Abstract
Gas permeability data are presented for mixed matrix membranes (MMMs) of few-layer graphene in the polymer of intrinsic microporosity PIM-1, and the results compared with previously reported data for two other nanofillers in PIM-1: multiwalled carbon nanotubes functionalized with poly(ethylene glycol) (f-MWCNTs) and fused silica. For few-layer graphene, a significant enhancement in permeability is observed at very low graphene content (0.05 vol.%), which may be attributed to the effect of the nanofiller on the packing of the polymer chains. At higher graphene content permeability decreases, as expected for the addition of an impermeable filler. Other nanofillers, reported in the literature, also give rise to enhancements in permeability, but at substantially higher loadings, the highest measured permeabilities being at 1 vol.% for f-MWCNTs and 24 vol.% for fused silica. These results are consistent with the hypothesis that packing of the polymer chains is influenced by the curvature of the nanofiller surface at the nanoscale, with an increasingly pronounced effect on moving from a more-or-less spherical nanoparticle morphology (fused silica) to a cylindrical morphology (f-MWCNT) to a planar morphology (graphene). While the permeability of a high-free-volume polymer such as PIM-1 decreases over time through physical ageing, for the PIM-1/graphene MMMs a significant permeability enhancement was retained after eight months storage.
Keywords: mixed matrix membranes, polymers of intrinsic microporosity, graphene, gas permeation
1. Introduction
Membranes are currently used for a variety of industrial gas separations, including nitrogen generation from air and the removal of CO2 from natural gas [1]. However, for many potential large-scale applications, such as the capture of CO2 from power station flue gases, there is a need for new materials that offer high permeability combined with good selectivity. High-free-volume polymers, such as polymers of intrinsic microporosity (PIMs), have attracted attention for their high gas permeabilities [2,3]. However, they are susceptible to physical ageing, which leads to a reduction in permeability over time [4,5]. The addition of an inorganic, metal-organic or organic filler to a polymer, to form a mixed matrix membrane (MMM) [6], can give synergistic improvements in the permeation properties, help to control ageing effects, and enhance the mechanical performance. Here, the effect of graphene on the gas permeability of PIM-1 (figure 1a) [7–9], the archetypal solution-processable PIM, is compared with data of Khan et al. [10,11] for multiwalled carbon nanotoubes functionalized with poly(ethylene glycol) (f-MWCNTs) and data of Ahn et al. [12] for a fused silica nanofiller.
Figure 1.
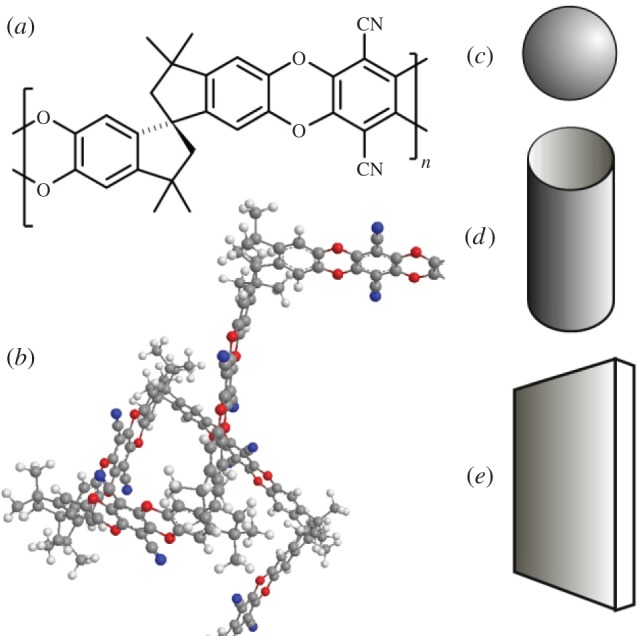
(a) Chemical structure of the polymer of intrinsic microporosity PIM-1. (b) Molecular model of a fragment of PIM-1 showing its contorted structure. Idealized nanofiller morphologies (c) spherical, (d) cylindrical and (e) planar. (Online version in colour.)
