Abstract
Background
The recently published SSO–ASTRO consensus guideline on margins concluded “no ink on tumor” is the standard for an adequate margin. This study was conducted to determine how this guideline is aligned with current clinical practice.
Methods
A survey was sent to 3057 members of the American Society of Breast Surgeons. Questions assessed respondents’ clinical practice type and duration, familiarity with the guideline, and preferences for margin re-excision.
Results
Of those surveyed, 777 (25 %) responded. Most (92 %) indicated familiarity with the guideline. Of these respondents, the majority (n = 678, or 94.7 %) would re-excise all or most of the time when tumor extended to the inked margin. Very few (n = 9, or 1.3 %) would re-excise all or most of the time when tumor was within 2 mm of the margin. Over 12 % (n = 90) would re-excise all or most of the time for a triple-negative tumor within 1 mm of the margin, whereas 353 (49.6 %) would re-excise all or most of the time when imaging and pathology were discordant, and tumor was within 1 mm of multiple margins. Finally, 330 (45.8 %) would re-excise all or most of the time when multiple foci of ductal carcinoma in situ extended to within 1 mm of multiple inked margins.
Conclusions
Surgeons are in agreement to re-excise margins when tumor touches ink and generally not to perform re-excisions when tumor is close to (but not touching) the inked margin. For more complex scenarios, surgeons are utilizing their individual clinical judgment to determine the need for re-excision.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 trial and five other large, randomized trials demonstrated that overall survival is equivalent in patients with early-stage breast cancer undergoing mastectomy or breast-conserving therapy (BCT).1-6 Each trial defined BCT as excision of the primary tumor with a margin of normal breast tissue followed by whole-breast irradiation (WBI). However, width of the margin of normal breast tissue required at excision varied across trials. While the NSABP B-06 trial defined a negative margin as “no tumor on ink” the Milan trials required quadrantectomy with excision of a 2- to 3-cm margin of grossly normal tissue as well as overlying skin and underlying fascia.1,2 BCT requires tumor excision with negative margins to reduce risk of ipsilateral breast tumor recurrence (IBTR). However, the width of normal tissue required for a negative margin to be deemed adequate has varied widely in clinical practice.7,8 In nearly half of patients who return to the operating room for margin re-excision, the reason is to achieve a wider margin of normal breast tissue.9 Returning to the operating room for re-excision of margins has been associated with increased surgical complications, increased stress and anxiety for patients and their families, increased healthcare costs, and even an increased rate of contralateral prophylactic mastectomy.10-12
Recently, the Society of Surgical Oncology (SSO) and the American Society for Radiation Oncology (ASTRO)published a consensus guideline on margins for breast-conserving surgery (BCS) with WBI in stage I and II invasive breast cancer.13 This was done in collaboration with the American Society of Breast Surgeons (ASBrS), American Society of Clinical Oncology (ASCO), College of American Pathologists, a patient advocate and funding from Susan G. Komen, and Dr. Houssami from the School of Public Health in Sydney, Australia. The SSO and ASTRO convened a multidisciplinary panel to address the question, “What margin width minimizes the risk of IBTR in patients with invasive cancer receiving WBI?” This guideline was developed based on results from a meta-analysis performed by Houssami et al. that included more than 28,000 patients from 33 studies.14 Data for patients who received neoadjuvant chemotherapy, patients who did not undergo radiation therapy, and patients for whom radiation other than WBI was planned were excluded from the analysis. This multidisciplinary panel concluded that the standard for an adequate margin in patients undergoing BCS with WBI should be “no ink on tumor.”
In response to publication of this guideline, some have advocated for a multidisciplinary evaluation of each case to determine the adequacy of margin width on the basis of clinical, pathologic, and treatment variables.15 However, little is known about national practice patterns since publication of the SSO–ASTRO guideline on margins. The current study was conducted to evaluate current practice patterns among breast surgeons since publication of the guideline.
