cadherin is required for terminal differentiation and cell survival of alveoli in the lactating gland (Boussadia et al., 2002). E-cadherin loss induces lobuloalveolar collapse and massive apoptosis.
Collectively, these genetic approaches demonstrated a requirement for E-cadherin during lactation but did not test the consequences of E-cadherin loss in the virgin gland. Due to low levels of Cre-mediated deletion during puberty and the lack of reliable markers for recombined cells, the fate of E-cadherin− cells during branching morphogenesis remained unknown. To address this question, a recent study used fluorescently labeled genetic mosaic analysis, 3D organotypic culture, and orthotopic transplantation to test the consequences of E-cadherin loss on mammary branching and maintenance of epithelial architecture (Fig. 2A–C; Shamir et al., 2014). The Cre biosensor mT/mG was used to distinguish the fate of E-cadherin− cells in genetic mosaic epithelium. E-cadherin deletion in transplanted donor tissue results in failure of E-cadherin− cells to contribute to ductal outgrowths, which are elaborated by E-cadherin+ cells (Fig. 2B; Shamir et al., 2014). E-cadherin− cells are viable for at least 6 weeks in vivo but are excluded both from elongating, multilayered buds and from polarized, bilayered ducts. E-cadherin deletion in polarized epithelial ducts results in the extrusion of E-cadherin− cells apically, into the ductal lumen, and basally, onto the ductal surface (Fig. 2C; Shamir et al., 2014). These data reveal a requirement for E-cadherin in branching morphogenesis and in duct homeostasis in vivo.
Consistent with in vivo findings, in 3D culture, E-cadherin− cells can locally invade past basement membrane (Fig. 3C); however, they rarely disseminate (Shamir et al., 2014). By ultrastructural analysis, cells in these invasive groups are still connected by desmosomes (Shamir et al., 2014). E-cadherin loss thus disrupts epithelial polarity and organization but is not sufficient to induce dissemination in vivo or in vitro. It remains an open question whether the observed cell–cell junctions functionally limit the dissemination of E-cadherin− cells. Interestingly, basally positioned E-cadherin− cells are consistently covered by myoepithelial cells, both in 3D culture and in vivo. This result raises the alternate possibility that myoepithelial cells resist the dissemination of E-cadherin− luminal epithelial cells. This scenario is plausible as the presence of an intact myoepithelium is the feature that clinically distinguishes ductal carcinoma in situ from invasive breast cancer (Hu et al., 2008; Polyak, 2010; Polyak & Hu, 2005). Furthermore, even focal disruptions in the myoepithelium correlate with poor patient outcomes in breast cancer (Man et al., 2003), and myoepithelial cells have been proposed as cellular suppressors of tumor cell invasion (Polyak & Hu, 2005; Sternlicht & Barsky, 1997). Future studies are needed to test this concept through myoepithelium-specific genetic perturbation.
Figure 3.
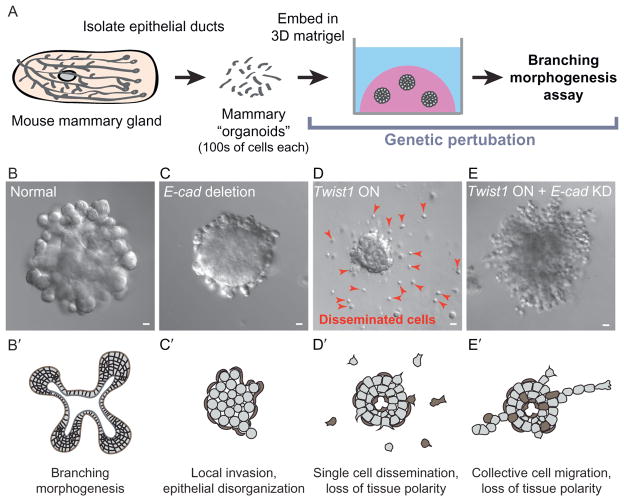
E-cadherin is required for Twist1-induced single cell dissemination. 3D organotypic culture of primary mouse mammary epithelium was used to isolate the effects of single gene changes associated with the epithelial–mesenchymal transition. (A) In the assay, fragments of mammary ducts, called “organoids,” are explanted into a basement membrane-rich extracellular matrix. Genetic perturbations, including Cre-lox-based gene deletion, shRNA knockdown, and tet-inducible gene expression, are performed in vitro. (B) Normal organoids branch in response to growth factor. (C) E-cadherin deletion blocks branching and induces epithelial disorganization. (D) Twist1 expression blocks branching and induces single cell dissemination. (E) E-cadherin knockdown inhibits Twist1-induced single cell dissemination. Bars, 20 μm. Images ©Shamir et al. (2014). Originally published in The Journal of Cell Biology, doi: http://dx.doi.org/10.1083/jcb.201306088.
2.2.4 P-cadherin
P-cadherin is a classical cadherin specifically expressed in myoepithelial cells (Daniel et al., 1995). The exception is during late pregnancy and lactation, when luminal epithelial cells secrete high levels of a soluble fragment of P-cadherin in human milk (Soler, Russo, Russo, & Knudsen, 2002). P-cadherin-deficient mice are viable, and the females are both fertile and can nurse their young (Radice et al., 1997). This result was somewhat surprising given the normally high levels of expression of P-cadherin in the placenta and in the myoepithelium (Radice et al., 1997). However, P-cadherin loss induces precocious differentiation in the postpubescent virgin mammary gland, with appearance of alveolar-like buds that express milk proteins (Radice et al., 1997). Over time, P-cadherin− females develop focal hyperplasia in the luminal epithelial compartment; however, P-cadherin− mice do not develop carcinomas (Radice et al., 1997). P-cadherin therefore serves as an important regulator of proliferation and differentiation in the mammary gland. Importantly, these data demonstrate that loss of a myoepithelium-specific protein can induce a phenotype in the luminal epithelial cells, revealing a functional cross talk between epithelial lineages. It remains unclear whether the consequences of P-cadherin loss result directly from a defect in P-cadherin-mediated signaling or from a more general disruption in cell–cell adhesion and tissue organization.
2.2.5 Catenins
Inside the cell, cadherins are linked to the actin cytoskeleton by the cadherin–catenin core complex, which includes α-catenin, β-catenin, and p120-catenin (Takeichi, 2014). Genetic testing of the roles for catenins in the mammary gland supports the concept that the adherens junction is required for pubertal development and epithelial cell survival during lactation.