Simple models of MMMs predict that the addition of an impermeable nanofiller to a permeable matrix will reduce permeability compared with the unfilled material. For example, the Maxwell model [13] predicts that the permeability PMMM of a MMM containing a volume fraction ϕF of impermeable, spherical filler particles within a polymer of permeability PP, is given by equation (??).
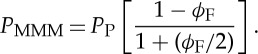 |
1.1 |
Pinnau & He [14] reported that the addition of non-porous fillers to glassy polymers can actually increase permeability. This counter-intuitive effect has been confirmed for non-porous inorganic fillers, such as fumed silica [12], MgO [15] and TiO2 [16], in various polymers.
The permeability of a glassy polymer is linked to the amount and distribution of free volume within the material. Glasses are in a non-equilibrium state, and many factors may influence the way the molecules pack together and, thus, the free-volume distribution. High-free-volume glassy polymers have high permeabilities as a result of frustrated packing of the constituent macromolecules. In a PIM, this is a consequence of the molecular structure of the polymer chain, which is composed of fused-ring sequences interrupted by sites of contortion such as spiro-centres (figure 1b). The randomly twisted shape makes it difficult for molecules to pack, and because there are no single bonds in the backbone about which rotation can occur, they cannot undergo the types of conformational change that enable conventional polymers to rearrange and fill space.
The presence of a nanofiller surface restricts the conformational freedom of polymer chains in its vicinity, which may further frustrate the ability of the chains to pack together, thus increasing free volume and enhancing permeability. It is a reasonable hypothesis that the effect of a nanofiller will depend not only on its available surface area but also on the curvature of the surface at the nanoscale. Here we compare an essentially planar filler (graphene), with a cylindrical nanofiller (f-MWCNTs), and a filler composed of more-or-less spherical nanoparticles (fumed silica) (figure 1c–e).
The term ‘graphene’ may be applied to a wide range of materials. A classification framework for graphene-based materials has been suggested [17] and a recommended nomenclature proposed [18]. The graphene family includes graphene itself (a single-atom-thick sheet), bilayer graphene, few-layer graphene, etc. Material with more than ca 10 regularly stacked layers is generally regarded as graphitic. For the present work, few-layer graphene was considered a suitable planar nanofiller for comparison with multiwalled carbon nanotubes.
2. Experimental
(a). Materials
Tetrafluoroterephthalonitrile (TFTPN, 98%, Aldrich) was purified by sublimation; it was heated to around 150°C and the pure product collected without vacuum. 5,5′,6,6′-Tetrahydroxy-3,3,3′,3′-tetramethyl-1,1′-spirobisindane (TTSBI, 98%, Alfa Aesar) was dissolved in methanol and re-precipitated from dichloromethane before use. Anhydrous K2CO3 (99.0%, Fisher) was dried in an oven at 110°C overnight before use. Anhydrous dimethylacetamide (DMAc), toluene and methanol (MeOH), were purchased from Sigma-Aldrich and used as received. Natural graphite was purchased from NGS Naturgraphite GmbH.
(b). Methods
Gel permeation chromatography (GPC) measurements were carried out using a Viscotek GPC max VE 2001 instrument with two PL mixed B columns and a refractive index detector. Tetrahydrofuran was used as solvent at a flow rate of 1 cm3 min−1 and the injection volume was 100 μl. Polystyrene standards of known molar mass were used for calibration.
1H nuclear magnetic resonance (NMR) spectroscopy was carried out using a Bruker 400 MHz spectrometer. For NMR sample preparation, PIM-1 (≈5 mg) was dissolved in deuterochloroform (CDCl3, Aldrich, 99.8% atom D) and transferred into a 5 mm NMR tube.
Ultraviolet–visible (UV–Vis) spectroscopy was carried out using a Cary 60 UV–Vis spectrophotometer at room temperature.
