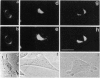
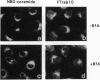
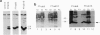Abstract
Small GTP-binding proteins of the YPT/SEC4/Rab family have been shown to play an essential role in intracellular membrane trafficking. In mammals, Rab8 and Rab10 are the two small GTP-binding proteins identified so far that are closest to SEC4, an essential gene product involved in post-Golgi constitutive secretion in the yeast Saccharomyces cerevisiae. To study the localization of Rab proteins, we have expressed the cDNAs with an influenza virus hemagglutinin (HA) epitope tag at the N terminus. The feasibility of this method was tested by using yeast SEC4. HA-tagged SEC4 functionally complemented a temperature-sensitive sec4 mutant similarly to wild-type SEC4, indicating that the modified protein retained functional integrity. Monoclonal antibody 12CA5, raised against the HA tag, was used to determine the expression and localization of HA-tagged proteins after transfection. In stably transfected CHO and Swiss 3T3 cells, HA-tagged Rab8 was localized to the cell periphery, with the highest concentration in the ruffling areas. In contrast, epitope-tagged Rab10 expressed in CHO and BHK cells was concentrated on membranes in the perinuclear region. By light microscopy, the staining partially overlapped with that of a Golgi marker, beta-COP. Thus, despite the high degree homology of Rab8 and Rab10 (66% identity), the two proteins are localized to distinct cellular compartments. This approach should provide a general tool for the analyses of other members of the YPT/SEC4/rab gene family.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini G., Hohl T., Lin H. Y., Lodish H. F. Cloning of a Rab3 isotype predominantly expressed in adipocytes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5049–5052. doi: 10.1073/pnas.89.11.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D., Suhan J. P., McCormick F., Feramisco J. R. Localization of phospholipase A2 in normal and ras-transformed cells. J Cell Biol. 1988 May;106(5):1649–1658. doi: 10.1083/jcb.106.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Kupfer A., Singer S. J. Membrane insertion at the leading edge of motile fibroblasts. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1367–1371. doi: 10.1073/pnas.80.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Distribution of receptors for transferrin and low density lipoprotein on the surface of giant HeLa cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):454–458. doi: 10.1073/pnas.80.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989 May;8(5):1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Gorvel J. P., Stelzer E., Simons K., Gruenberg J., Zerial M. Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature. 1991 Oct 24;353(6346):769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Simons K., Zerial M. The complexity of the Rab and Rho GTP-binding protein subfamilies revealed by a PCR cloning approach. Gene. 1992 Mar 15;112(2):261–264. doi: 10.1016/0378-1119(92)90387-5. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Vingron M., Sander C., Simons K., Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990 Dec;10(12):6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Bloom G. S., Kreis T. E., Klausner R. D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990 Dec;111(6 Pt 1):2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J. P., Fournier S., Parulekar M., Trifaró J. M. Detection of low molecular mass GTP-binding proteins in chromaffin granules and other subcellular fractions of chromaffin cells. FEBS Lett. 1989 Apr 10;247(1):127–131. doi: 10.1016/0014-5793(89)81254-2. [DOI] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Anzai K., Scheller R. H. rab15, a novel low molecular weight GTP-binding protein specifically expressed in rat brain. J Biol Chem. 1992 Mar 25;267(9):5768–5775. [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech M., John J., Pizon V., Chardin P., Tavitian A., Clark R., McCormick F., Wittinghofer A. Inhibition of GTPase activating protein stimulation of Ras-p21 GTPase by the Krev-1 gene product. Science. 1990 Jul 13;249(4965):169–171. doi: 10.1126/science.2164710. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990 Jun 7;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Haubruck H., Engelke U., Mertins P., Gallwitz D. Structural and functional analysis of ypt2, an essential ras-related gene in the fission yeast Schizosaccharomyces pombe encoding a Sec4 protein homologue. EMBO J. 1990 Jun;9(6):1957–1962. doi: 10.1002/j.1460-2075.1990.