α-Catenin connects to the cadherin complex through β-catenin and also binds to F-actin. Loss of α-catenin causes embryonic lethality due to disruption of the trophoblast epithelium (Torres et al., 1997). As such, mammary-specific α-catenin gene deletion was induced in α-catenin fl/fl mice by expressing Cre under control of either the WAP or MMTV promoter (Nemade et al., 2004). Loss of α-catenin impaired functional differentiation and polarization in alveolar epithelial cells (Nemade et al., 2004). Reduced milk protein gene expression resulted in failure to thrive among offspring of these mice. Epithelial clusters at parturition lacked central lumens and lipid droplets and displayed increased cell death, similar to the mammary epithelium at involution (Nemade et al., 2004). This combination of impaired milk production and increased apoptosis closely parallels the phenotypic consequences of conditional E-cadherin deletion (Boussadia et al., 2002). Moreover, as with E-cadherin, deletion of α-catenin does not result in hyper-proliferation or tumor formation.
Importantly, α-catenin deletion induced by MMTV-Cre has no detectable effect on branching morphogenesis (Nemade et al., 2004). Nevertheless, the known mosaic expression of the MMTV-Cre transgene (Wagner et al., 2001) suggests the possibility that cell competition with α-catenin+ cells may have obscured a defect. Fluorescent labeling of genetically modified E-cadherin− cells recently enabled demonstration of a previously unrecognized requirement for E-cadherin in branching morphogenesis (Shamir et al., 2014). Analogous approaches will likely reveal additional roles for adhesion molecules such as α-catenin in developmental stages other than lactation, during which the MMTV and WAP promoters are most active.
β-Catenin’s role in mammary development and cancer has largely been studied in the context of its signaling function as an essential element in the canonical Wnt pathway, not its structural function as a component of the adherens junction (Hatsell, Rowlands, Hiremath, & Cowin, 2003). For example, a stabilized, constitutively active form of β-catenin expressed in luminal epithelial cells (MMTV::Δ N89-β-catenin mice) induces precocious lobuloalveolar development and differentiation in male and female virgin mice, incomplete involution following cessation of lactation, and development of adenocarcinomas in all female mice early in life (Imbert, Eelkema, Jordan, Feiner, & Cowin, 2001). A constitutively active form of β-catenin expressed in basal myoepithelial cells (K5::Δ N57-β-catenin mice) induces precocious lobuloalveolar development in pregnancy, sustained luminal cell proliferation during lactation and accelerated involution, and development of basal-type hyperplasia and invasive carcinomas (Teuliere et al., 2005). Consistent with these findings, expression of a dominant-negative β-catenin (β-eng) that retains normal cell–cell adhesion properties but lacks the cytoplasmic signaling domain inhibits lobuloalveolar development and induces apoptosis (Tepera, McCrea, & Rosen, 2003). We are not aware of any studies that have specifically tested the consequences of β-catenin deletion on mammary epithelial development. Importantly, deletion of E-cadherin or α-catenin in normal mammary ducts in vivo has not been reported to result in nuclear localization of β-catenin or Wnt activation (Boussadia et al., 2002; Nemade et al., 2004). Disruption of the adherens junction in otherwise normal mammary epithelial cells therefore does not necessarily activate β-catenin signaling.
p120-catenin (p120) regulates cadherin levels at the cell surface by physically blocking cadherin endocytosis and subsequent degradation (Kowalczyk & Reynolds, 2004). The requirement for p120 in the mammary gland was tested through MMTV-Cre-mediated recombination in p120 fl/fl mice. p120− cells are excluded from TEBs upon genetic mosaic ablation at puberty, followed by rapid elimination of p120− cells and a transient delay in ductal outgrowth that is accomplished by p120+ cells (Kurley et al., 2012). Sorting of both luminal and myoepithelial p120− cells is observed in nascent TEBs, with shedding of K8+p120− cells into the lumen and accumulation of SMA+p120− cells in the subcapsular space. This observation suggests a functional requirement for catenin-based cell–cell junctions in myoepithelial cells. Finally, transplanted p120− cells fail to reconstitute the mammary gland. These findings directly parallel the consequences of conditional E-cadherin deletion and demonstrate the importance of labeling of the recombined cells: in genetic mosaics, p120− and E-cadherin− cells are excluded apically and basally from normal epithelium and fail to contribute to ductal outgrowths, whereas wild-type cells in the same epithelium successfully reconstitute the ductal network (Kurley et al., 2012; Shamir et al., 2014). p120 and E-cadherin thus have nonredundant roles in mammary cell–cell adhesion and in the collective migration required for normal development. Moreover, as loss of p120 induces concurrent depletion of E-cadherin (Kurley et al., 2012), the absence of cadherin stability may serve as the ultimate driver of the phenotype.
2.3 Desmosomes
Desmosomes are observed at luminal–luminal, myoepithelial–myoepithelial, and luminal–myoepithelial cell–cell contacts, and their number shifts dynamically during morphogenesis (Pitelka et al., 1973). Resting or nonsecretory mammary ducts have an abundance of desmosomes (Pitelka et al., 1973). In contrast, during lactation and involution, alveoli lose nearly all desmosomes and adherens junctions and establish a new network of tight junctions (Pitelka et al., 1973). Interestingly, desmosomes appear to be the junctions that remain in multiple contexts of reduced cell–cell adhesion: (a) in the normal, stratified, elongating bud (Ewald et al., 2012) and (b) in chains of migratory, but collective, E-cadherin− cells (Shamir et al., 2014). Nevertheless, the requirement for desmosomal proteins in mammary epithelial development has not been tested genetically.
Desmocollins (Dscs) and desmogleins (Dsgs), the desmosomal cadherins, each exist as three genetic isoforms that are differentially expressed in the mammary epithelial bilayer: Dsc2 and Dsg2 are ubiquitously expressed, Dsc3 and Dsg3 are expressed only in myoepithelial cells, and Dsc1 and Dsg1 are absent (Runswick et al., 2001). The function of desmosomal adhesion in cell positioning was tested in vitro by treating human mammary epithelial cells with blocking peptides against cell adhesion recognition (CAR) sites on the desmosomal cadherins (Runswick et al., 2001). Combined, but not individual, Dsc2 and Dsg2 CAR peptides inhibit luminal–luminal cell adhesion, and combined, but not individual, Dsc3 and Dsg3 peptides disrupt proper cell type-specific positioning, suggesting a role for specific isoforms in mediating polarized sorting of luminal and myoepithelial cells (Runswick et al., 2001). In contrast, a separate study concluded that downregulation of Dsc3 and Dsg3 facilitates branching morphogenesis in a primary mammary 3D culture model (Basham et al., 2013). Further elucidation of the precise role of desmosomal adhesion in vivo will likely require genetic ablation of various desmosomal proteins both in isolation and in combination. Major open questions include whether desmosome number is tightly regulated across distinct morphogenetic events and whether desmosomes play an essential compensatory function for adherens junctions.