(c). Synthesis of PIM-1
PIM-1 was synthesized by a method based on that of Du et al. [19]. TFTPN (2.001 g, 0.01 mol), TTSBI (3.45 g, 0.01 mol), anhydrous K2CO3 (0.03 mol), DMAc (20 ml) and toluene (10 ml) were added to a round-bottomed flask equipped with a mechanical stirrer, nitrogen inlet and Dean–Stark trap. The mixture was refluxed at 160°C for 40 min, then the viscous solution was added to methanol to precipitate the product. The yellow product was dissolved in chloroform and reprecipitated from methanol. Further purification was carried out by refluxing the precipitate in deionized water overnight, then it was dried overnight in a vacuum oven at 110°C. GPC: Mn=43000, Mw=170000, Mw/Mn=4.0. 1H NMR (400 MHz; CDCl3): δ6.81 (br, s, 2H), 6.42 (br, s, 2H), 2.33 (br, s, 2H), 2.17 (br, s, 2H), 1.36 (br, s, 6H), 1.31 (br, s, 6H). IR (ATR; cm−1): 3000–2800, 2238, 1443, 1262, 1107, 1009, 751. Elemental analysis, calculated for C29H20N2O4 (wt.%): C, 75.64; H, 4.38; N, 6.08. Found: C, 71.99; H, 4.17; N, 6.02.
(d). Preparation of PIM-1/graphene solutions and membrane casting
A stock PIM-1/graphene dispersion was prepared as follows: natural graphite (600 mg) and PIM-1 (1.4 g) were added to chloroform (100 ml). The solution was sonicated for 84 h (Elmasonic P 70H, 220 W effective ultrasonic power, 37 kHz ultrasonic frequency) and then centrifuged twice at 6000 r.p.m. for 20 min to remove graphitic particles. Although prolonged sonication of a polymer solution might be expected to cause cleavage of polymer chains, control experiments with PIM-1 solutions showed little effect on number-average molar mass, Mn, and only a modest decrease in weight-average molar mass, Mw. To form the membrane with the highest graphene content (sample KA1-7-12(3)), a 5 ml portion of the stock solution was transferred into a 7.8 cm diameter flat-bottomed glass petri dish, and the solvent was allowed to evaporate over 3 days. For preparation of membranes with lower graphene contents, portions of the stock PIM-1/graphene solution were mixed with portions of a pure PIM-1 solution of concentration 35 mg ml−1. For samples KA-1-7(1), KA-1-7(2) and KA-1-7(3), 2 ml of stock solution were mixed with 2, 5 and 10 ml, respectively, of PIM-1 solution. For samples KA-1-7(5) and KA-1-7(6), 4 ml of stock solution were mixed with 20 and 40 ml, respectively, of PIM-1 solution. A 7.8 cm petri dish was used for samples KA-1-7(1) and KA-1-7(6), and a 6.8 cm dish for other samples.
(e). Determination of graphene content
For determination of the graphene content, membrane KA-1-7(5) (0.23 g) was redissolved in 23 ml of chloroform. The graphene concentration of the solution, CG, was determined by UV–Vis spectroscopy. The absorption was measured at 660 nm and CG evaluated using a value of α=3620 ml mg−1 m−1 for the absorption coefficient, as determined by the Coleman group [20,21]. This gave a graphene weight fraction of 0.0018. Hence, it was determined that the stock PIM-1/graphene solution contained 0.349 mg ml−1 graphene, as well as 14 mg ml−1 PIM-1. The graphene contents of membranes used for permeation measurements were then calculated from the known dilutions. For calculation of the percentage graphene by volume, the graphene was assumed to have the same density as graphite (2.2 g cm−3).
(f). Gas permeation measurements
Single gas permeation measurements were carried out in a fixed volume/pressure increase apparatus (GKSS, Germany) at 25°C at a feed pressure of 1 bar, as described previously [22,23].