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella B. T., Maltese W. A. rab GTP-binding proteins with three different carboxyl-terminal cysteine motifs are modified in vivo by 20-carbon isoprenoids. J Biol Chem. 1992 Feb 25;267(6):3940–3945. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Glickman J., Donaldson J. G., Robbins J., Kreis T. E., Seamon K. B., Sheetz M. P., Klausner R. D. Forskolin inhibits and reverses the effects of brefeldin A on Golgi morphology by a cAMP-independent mechanism. J Cell Biol. 1991 Feb;112(4):567–577. doi: 10.1083/jcb.112.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS P. I. Dynamics of surface modification in myxovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1962;27:351–365. doi: 10.1101/sqb.1962.027.001.033. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P. A., Südhof T. C., Jahn R., De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991 Nov;115(3):625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Carnell L., Moore H. H. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol. 1992 Jul;118(2):267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar C. M., Prange R., Gallwitz D. A carboxyl-terminal cysteine residue is required for palmitic acid binding and biological activity of the ras-related yeast YPT1 protein. EMBO J. 1988 Apr;7(4):971–976. doi: 10.1002/j.1460-2075.1988.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrdal S. E., Auersperg N. p21ras. Heterogeneous localization in transformed cells. Exp Cell Res. 1985 Aug;159(2):441–450. doi: 10.1016/s0014-4827(85)80017-3. [DOI] [PubMed] [Google Scholar]
- Nagata K., Satoh T., Itoh H., Kozasa T., Okano Y., Doi T., Kaziro Y., Nozawa Y. The ram: a novel low molecular weight GTP-binding protein cDNA from a rat megakaryocyte library. FEBS Lett. 1990 Nov 26;275(1-2):29–32. doi: 10.1016/0014-5793(90)81431-m. [DOI] [PubMed] [Google Scholar]
- Ngsee J. K., Elferink L. A., Scheller R. H. A family of ras-like GTP-binding proteins expressed in electromotor neurons. J Biol Chem. 1991 Feb 5;266(4):2675–2680. [PubMed] [Google Scholar]
- Nimmo E. R., Sanders P. G., Padua R. A., Hughes D., Williamson R., Johnson K. J. The MEL gene: a new member of the RAB/YPT class of RAS-related genes. Oncogene. 1991 Aug;6(8):1347–1351. [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Sepanski M. A., Martin O. C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989 Nov;109(5):2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B. Small GTP-binding proteins associated with secretory vesicles of Paramecium. J Protozool. 1991 Sep-Oct;38(5):495–501. doi: 10.1111/j.1550-7408.1991.tb04823.x. [DOI] [PubMed] [Google Scholar]
- Philips M. R., Abramson S. B., Kolasinski S. L., Haines K. A., Weissmann G., Rosenfeld M. G. Low molecular weight GTP-binding proteins in human neutrophil granule membranes. J Biol Chem. 1991 Jan 15;266(2):1289–1298. [PubMed] [Google Scholar]
- Powell S. K., Orci L., Craik C. S., Moore H. P. Efficient targeting to storage granules of human proinsulins with altered propeptide domain. J Cell Biol. 1988 Jun;106(6):1843–1851. doi: 10.1083/jcb.106.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992 Jan;116(1):85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rubins J. B., Panchenko M., Shannon T. M., Dickey B. F. Identification of ras and ras-related low-molecular-mass GTP-binding proteins associated with rat lung lamellar bodies. Am J Respir Cell Mol Biol. 1992 Mar;6(3):253–259. doi: 10.1165/ajrcmb/6.3.253. [DOI] [PubMed] [Google Scholar]
- Sakurada K., Uchida K., Yamaguchi K., Aisaka K., Ito S., Ohmori T., Takeyama Y., Ueda T., Hori Y., Ohyanagi H. Molecular cloning and characterization of a ras p21-like GTP-binding protein (24KG) from rat liver. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1224–1232. doi: 10.1016/0006-291x(91)90672-t. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielh E., Touchot N., Zahraoui A., Tavitian A. Nucleotide sequence of a rat cDNA: rab1B, encoding a rab1-YPT related protein. Nucleic Acids Res. 1989 Feb 25;17(4):1770–1770. doi: 10.1093/nar/17.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N. C., Goud B., Kabcenell A. K., Novick P. J. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989 Jun;8(6):1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. Complete coding sequences of the ras related rab 3 and 4 cDNAs. Nucleic Acids Res. 1988 Feb 11;16(3):1204–1204. doi: 10.1093/nar/16.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989 Jul 25;264(21):12394–12401. [PubMed] [Google Scholar]
- van der Meulen J., Bhullar R. P., Chancellor-Maddison K. A. Association of a 24-kDa GTP-binding protein, Gn24, with human platelet alpha-granule membranes. FEBS Lett. 1991 Oct 7;291(1):122–126. doi: 10.1016/0014-5793(91)81118-r. [DOI] [PubMed] [Google Scholar]