3. CELL–CELL ADHESION IN BREAST CANCER
3.1 E-cadherin: An invasion suppressor?
E-cadherin is considered a suppressor of tumor invasion and metastasis across epithelial cancers (Beavon, 2000). E-cadherin function can be lost through mutation, gene deletion, or transcriptional repression (Berx & Van Roy, 2001). Classic experiments in human carcinoma cell lines demonstrated that those with an “epithelioid” morphology generally are E-cadherin+ and non-invasive into collagen gels, whereas those with a “fibroblastoid” morphology are E-cadherin− and invasive (Frixen et al., 1991). Furthermore, forced E-cadherin expression was sufficient to inhibit the invasiveness of human breast carcinoma cells (Frixen et al., 1991; Vleminckx, Vakaet, Mareel, Fiers, & van Roy, 1991).
In the breast, E-cadherin loss is a characteristic feature of ILC (Berx et al., 1995; Moll, Mitze, Frixen, & Birchmeier, 1993). In the mouse, loss of E-cadherin synergizes with loss of p53 to accelerate tumor formation and metastasis (Derksen et al., 2006). At the tissue level, loss of E-cadherin and p53 is accompanied by anoikis resistance and increased angiogenesis, and the tumor itself has a remarkable histologic resemblance to human ILC (Derksen et al., 2006). This study revealed that E-cadherin can function as both a tumor and metastasis suppressor. Nevertheless, loss of E-cadherin is commonly observed in benign lobular carcinoma in situ as well as ILC, suggesting that E-cadherin loss is not sufficient for invasion (Vos et al., 1997). Interestingly, germline E-cadherin mutations are rarely observed in patients with invasive lobular breast cancer but are implicated in hereditary diffuse gastric cancer (Paredes et al., 2012).
Importantly, while E-cadherin loss is characteristic of ILC, lobular carcinomas represent only about 10% of breast tumors (Arpino, Bardou, Clark, & Elledge, 2004). Most primary breast tumors and their distant metastases are in fact E-cadherin positive (Kowalski et al., 2003). Specifically, E-cadherin is detectable in most ductal carcinomas, the most common histologic subtype of breast cancer (Moll et al., 1993). The dual presence of E-cadherin in the primary tumor and the metastatic sites can still be harmonized with a view of E-cadherin as an obligate invasion suppressor if there is a transient loss of E-cadherin as the cancer cells are traveling to distant organs (Polyak & Weinberg, 2009). Arguing against this concept is the observation that E-cadherin levels are normal or higher in tumor microemboli of inflammatory breast cancer, a highly aggressive and poor prognosis breast cancer subtype (Kleer, van Golen, Braun, & Merajver, 2001).
The natural next question is whether E-cadherin levels in the primary tumor correlate with lymph node involvement, metastasis, and clinical outcomes. Several studies have identified an association between reduced or absent E-cadherin staining and increased invasiveness, higher rates of metastasis, and poor clinical outcomes (Berx & van Roy, 2009; Hunt et al., 1997; Oka et al., 1993; Siitonen et al., 1996). Conversely, multiple studies have found an association between E-cadherin status and histologic type without detecting a significant, independent correlation between E-cadherin status and outcomes (Gamallo et al., 1993; Lipponen et al., 1994). Finally, some studies have noted an independent correlation between strong E-cadherin staining and poor patient survival (Tan et al., 1999). Collectively, these data reveal that E-cadherin expression usefully distinguishes histologic subtypes but that its relationship to clinical outcomes, such as metastasis or survival, is not yet sufficiently well understood. From an underlying biological mechanism perspective, it is possible that complex spatial and temporal dynamics in E-cadherin expression could mask real correlations with survival. Alternatively, it is possible that there are both E-cadherin+ and E-cadherin− paths to metastasis.
3.2 Cadherin switching
One mechanism for regulating cadherin dynamics during epithelial invasion is cadherin switching, in which cells induce expression of a cadherin isoform not normally present in that cell type (Wheelock, Shintani, Maeda, Fukumoto, & Johnson, 2008). The most commonly observed example is gain of N-cadherin, with or without concurrent loss of E-cadherin (Wheelock et al., 2008). N-cadherin expression is normally restricted to neural tissue and mesenchymal cells. Its expression in human mammary tumor cell lines correlates with increased invasiveness and motility in vitro (Nieman, Prudoff, Johnson, & Wheelock, 1999) and increased metastasis in nude mice in vivo (Hazan, Phillips, Qiao, Norton, & Aaronson, 2000). In these tumor cell lines, N-cadherin-mediated induction of cell migration was found to be independent of E-cadherin status; forced E-cadherin expression in N-cadherin+ cells did not reduce motility (Nieman et al., 1999).
Based on these observations, the expectation was that ectopic N-cadherin expression in mammary epithelial cells would promote invasion in vivo, regardless of E-cadherin expression. Surprisingly, N-cadherin expression in the pregnant and lactating mammary epithelium, driven by the MMTV promoter, has no detectable phenotype (Knudsen, Sauer, Johnson, & Wheelock, 2005). Mid- and late-stage pregnant mice have normal mammary gland morphology and function, retain E-cadherin and β-catenin expression, and do not develop spontaneous tumors (Knudsen et al., 2005). A separate study tested whether N-cadherin could functionally replace E-cadherin in the mammary gland (Kotb et al., 2011). A knock-in of N-cadherin at the E-cadherin locus, combined with conditional deletion of the second E-cadherin allele during late pregnancy, induces massive apoptosis of alveolar cells and a lactation defect more severe than E-cadherin loss alone (Kotb et al., 2011). After multiple lactation cycles, mammary glands develop fibrocystic change that frequently progresses to tumor formation in combination with conditional deletion of one p53 allele (Kotb et al., 2011). Collectively, these studies demonstrate that expression of N-cadherin is insufficient to drive invasion or tumor formation and that E-cadherin is specifically required for epithelial integrity and cannot be substituted by N-cadherin.
A second plausible basis for the cadherin switch is the gain of P-cadherin in luminal epithelial cells. P-cadherin is the dominant classical cadherin in myoepithelial cells, and its expression is low or undetectable in normal luminal epithelial cells (Chanson et al., 2011; Daniel et al., 1995). Human breast cancers typically originate in luminal cells but frequently express P-cadherin, and P-cadherin expression is associated with high histologic grade and poor clinical outcomes (Paredes et al., 2005; Soler, Knudsen, Salazar, Han, & Keshgegian, 1999). To test the effect of inappropriate P-cadherin in normal adult luminal cells, transgenic mice were generated with P-cadherin expression driven by the MMTV promoter. Mammary glands with ectopic P-cadherin expression maintain membrane-localized E-cadherin and β-catenin; have no apparent defects in branching morphogenesis, ductal architecture, lactation, or involution; and do not develop mammary tumors (Radice, Sauer, Kostetskii, Peralta Soler, & Knudsen, 2003).