3. Results and discussion
(a). PIM-1/graphene MMMs
Self-supported PIM-1/graphene MMMs were obtained by slow evaporation of the solvent from PIM-1/graphene dispersions in chloroform. A stock PIM-1/graphene dispersion was prepared by ultrasound-assisted exfoliation of natural graphite into a PIM-1 solution. Dilution of the stock dispersion with fresh PIM-1 solution enabled dispersions of various concentrations to be obtained, and hence MMMs with various graphene contents as indicated in table 1. While chloroform alone is not a particularly good solvent for graphene exfoliation, the PIM-1 acts to stabilize the dispersion through interactions with the graphene [24]. Raman spectroscopy [25–27] of flakes in a PIM-1/graphene MMM shows restacked few-layer graphene, and excludes the presence of species with graphitic AB stacking (see the electronic supplementary material).
Table 1.
Graphene content, thickness and gas permeabilities of PIM-1 and PIM-1/graphene membranes after methanol-treatment and after ageing for ca eight months.
| graphene content |
permeability (barrer) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| membrane | (wt/%) | (vol.%) | (days) | (μm) | CO2 | H2 | He | O2 | CH4 | N2 |
| PIM-1 | 0 | 0 | 0 | 59 | 5120 | 3210 | 1610 | 1130 | 340 | 270 |
| KA1-7(6) | 0.00096 | 0.046 | 0 | 352 | 12 700 | 4660 | 1770 | 2260 | 1450 | 870 |
| KA1-7(3) | 0.0018 | 0.088 | 0 | 100 | 9840 | 4730 | 1890 | 1850 | 800 | 570 |
| KA1-7(2) | 0.0034 | 0.164 | 0 | 52 | 7830 | 4470 | 1830 | 1560 | 550 | 410 |
| KA1-7(1) | 0.0071 | 0.338 | 0 | 24 | 3410 | 3860 | 1950 | 820 | 160 | 170 |
| KA1-7-12(3) | 0.0243 | 0.338 | 0 | 86 | 5150 | 3210 | 1390 | 1040 | 390 | 270 |
| PIM-1 | 0 | 0 | 244 | 57 | 3670 | 2720 | 1220 | 730 | 200 | 160 |
| KA1-7(6) | 0.00096 | 0.046 | 226 | 351 | 9240 | 3970 | 1550 | 1800 | 980 | 620 |
| KA1-7(3) | 0.0018 | 0.088 | 236 | 94 | 6660 | 3450 | 1420 | 1250 | 460 | 340 |
| KA1-7(2) | 0.0034 | 0.164 | 236 | 50 | 5680 | 3210 | 1350 | 1,070 | 330 | 260 |
Single gas permeabilities for PIM-1/graphene MMMs, and for the batch of PIM-1 used in this work, are included in table 1. The measurements are for membranes which have been soaked in methanol then dried. The methanol-treatment is used to remove residual solvents and to reverse effects of the previous membrane history. Single gas permeabilities decrease in the sequence CO2 > H2 > He ≈ O2 > CH4 > N2. In the simplest model of membrane permeation, the solution-diffusion model, permeability coefficient P is the product of a diffusion coefficient D and a solubility or sorption coefficient S (equation (3.1)).
| 3.1 |
The permeability sequence CO2 > H2 is the reverse of that observed for the majority of glassy polymers, and may be attributed to strong sorption enhancing CO2 permeability. It should be noted, however, that if the sorption term is too strong, diffusion may be restricted and the permeability decreased, as has been observed for amine-modified PIM-1 [23].
A significant enhancement in gas permeability is observed at low graphene content. This is discussed further and compared with the effect of other nanofillers below.