Taken together, misexpression of either N-cadherin or P-cadherin in normal mammary epithelium, without loss of E-cadherin, is not sufficient to disrupt mammary gland function or induce tumor formation. It is worth investigating whether another cadherin, such as cadherin 6 or 11, would be capable of inducing invasion or dissemination in the mammary epithelium (Gheldof & Berx, 2013; Jia, Liu, Hansen, Ter Beest, & Zegers, 2011). For example, experimental induction of cadherin 8, which is normally expressed in neurons, is sufficient to induce cyst formation in kidney epithelium (Kher et al., 2011). Alternately, the capacity of inappropriate cadherin expression to promote invasion and metastasis may require a decrease in E-cadherin levels and/or other as yet unknown molecular events. The cadherin switching phenomena observed in culture remain an attractive conceptual framework for regulating cell motility, but they have proven difficult to replicate in vivo in the mammary epithelium.
3.3 Rethinking the epithelial-to-mesenchymal transition
A second, related model is that cells fundamentally change their identity and undergo an epithelial-to-mesenchymal transition (EMT) in order to detach and migrate away from a tissue as a single cell (Greenburg & Hay, 1982; Hay & Zuk, 1995; Thiery, 2002). This concept is grounded in observations of cellular and molecular dynamics during embryogenesis (Acloque, Adams, Fishwick, Bronner-Fraser, & Nieto, 2009; Thiery, Acloque, Huang, & Nieto, 2009), such as neural crest formation, and has been proposed as a mechanism for cancer metastasis (Polyak & Weinberg, 2009; Thiery et al., 2009; Yang & Weinberg, 2008). The EMT paradigm originated from classic experiments by Elizabeth Hay, who observed that mature epithelia explanted into collagen I gels lost polarity and migrated into the matrix as single cells with a strong morphological resemblance to embryonic fibroblasts, a mesenchymal cell type (Greenburg & Hay, 1982; Hay & Zuk, 1995). These morphological observations formed the basis for the concept of “loss of the epithelial phenotype,” which expanded to include loss of epithelium-specific gene expression (Thiery, 2002). In turn, E-cadherin is often considered as the archetypal “caretaker” of the epithelial phenotype, and its loss has been used to invoke a broader change in cellular differentiation state (Peinado, Olmeda, & Cano, 2007).
A challenge to testing the EMT model in human cancer is that different investigators have used different criteria to define whether an EMT has occurred (see also the “Epithelial-Mesenchymal transitions: from cell plasticity to concept elasticity” by Pierre Savagner in this volume). While Hay proposed a fundamentally morphological definition, most current investigators define the molecular basis of EMT in terms of a transcriptional program consisting of downregulation of E-cadherin, catenins, and cytokeratins and upregulation of the mesenchymal markers N-cadherin, vimentin, and fibronectin (Peinado et al., 2007). Just as no single gene is specific to all epithelial tissues, no single marker definitively identifies an EMT in all circumstances. Nevertheless, EMT transcription factors such as Snail1, Slug, and Twist1 are collectively thought to operate through these molecular changes, and their expression has been correlated with invasion and metastasis in breast cancer patients and in experimental cancer models (Blanco et al., 2002; Martin, Goyal, Watkins, & Jiang, 2005; Mironchik et al., 2005; Peinado et al., 2007; Yang et al., 2004).
The varied use of morphological versus molecular criteria, or a combination of the two, has resulted in tension across studies as to when and whether an EMT has occurred. The concept of an epithelium is, at root, very morphological, and cell escape during metastasis would appear to require a dramatic break from this morphology. Nevertheless, the frequent absence of concurrent EMT-associated molecular changes in metastatic tumors has led some investigators to propose a “partial” EMT. For example, the commonly used MMTV-PyMT mouse model of breast cancer, which histologically resembles invasive ductal carcinoma and is highly metastatic to the lung, does not induce an EMT, as tumors are positive for E-cadherin and cytokeratins and negative for fibronectin (Guy, Cardiff, & Muller, 1992; Trimboli et al., 2008). Even a mouse model of ILC, which is E-cadherin negative, retains expression of the luminal epithelial cytokeratin K8 and does not upregulate vimentin (Derksen et al., 2006). In addition, human primary breast tumor cells positive for the EMT transcription factor Slug can express high levels of E-cadherin (Come et al., 2006). Such discrepancies indicate that “EMT” has different meanings depending on the investigator’s chosen definition and does not precisely or universally explain how epithelial tumor cells disseminate. Furthermore, lineage analysis of mesenchymal gene expression within epithelial cells revealed that mouse models of breast cancer can present clear evidence of a molecular EMT during metastasis (e.g., WAP-Myc), whereas other models accomplish metastasis while maintaining epithelial gene expression (e.g., MMTV-Neu and MMTV-PyMT) (Trimboli et al., 2008). The concept of EMT in human breast cancer remains controversial, with some pathologists arguing that they do not see evidence to support a shift toward mesenchymal cell identity in cancer cells (Tarin, 2005). The most direct evidence for the molecular changes associated with EMT are in Myc-overexpressing experimental mouse models (Trimboli et al., 2008) and in the claudin-low subtype of human breast cancer (Hennessy et al., 2009; Taube et al., 2010). Furthermore, the molecular program of EMT may not correlate with a transition to single cell organization, as a recent study exhaustively characterized the invasive boundary of breast tumors with the stroma and observed frequent collective invasion and essentially no isolated single cancer cells (Bronsert et al., 2014).
To resolve the direct effects of EMT-associated molecular changes on cell- and tissue-level behavior, a recent study directly contrasted the effect of E-cadherin deletion with Twist1 expression in an organotypic culture model of primary mouse mammary epithelium (Fig. 3A–E; Shamir et al., 2014). Loss of E-cadherin induced single-file invasion past basement membrane but only rare dissemination in 3D culture or in vivo (Fig. 3C). In contrast, Twist1 induced rapid and robust dissemination of single cells that were cytokeratin positive and migrated with dynamic, forward-oriented protrusions (Fig. 3D; Shamir et al., 2014). Contrary to expectation, Twist1 did not result in a loss of epithelial-specific gene expression or significantly regulate any canonical EMT genes, such as E-cadherin, N-cadherin, and vimentin (Shamir et al., 2014). Instead, Twist1 regulated a set of genes that collectively reprogram the composition of the extracellular space and the cell’s interactions with the extracellular matrix (ECM) (Shamir et al., 2014). Surprisingly, E-cadherin was still localized to the membranes of cells at every stage of dissemination, and E-cadherin knockdown inhibited dissemination of Twist1+ cells (Shamir et al., 2014). Rather, loss of E-cadherin promoted more cohesive migration of chains of Twist1+, cytokeratin+ cells (Fig. 3E). The exact levels of E-cadherin may thus critically regulate whether Twist1 induces single cell dissemination or collective epithelial invasion.
3.4 “Unexpected” roles for E-cadherin
Classic portrayals of E-cadherin as solely a tumor and/or invasion suppressor may be too narrow. In certain contexts, E-cadherin appears to promote cell migration, invasion, and even tumor progression (Rodriguez, Lewis-Tuffin, & Anastasiadis, 2012). In the developing zebrafish embryo, E-cadherin-mediated traction forces are required for single cell migration of chemokine-guided germ cells, and interfering with E-cadherin disrupts F-actin dynamics and organization at the cellular front and inhibits cell motility (Kardash et al., 2010). This study demonstrated that the precise levels of E-cadherin are important, as too much E-cadherin results in slower than normal migration, whereas loss of E-cadherin results in no migration (Kardash et al., 2010). Analogous regulation may have implications for the mechanisms of tumor cell motility in vivo. A recent study of developmental collective cell migration of the border cells in Drosophila revealed a novel role for E-cadherin as an integrator of mechanical signals and demonstrated its requirement for directional migration of cell clusters (Cai et al., 2014).
E-cadherin is also associated with aggressive cancer in multiple organs. In addition to being normal or increased in inflammatory breast cancer, E-cadherin is expressed in most ovarian carcinomas, which are derived from normally E-cadherin− ovarian surface epithelium (Auersperg et al., 1999; Kleer et al., 2001). Among a small subset of glioblastomas, E-cadherin is associated with decreased survival and correlates with increased invasiveness in vivo in orthotopic mouse xenografts (Lewis-Tuffin et al., 2010). The function of E-cadherin in such tumors and validation of its potential role in promoting invasion remains a major open area for future research (Rodriguez et al., 2012). It is also important to distinguish whether E-cadherin’s role in cell migration is primarily through direct control of intercellular adhesion or instead through regulation of intracellular signaling networks (Chu, Boley, Moraes, Barsky, & Robertson, 2013).
4. CAN A MIGRATORY SINGLE CELL OR CELL CLUSTER BE “EPITHELIAL”?
As our conception of metastasis broadens to include the invasion of E-cadherin+ cancer cells, an important definitional question arises: can we consider the resulting migratory single cells and cell groups to be epithelial? The answer to this question really rests on whether we define epithelial identity at the tissue or cellular level. In a homeostatic context, individual cells are components of an epithelial tissue and could not accomplish any of its essential barrier, secretory, or absorptive functions in isolation. At this functional level, it seems clear that an isolated single cell cannot be a functional barrier or an absorptive epithelium.
Yet the answer is subtler when considering the defining morphologic and molecular properties of an epithelium. Tight cell–cell adhesion represents one such property and has traditionally been thought of as serving mostly a structural or barrier function in epithelial ducts. However, mammary epithelial cells maintain at least some intercellular adhesive junctions during active migratory processes (Ewald et al., 2012; Shamir et al., 2014). Data from genetic mouse models and patient samples now increasingly support a more nuanced portrait of the roles of cell–cell adhesion proteins in normal and pathologic cellular contexts. In particular, there is not a simple relationship between loss of E-cadherin and invasive or disseminative behavior in normal or tumor mammary epithelium (Rodriguez et al., 2012). However, it is clear that migratory single cells can maintain membrane-localized E-cadherin and β-catenin and a normal complement of mRNAs for the genes encoding core epithelial functions (Shamir et al., 2014). Consistent with this observation, E-cadherin is expressed in most invasive breast cancers and, remarkably, is required for efficient single cell dissemination of Twist1+ cells (Kowalski et al., 2003; Shamir et al., 2014).
Given these observations, should we consider disseminated cells or groups “epithelial”? The community will have to settle this issue, likely through active new research and careful consideration of definitions. Our answer, based on our data, is that epithelial cells clearly are capable of migration within their tissue during developmental contexts (Ewald et al., 2008, 2012); that epithelial cells can be induced to disseminate into the ECM by changes in the composition, organization, or adhesive density of the micro-environment (Beck, Singh, Rothenberg, Elisseeff, & Ewald, 2013; Nguyen-Ngoc et al., 2012; Nguyen-Ngoc & Ewald, 2013); that simple molecular interventions, such as Twist1 expression, can induce dissemination of cells that maintain epithelial gene expression (Shamir et al., 2014); and that metastasis can occur in cells that retain many features of their epithelial origin (Cheung, Gabrielson, Werb, & Ewald, 2013; Nguyen-Ngoc et al., 2012). It may therefore be most useful to expand our concept of epithelial cells and tissues to include different characteristic states of epithelial function and behavior, including specifically a migratory or morphogenetically active epithelial state (Ewald et al., 2008). Considered from this perspective, it becomes important to determine the relative frequency with which tumors recapitulate epithelial migratory states versus mesenchymal fates or migratory states.
4.1 Dissemination: A transition in the substrate for migration
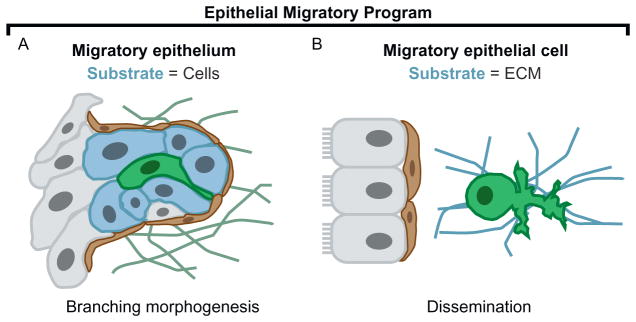
Epithelium is most often conceptualized in terms of quiescent ducts or sheets. However, building epithelial ductal networks involves tube elongation and branching, a dynamic process driven by the collective migration of adherent groups of cells (Andrew & Ewald, 2010). In the mammary gland, ultrastructural analysis and confocal imaging of stratification and bud elongation have revealed that cells within these groups lack apicobasal polarity, have reduced cell–cell junctions, are individually motile, and can appear elongated and even protrusive (Ewald et al., 2012). The presence of such features in normal cells during development suggests that motility rests within the spectrum of normal epithelial cell behavior. Epithelial dissemination may therefore not be an “invented” feature of metastatic cancer; rather, epithelial cells may be able to activate and modulate a conserved migratory program. In turn, dissemination might best be explained by a transition in the substrate for migration: in a normal migratory epithelium, the substrate is adjacent cells; in a disseminating migratory epithelial cell, the substrate is the surrounding ECM (Fig. 4A–B). Consistent with this concept, mammary epithelial cell dissemination induced by Twist1 primarily involves transcriptional changes in genes that reside in the extracellular compartment. Thus, dissemination may primarily involve a change in the type of adhesion proteins on the cell surface and the composition of the immediate extracellular environment.