(b). Comparison of nanofillers
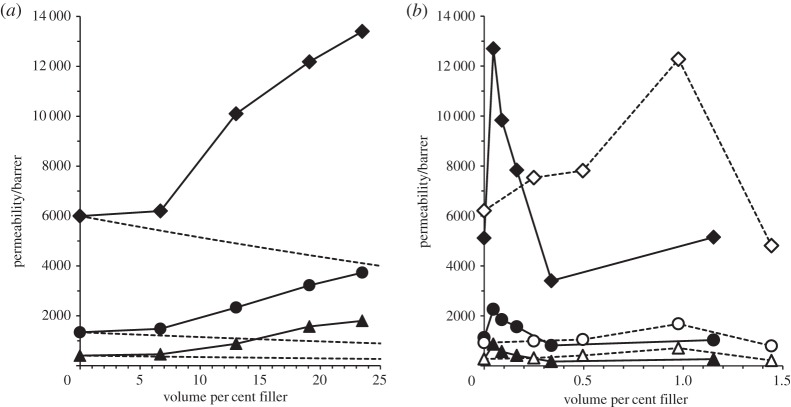
Data of Ahn et al. [12] for methanol-treated MMMs of hydrophobic fumed silica (Cabosil TS 530) in PIM-1 are compared with the Maxwell model (equation (??)) in figure 2a. It can be clearly seen that while the Maxwell model predicts a decrease in gas permeability, the experimental results show a significant increase in the effective permeability of the MMM with increasing filler loading over the range 7–24 vol.%. Filler loadings above 24 vol.% were not investigated; it is difficult to achieve homogeneous dispersions at higher loadings. The nanoparticles used in that study were reported to have particle diameters in the range 11.1–13.3 nm, although there was evidence of larger aggregates in the MMMs. Filler density and Brunauer–Emmett–Teller (BET) surface area were reported as 2.2 g cm−3 and 205−245 m2 g−1, respectively.
Figure 2.
Dependence of CO2 (diamonds), O2 (circles) and N2 (triangles) permeability on nanofiller loading for MMMs of PIM-1 with (a) fused silica [12] and (b) graphene (solid symbols) (this work) and f-MWCNTs (open symbols) [10]. The dashed lines in (a) indicate the prediction of the Maxwell model for an impermeable filler.
Permeability data from Khan et al. [10] for methanol-treated PIM-1/f-MWCNT MMMs are shown in figure 2b. The MWCNTs used for functionalization (FutureCarbon GmbH) were reported to have 8–12 walls and diameter in the range 12–15 nm. The BET surface area was 250 m2 g−1, similar to the fused silica described above. For the plot in figure 2b, a density of 2.1 g cm−3 was assumed in order to calculate vol.% filler; in practice, the density may vary depending on the diameter and number of walls [28]. It can be seen that gas permeabilities increase up to ca 1 vol.% filler, and then decrease.
Permeability data for methanol-treated PIM-1/graphene MMMs from the present work are included in figure 2b. For calculation of vol.% filler, the density of bulk graphite (2.2 g cm−3) was assumed. A similar pattern is seen with graphene as for f-MWCNTs, with an initial increase followed by a decrease with increasing filler content. While PIM-1 itself can exhibit substantial differences in permeability depending on sample history, it is noteworthy that broadly similar results were obtained by three different laboratories working with three different fillers, the CO2 permeability for methanol-treated PIM-1 being in the range 5000–6200 barrer, and the highest CO2 permeability achieved for these MMMs being about double, in the range 12 200–13 400 barrer. The significant difference between the fillers is the filler loading at which the greatest permeability enhancement is seen, which increases from ca 0.05 vol.% for few-layer graphene, to ca 1 vol.% for f-MWCNTs, to ca 24 vol.% for fused silica.
The maximum in permeability observed with increasing content of graphene or f-MWCNTs, and which would presumably also be seen if it were possible to push fused silica loadings to higher levels, may be attributed to competition between an enhancement in permeability through disruption of polymer chain packing, and a reduction in permeability due to the added bulk of the filler, perhaps coupled with the effects of aggregation of the filler at higher concentration. The differences in nanofiller loadings at which the maximum occurs may in part reflect differences in accessible surface area. However, for the MWCNT and fused silica nanofillers the surface areas appear to be similar, but there is a large difference in the nanofiller content at which the permeability enhancement is observed, suggesting that the morphology of the nanofiller plays an important role. For a single graphene sheet the theoretical surface area is 2630 m2 g−1 (1315 m2 g−1 per side [29]), but it will be much less for few-layer graphene in the MMMs. It, therefore, seems likely that the sheet-like morphology is at least partly responsible for the very low graphene content (0.05 vol.%) at which permeability enhancement is observed. These results are consistent with the hypothesis that on moving from a spherical, to a cylindrical, to a planar nanofiller, pronounced effects on polymer chain packing, and thus on permeability, may be achieved at increasingly lower nanofiller contents.
(c). Effect of ageing
Khan et al. [11] observed that thin-film composite membranes with a PIM-1/f-MWCNT active layer on a porous polyacrylonitrile support showed better long-term stability than membranes prepared with PIM-1 alone as the active layer. In this work, the gas permeabilities of self-supported PIM-1/graphene membranes were re-measured after ca eight months storage under ambient conditions (table 1). The changes in CO2 permeability for different graphene loadings are illustrated in figure 3. While a loss of permeability is observed over time in all cases, a substantial permeability enhancement is retained after eight months for the PIM-1/graphene MMMs, as compared to neat PIM-1.
Figure 3.
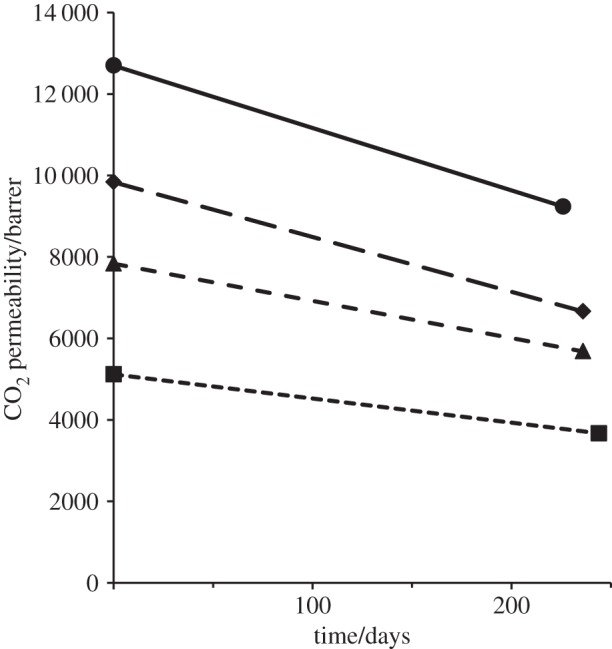
Effect of ageing for ca eight months on the CO2 permeability of PIM-1 (square) and PIM-1/graphene MMMs at nanofiller loadings of 0.046 vol.% (circle), 0.088 vol.% (diamond) and 0.164 vol.% (triangle). The lines are guides to the eye.
4. Conclusion
For this series of samples, the addition of just 0.05 vol.% few-layer graphene to PIM-1 gave a substantial enhancement in gas permeability; for CO2, it was more than twofold. Even after eight months of storage the permeability was substantially higher than that observed for the unfilled polymer. Other nanofillers exhibit similar permeability enhancements, but higher loadings are required. This is consistent with the hypothesis that packing of the polymer chains is influenced by the curvature of the nanofiller surface at the nanoscale, with an increasingly pronounced effect on moving from a more-or-less spherical nanoparticle morphology (fused silica) to a cylindrical morphology (f-MWCNT) to a planar morphology (graphene).
Supplementary Material
Authors' contributions
Polymer synthesis and membrane preparation was carried out in Manchester by K.A., with support from W.J.H., under the supervision of J.M.G. and P.M.B. Graphene characterization was carried out by Y.S., under the supervision of C.C. Gas permeation measurements were carried out in Italy by P.B. and G.C. and J.C.J. The article was drafted by P.M.B. and all other authors contributed to its revision.