Figure 4.
Is there are conserved epithelial migratory program? Dissemination might be explained by a switch in the substrate of migration from adjacent cells to ECM. (A) Epithelial cells collectively migrate as an elongating bud during mammary branching morphogenesis. Within the multilayer, an individual epithelial cell (green; dark gray shade in the print version) can appear elongated and protrusive. (B) During Twist1-induced dissemination, a single epithelial cell (green; dark gray shade in the print version) migrates through the ECM with amoeboid morphology and extensive protrusions at the leading front.
4.2 Balancing cell–cell and cell–matrix adhesion
Epithelial cells do not exist in isolation; rather, the epithelium is surrounded by connective tissue, which changes in organization and composition in cancer. Cell adhesion to the ECM via integrins has an established role in regulating mammary epithelial differentiation and morphogenesis (Muschler & Streuli, 2010). The composition and mechanical properties of the ECM also modulate cell behaviors in normal development and tumor progression (Ghajar & Bissell, 2008; Schedin & Keely, 2011). A collagen I-rich ECM, which models a more adhesive, tumorigenic microenvironment, induces protrusive invasion and dissemination even in normal mammary epithelium (Nguyen-Ngoc et al., 2012). Furthermore, the microenvironment can modulate phenotypic outcomes of genetic changes. For example, loss of P-cadherin induces hyperbranching of mammary epithelium cultured in a basement membrane-rich ECM but promotes excess, sustained myoepithelial dissemination when the same epithelium is cultured in collagen I (Nguyen-Ngoc et al., 2012).
We propose that it is useful to integrate these data within a free energy model for dissemination, such that cells leave the epithelial group whenever the energy of cell–matrix adhesion exceeds the energy of cell–cell adhesion. Our model allows for different categories of dissemination-inducing signals: broadly, cell–intrinsic cues and cell–extrinsic cues. The former encompasses transcriptional changes regulating the cell’s interaction with the extracellular space, such as those induced by Twist1 (Shamir et al., 2014). The latter includes many examples of microenvironmental cues, such as a collagen I ECM or synthetic matrices containing adhesive peptides, which can promote invasion and dissemination in normal epithelial tissues (Beck et al., 2013; Nguyen-Ngoc et al., 2012). Notably, microenvironment-driven dissemination is the origin of the EMT concept described by Elizabeth Hay (Greenburg & Hay, 1982). Thus, we anticipate that there are multiple avenues to reach the same cellular outcome of dissemination.
4.3 Novel functions for classic molecules: Next steps in adhesion biology
To date, our knowledge of cell–cell adhesion in normal mammary gland development and homeostasis has been mostly confined to E-cadherin and P-cadherin, in part due to the technical and financial difficulties of developing new loss-of-function reagents. CRISPR/Cas9 technology now offers the opportunity to systematically and rapidly assay the function of many classes of adhesion molecules, including various components of adherens junctions and desmosomes that are dysregulated in breast cancer (Leary et al., 2008). Due to the temporal constraints of in vivo gene inactivation approaches with the MMTV and WAP promoters, there is much that remains unknown about the function of adhesion proteins during pubertal branching morphogenesis. Genetic perturbation in organotypic cultures, combined with in vivo validation with fluorescent reporters, has introduced a valuable tool to circumvent these limitations. Inducible shRNA or gene deletion techniques further enable the study of loss of cell–cell adhesion molecules in established ducts.
Looking forward, a major unanswered question is how do adhesion proteins regulate and promote migration? The precise levels of particular cell–cell junctions may play an unappreciated role in modulating epithelial cell behaviors. For example, mammary end buds contain a reduced number of desmosomes, but the requirement for desmosomes in bud elongation remains untested, and the presence of too many desmosomes may even inhibit branching (Basham et al., 2013). Moreover, in cancer, the idea that adhesion proteins principally act as invasion suppressors is incompatible with many experimental and clinical observations. Cellular context, including posttranslational modifications and protein turnover, may critically regulate junction dynamics and cell motility and can collaborate with the microenvironment to alter tissue-level phenotypes. We envision that future investigations in adhesion biology have the potential to uncover novel roles for many epithelial adhesion molecules in both development and disease.
Acknowledgments
E. R. S. was supported by an Isaac and Lucille Hay Graduate Fellowship, endowed by Elizabeth “Betty” Hay. E. R. S. and A. J. E. were supported by a Research Scholar Grant (RSG-12-141-01-CSM) from the American Cancer Society, by funds from the NIH/NCI (P30 CA006973), and by a grant from the Breast Cancer Research Foundation. The authors thank Jamie Davies and David Garrod for stimulating discussions of epithelial identity and epithelial morphogenesis.