Competing interests
We have no competing interests.
Funding
This research is funded by EPSRC grant no. EP/K016946/1 (graphene-based membranes) and the Italian national research program ‘Programma Operativo Nazionale Ricerca e Competitività 2007–2013, project PON01_01840 MicroPERLA’. K.A. is supported by the Deanship of Graduate Studies and Research, Taibah University, Saudi Arabia.
References
- 1.Bernardo P, Drioli E, Golemme G. 2009. Membrane gas separation: a review/state of the art. Ind. Eng. Chem. Res. 48, 4638–4663. ( 10.1021/ie8019032) [DOI] [Google Scholar]
- 2.Budd PM, McKeown NB. 2010. Highly permeable polymers for gas separation membranes. Polym. Chem. 1, 63–68. ( 10.1039/b9py00319c) [DOI] [Google Scholar]
- 3.McKeown NB, Budd PM. 2010. Exploitation of intrinsic microporosity in polymer-based materials. Macromolecules 43, 5163–5176. ( 10.1021/ma1006396) [DOI] [Google Scholar]
- 4.Harms S, Raetzke K, Faupel F, Chaukura N, Budd PM, Egger W, Ravelli L. 2012. Aging and free volume in a polymer of intrinsic microporosity (PIM-1). J. Adhes. 88, 608–619. ( 10.1080/00218464.2012.682902) [DOI] [Google Scholar]
- 5.Koschine T, Raetzke K, Faupel F, Khan MM, Emmler T, Filiz V, Abetz V, Ravelli L, Egger W. 2015. Correlation of gas permeation and free volume in new and used high free volume thin film composite membranes. J. Polym. Sci. B Polym. Phys. 53, 213–217. ( 10.1002/polb.23616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezakazemi M, Ebadi Amooghin A, Montazer-Rahmati MM, Ismail AF, Matsuura T. 2014. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): an overview on current status and future directions. Prog. Polym. Sci. 39, 817–861. ( 10.1016/j.progpolymsci.2014.01.003) [DOI] [Google Scholar]
- 7.Budd PM, Ghanem BS, Makhseed S, McKeown NB, Msayib KJ, Tattershall CE. 2004. Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials. Chem. Commun. 230–231. ( 10.1039/b311764b) [DOI] [PubMed] [Google Scholar]
- 8.Budd PM, Msayib KJ, Tattershall CE, Ghanem BS, Reynolds KJ, McKeown NB, Fritsch D. 2005. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 251, 263–269. ( 10.1016/j.memsci.2005.01.009) [DOI] [Google Scholar]
- 9.Budd PM. et al. 2008. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: polybenzodioxane PIM-1. J. Membr. Sci. 325, 851–860. ( 10.1016/j.memsci.2008.09.010) [DOI] [Google Scholar]
- 10.Khan MM, Filiz V, Bengtson G, Shishatskiy S, Rahman MM, Lillepaerg J, Abetz V. 2013. Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J. Membr. Sci. 436, 109–120. ( 10.1016/j.memsci.2013.02.032) [DOI] [Google Scholar]
- 11.Khan Muntazim M, Filiz V, Bengtson G, Shishatskiy S, Rahman M, Abetz V. 2012. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res. Lett. 7, 504 ( 10.1186/1556-276X-7-504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn J, Chung W-J, Pinnau I, Song J, Du N, Robertson GP, Guiver MD. 2010. Gas transport behavior of mixed-matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 346, 280–287. ( 10.1016/j.memsci.2009.09.047) [DOI] [Google Scholar]
- 13.Maxwell JC. 1873. Treatise on electricity and magnetism. London, UK: Oxford University Press. [Google Scholar]
- 14.Pinnau I, He Z. 2001. Filled superglassy membrane. US patent 6,316,684.