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. The Journal of Clinical Investigation. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Developmental Biology. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Research. 2004;6:R149–R156. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham KJ, Kieffer C, Shelton DN, Leonard CJ, Bhonde VR, Vankayalapati H, et al. Chemical genetic screen reveals a role for desmosomal adhesion in mammary branching morphogenesis. The Journal of Biological Chemistry. 2013;288:2261–2270. doi: 10.1074/jbc.M112.411033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavon IR. The E-cadherin-catenin complex in tumour metastasis: Structure, function and regulation. European Journal of Cancer. 2000;36:1607–1620. doi: 10.1016/s0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Beck JN, Singh A, Rothenberg AR, Elisseeff JH, Ewald AJ. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials. 2013;34:9486–9495. doi: 10.1016/j.biomaterials.2013.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, et al. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. The EMBO Journal. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berx G, Van Roy F. The E-cadherin/catenin complex: An important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Research. 2001;3:289–293. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harbor Perspectives in Biology. 2009;1:a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. Correlation of snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mechanisms of Development. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, et al. Cancer cell invasion and EMT marker expression: A three-dimensional study of the human cancer-host interface. The Journal of Pathology. 2014;234:410–422. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, et al. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, et al. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson L, Brownfield D, Garbe JC, Kuhn I, Stampfer MR, Bissell MJ, et al. Self-organization is a dynamic and lineage-intrinsic property of mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3264–3269. doi: 10.1073/pnas.1019556108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Boley KM, Moraes R, Barsky SH, Robertson FM. The paradox of E-cadherin: Role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism. Oncotarget. 2013;4:446–462. doi: 10.18632/oncotarget.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, et al. Snail and slug play distinct roles during breast carcinoma progression. Clinical Cancer Research. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Strickland P, Friedmann Y. Expression and functional role of E-and P-cadherins in mouse mammary ductal morphogenesis and growth. Developmental Biology. 1995;169:511–519. doi: 10.1006/dbio.1995.1165. [DOI] [PubMed] [Google Scholar]
- Davies JA, Garrod DR. Molecular aspects of the epithelial phenotype. Bioessays. 1997;19:699–704. doi: 10.1002/bies.950190810. [DOI] [PubMed] [Google Scholar]
- Delmas V, Pla P, Feracci H, Thiery JP, Kemler R, Larue L. Expression of the cytoplasmic domain of E-cadherin induces precocious mammary epithelial alveolar formation and affects cell polarity and cell-matrix integrity. Developmental Biology. 1999;216:491–506. doi: 10.1006/dbio.1999.9517. [DOI] [PubMed] [Google Scholar]
- Derksen PWB, Braumuller TM, van der Burg E, Hornsveld M, Mesman E, Wesseling J, et al. Mammary-specific inactivation of E-cadherin and p53 impairs functional gland development and leads to pleomorphic invasive lobular carcinoma in mice. Disease Models & Mechanisms. 2011;4:347–358. doi: 10.1242/dmm.006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen PWB, Liu XL, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, et al. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. Journal of Cell Science. 2012;125:2638–2654. doi: 10.1242/jcs.096875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. The Journal of Cell Biology. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, et al. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. The American Journal of Pathology. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: Insights from imaging. Histochemistry and Cell Biology. 2008;130:1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Progress in Molecular Biology and Translational Science. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nature Reviews. Molecular Cell Biology. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. The Journal of Cell Biology. 1982;95:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. The Journal of Cell Biology. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for meta-static disease. Molecular and Cellular Biology. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. Journal of Mammary Gland Biology and Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- Hay ED, Zuk A. Transformations between epithelium and mesenchyme: Normal, pathological, and experimentally induced. American Journal of Kidney Diseases. 1995;26:678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. The Journal of Cell Biology. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Research. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nature Reviews. Molecular Cell Biology. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB. Key stages in mammary gland development: The mammary end bud as a motile organ. Breast Cancer Research. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg NA, Harrison CJ, Tickle C. Lumen formation in the developing mouse mammary gland. Journal of Embryology and Experimental Morphology. 1983;73:39–57. [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RJ, Ewald AJ. Cellular foundations of mammary tubulogenesis. Seminars in Cell and Developmental Biology. 2014;31:124–131. doi: 10.1016/j.semcdb.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RJ, Lechler T, Ewald AJ. Developmental stratification of the mammary epithelium occurs through symmetry-breaking vertical divisions of apically positioned luminal cells. Development. 2014;141:1085–1094. doi: 10.1242/dev.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt NC, Douglas-Jones AG, Jasani B, Morgan JM, Pignatelli M. Loss of E-cadherin expression associated with lymph node metastases in small breast carcinomas. Virchows Archiv. 1997;430:285–289. doi: 10.1007/BF01092751. [DOI] [PubMed] [Google Scholar]
- Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. The Journal of Cell Biology. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Liu F, Hansen SH, Ter Beest MB, Zegers MM. Distinct roles of cadherin-6 and E-cadherin in tubulogenesis and lumen formation. Molecular Biology of the Cell. 2011;22(12):2031–2041. doi: 10.1091/mbc.E11-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardash E, Reichman-Fried M, Maitre JL, Boldajipour B, Papusheva E, Messerschmidt EM, et al. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nature Cell Biology. 2010;12:47–53. 1–11. doi: 10.1038/ncb2003. [DOI] [PubMed] [Google Scholar]
- Kher R, Sha EC, Escobar MR, Andreoli EM, Wang P, Xu WM, et al. Ectopic expression of cadherin 8 is sufficient to cause cyst formation in a novel 3D collagen matrix renal tubule culture. American Journal of Physiology. Cell Physiology. 2011;301:C99–C105. doi: 10.1152/ajpcell.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, van Golen KL, Braun T, Merajver SD. Persistent E-cadherin expression in inflammatory breast cancer. Modern Pathology. 2001;14:458–464. doi: 10.1038/modpathol.3880334. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Sauer C, Johnson KR, Wheelock MJ. Effect of N-cadherin misexpression by the mammary epithelium in mice. Journal of Cellular Biochemistry. 2005;95:1093–1107. doi: 10.1002/jcb.20469. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Wheelock MJ. Cadherins and the mammary gland. Journal of Cellular Biochemistry. 2005;95:488–496. doi: 10.1002/jcb.20419. [DOI] [PubMed] [Google Scholar]
- Kotb AM, Hierholzer A, Kemler R. Replacement of E-cadherin by N-cadherin in the mammary gland leads to fibrocystic changes and tumor formation. Breast Cancer Research. 2011;13:R104. doi: 10.1186/bcr3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Reynolds AB. Protecting your tail: Regulation of cadherin degradation by p120-catenin. Current Opinion in Cell Biology. 2004;16:522–527. doi: 10.1016/j.ceb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Research. 2003;5:R217–R222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurley SJ, Bierie B, Carnahan RH, Lobdell NA, Davis MA, Hofmann I, et al. p120-catenin is essential for terminal end bud function and mammary morphogenesis. Development. 2012;139:1754–1764. doi: 10.1242/dev.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proceedings of the National academy of Sciences of the United States of America. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary RJ, Lin JC, Cummins J, Boca S, Wood LD, Parsons DW, et al. Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16224–16229. doi: 10.1073/pnas.0808041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, et al. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5:e13665. doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen P, Saarelainen E, Ji H, Aaltomaa S, Syrjanen K. Expression of E-cadherin (E-CD) as related to other prognostic factors and survival in breast cancer. The Journal of Pathology. 1994;174:101–109. doi: 10.1002/path.1711740206. [DOI] [PubMed] [Google Scholar]