- 15.Matteucci S, Kusuma VA, Kelman SD, Freeman BD. 2008. Gas transport properties of MgO filled poly(1-trimethylsilyl-1-propyne) nanocomposites. Polymer 49, 1659–1675. ( 10.1016/j.polymer.2008.01.004) [DOI] [Google Scholar]
- 16.Matteucci S, Kusuma VA, Sanders D, Swinnea S, Freeman BD. 2008. Gas transport in TiO2 nanoparticle-filled poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 307, 196–217. ( 10.1016/j.memsci.2007.09.035) [DOI] [Google Scholar]
- 17.Wick P. et al. 2014. Classification framework for graphene-based materials. Angew. Chem. Int. Edn. 53, 7714–7718. ( 10.1002/anie.201403335) [DOI] [PubMed] [Google Scholar]
- 18.Bianco A. et al. 2013. All in the graphene family—a recommended nomenclature for two-dimensional carbon materials. Carbon 65, 1–6. ( 10.1016/j.carbon.2013.08.038). [DOI] [Google Scholar]
- 19.Du N, Robertson GP, Pinnau I, Thomas S, Guiver MD. 2009. Copolymers of intrinsic microporosity based on 2,2′,3,3′-tetrahydroxy-1,1′-dinaphthyl. Macromol. Rapid Commun. 30, 584–588. ( 10.1002/marc.200800795) [DOI] [PubMed] [Google Scholar]
- 20.Khan U, O’Neill A, Lotya M, De S, Coleman JN. 2010. High-concentration solvent exfoliation of graphene. Small 6, 864–871. ( 10.1002/smll.200902066) [DOI] [PubMed] [Google Scholar]
- 21.O’Neill A, Khan U, Nirmalraj PN, Boland J, Coleman JN. 2011. Graphene dispersion and exfoliation in low boiling point solvents. J. Phys. Chem. C 115, 5422–5428. ( 10.1021/jp110942e) [DOI] [Google Scholar]
- 22.Mason CR, Maynard-Atem L, Al-Harbi NM, Budd PM, Bernardo P, Bazzarelli F, Clarizia G, Jansen JC. 2011. Polymer of intrinsic microporosity incorporating thioamide functionality: preparation and gas transport properties. Macromolecules 44, 6471–6479. ( 10.1021/ma200918h) [DOI] [Google Scholar]
- 23.Mason CR. et al. 2014. Enhancement of CO2 affinity in a polymer of intrinsic microporosity by amine modification. Macromolecules 47, 1021–1029. ( 10.1021/ma401869p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonciaruk A, Althumayri K, Harrison WJ, Budd PM, Siperstein FR. 2015. PIM-1/graphene composite: a combined experimental and molecular simulation study. Micropor. Mesopor. Mater. 209, 126–134. ( 10.1016/j.micromeso.2014.07.007) [DOI] [Google Scholar]
- 25.Haar S. et al. 2015. A supramolecular strategy to leverage the liquid-phase exfoliation of graphene in the presence of surfactants: unraveling the role of the length of fatty acids. Small 11, 1691–1702. ( 10.1002/smll.201402745) [DOI] [PubMed] [Google Scholar]
- 26.Ciesielski A. et al. 2014. Harnessing the liquid-phase exfoliation of graphene using aliphatic compounds: a supramolecular approach. Angew. Chem. Int. Edn. 53, 10 355–10 361. ( 10.1002/anie.201402696) [DOI] [PubMed] [Google Scholar]
- 27.Ferrari AC. et al. 2006. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 97, 187401 ( 10.1103/PhysRevLett.97.187401) [DOI] [PubMed] [Google Scholar]
- 28.Laurent C, Flahaut E, Peigney A. 2010. The weight and density of carbon nanotubes versus the number of walls and diameter. Carbon 48, 2994–2996. ( 10.1016/j.carbon.2010.04.010) [DOI] [Google Scholar]
- 29.Peigney A, Laurent C, Flahaut E, Bacsa RR, Rousset A. 2001. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 39, 507–514. ( 10.1016/s0008-6223(00)00155-x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.