- Man YG, Tai L, Barner R, Vang R, Saenger JS, Shekitka KM, et al. Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: Implications for tumor progression and invasion. Breast Cancer Research. 2003;5:R231–R241. doi: 10.1186/bcr653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Annals of Surgical Oncology. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- McNally S, Martin F. Molecular regulators of pubertal mammary gland development. Annals of Medicine. 2011;43:212–234. doi: 10.3109/07853890.2011.554425. [DOI] [PubMed] [Google Scholar]
- Mironchik Y, Winnard PT, Vesuna F, Kato Y, Wildes F, Pathak AP, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Research. 2005;65:10801–10809. doi: 10.1158/0008-5472.CAN-05-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. The American Journal of Pathology. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- Muschler J, Streuli CH. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harbor Perspectives in Biology. 2010;2:a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba D, Nakanishi Y, Hieda Y. Changes in adhesive properties of epithelial cells during early morphogenesis of the mammary gland. Development, Growth & Differentiation. 2001;43:535–544. doi: 10.1046/j.1440-169x.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Epithelial cell polarity from the outside looking in. News in Physiological Sciences. 2003;18:143–146. doi: 10.1152/nips.01435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemade RV, Bierie B, Nozawa M, Bry C, Smith GH, Vasioukhin V, et al. Biogenesis and function of mouse mammary epithelium depends on the presence of functional alpha-catenin. Mechanisms of Development. 2004;121:91–99. doi: 10.1016/j.mod.2003.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2595–E2604. doi: 10.1073/pnas.1212834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Ngoc KV, Ewald AJ. Mammary ductal elongation and myoepithelial migration are regulated by the composition of the extracellular matrix. Journal of Microscopy. 2013;251:212–223. doi: 10.1111/jmi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. The Journal of Cell Biology. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochimica et Biophysica Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Research. 1993;53:1696–1701. [PubMed] [Google Scholar]
- Paredes J, Albergaria A, Oliveira JT, Jerónimo C, Milanezi F, Schmitt FC. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clinical Cancer Research. 2005;11:5869–5877. doi: 10.1158/1078-0432.CCR-05-0059. [DOI] [PubMed] [Google Scholar]
- Paredes J, Figueiredo J, Albergaria A, Oliveira P, Carvalho J, Ribeiro AS, et al. Epithelial E- and P-cadherins: Role and clinical significance in cancer. Biochimica et Biophysica Acta. 2012;1826:297–311. doi: 10.1016/j.bbcan.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nature Reviews. Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK. Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. The Journal of Cell Biology. 1973;56:797–818. doi: 10.1083/jcb.56.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K. Molecular markers for the diagnosis and management of ductal carcinoma in situ. Journal of the National Cancer Institute. Monographs. 2010;2010:210–213. doi: 10.1093/jncimonographs/lgq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? Journal of Mammary Gland Biology and Neoplasia. 2005;10:231–247. doi: 10.1007/s10911-005-9584-6. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nature Reviews. Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, et al. Precocious mammary gland development in P-cadherin-deficient mice. The Journal of Cell Biology. 1997;139:1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Sauer CL, Kostetskii I, Peralta Soler A, Knudsen KA. Inappropriate P-cadherin expression in the mouse mammary epithelium is compatible with normal mammary gland function. Differentiation. 2003;71:361–373. doi: 10.1046/j.1432-0436.2003.7106005.x. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:855–859. doi: 10.1073/pnas.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GW, Hennighausen L. MMTV-Cre transgenes can adversely affect lactation: Considerations for conditional gene deletion in mammary tissue. Analytical Biochemistry. 2011;412:92–95. doi: 10.1016/j.ab.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin’s dark side: Possible role in tumor progression. Biochimica et Biophysica Acta. 2012;1826:23–31. doi: 10.1016/j.bbcan.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nature Cell Biology. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harbor Perspectives in Biology. 2011;3(1):a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir ER, Pappalardo E, Jorgens DM, Coutinho K, Tsai WT, Aziz K, et al. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. The Journal of Cell Biology. 2014;204:839–856. doi: 10.1083/jcb.201306088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. American Journal of Clinical Pathology. 1996;105:394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Developmental Biology. 1982;90:215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Soler AP, Knudsen KA, Salazar H, Han AC, Keshgegian AA. P-cadherin expression in breast carcinoma indicates poor survival. Cancer. 1999;86:1263–1272. doi: 10.1002/(sici)1097-0142(19991001)86:7<1263::aid-cncr23>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- Soler AP, Russo J, Russo IH, Knudsen KA. Soluble fragment of P-cadherin adhesion protein found in human milk. Journal of Cellular Biochemistry. 2002;85:180–184. [PubMed] [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Research. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Barsky SH. The myoepithelial defense: A host defense against cancer. Medical Hypotheses. 1997;48:37–46. doi: 10.1016/s0306-9877(97)90022-0. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nature Reviews. Molecular Cell Biology. 2014;15:397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- Tan DS, Potts HW, Leong AC, Gillett CE, Skilton D, Harris WH, et al. The biological and prognostic significance of cell polarity and E-cadherin in grade I infiltrating ductal carcinoma of the breast. The Journal of Pathology. 1999;189:20–27. doi: 10.1002/(SICI)1096-9896(199909)189:1<20::AID-PATH394>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tarin D. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Research. 2005;65:5996–6001. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepera SB, McCrea PD, Rosen JM. A beta-catenin survival signal is required for normal lobular development in the mammary gland. Journal of Cell Science. 2003;116:1137–1149. doi: 10.1242/jcs.00334. [DOI] [PubMed] [Google Scholar]
- Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze’ev A, Thiery JP, et al. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nature Reviews. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, et al. An alpha-E-catenin gene trap mutation defines its function in preimplantation development. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Research. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cellular and Molecular Life Sciences. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. Cellular pathology: As based upon physiological and pathological histology. In: Chance F, translator. Twenty lectures delivered in the Pathological institute of Berlin during the months of February, March and April, 1858. New York: R. M. De Witt; 1860. [Google Scholar]
- Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Vos CB, Cleton-Jansen AM, Berx G, de Leeuw WJ, ter Haar NT, van Roy F, et al. E-cadherin inactivation in lobular carcinoma in situ of the breast: An early event in tumorigenesis. British Journal of Cancer. 1997;76:1131–1133. doi: 10.1038/bjc.1997.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Research. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Research. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. Journal of Cell Science. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. The Journal of Cell Biology. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Developmental Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Yuan TC, Wang YP, Pao L, Anderson SM, Gu HH. Lactation defect in a widely used MMTV-Cre transgenic line of mice. PLoS One. 2011;6:e19233. doi: 10.1371/journal.pone.0019233. [DOI] [PMC free article] [PubMed] [Google Scholar]