METHODS
The current study was reviewed by the ASBrS Research Committee and approved by the ASBrS Board of Directors. A link to an 8-question survey (Fig. 1) was sent electronically to all ASBrS members. Questions assessed respondents’ clinical practice type and duration as well as familiarity with the guideline published in May of 2014. For respondents familiar with the guideline, preferences for re-excision of margins according to pathologic margin width and other factors were assessed for five different clinical scenarios, all involving patients planned for BCS with WBI. A request to participate in the survey was sent on October 14, 2014, and a reminder was sent on October 21, 2014. The survey was closed on October 30, 2014. Statistical analyses were performed using Chi squared and Fisher’s exact tests (Stata v13.1, College Station, TX).
FIG. 1.
Survey instrument
RESULTS
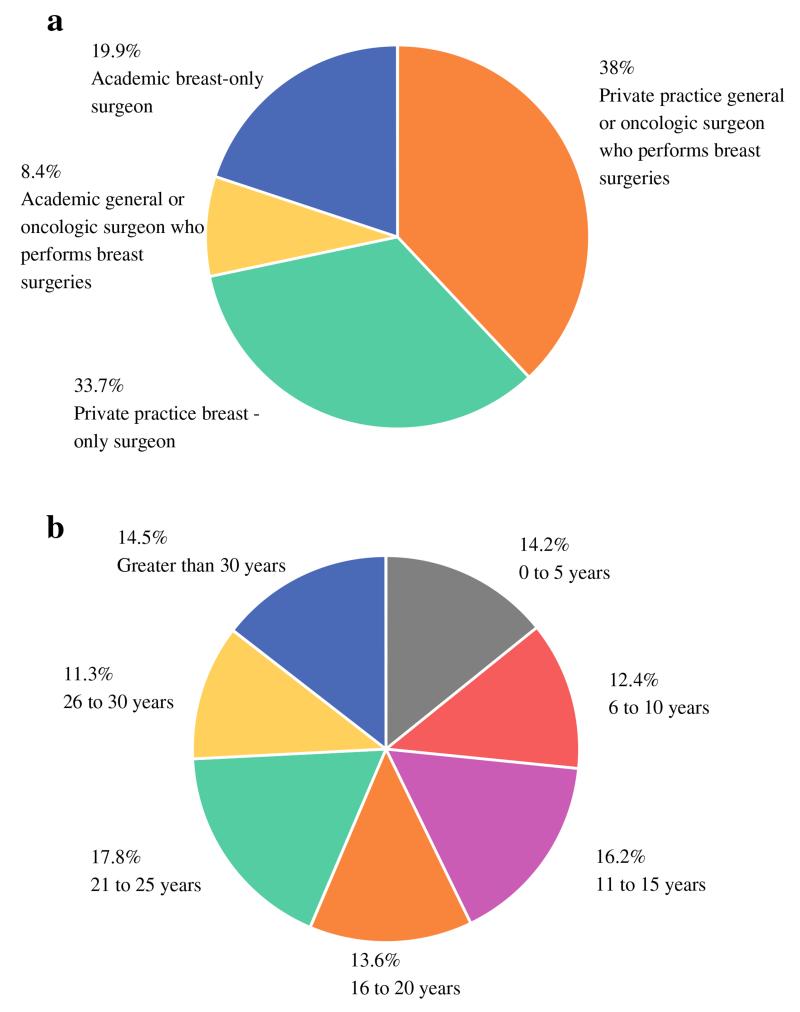
Of the 3057 ASBrS members invited to participate, 777 (25 %) responded to the survey. Of these respondents, 557 (71.7 %) were in private practice and 220 (28.3 %) were in an academic practice setting. A total of 360 respondents (46.4 %) were general or oncologic surgeons who perform breast surgery as part of their practice, and 417 (53.6 %) were surgeons focusing only on breast diseases (Fig. 2a). One hundred ten respondents (14.2 %) had been in practice for 5 years or less, 96 (12.4 %) for 6–10 years, 126 (16.2 %) for 11–15 years, 106 (13.6 %) for 16–20 years, 138 (17.8 %) for 21–25 years, 88 (11.3 %) for 26–30 years, and 113 (14.5 %) for more than 30 years (Fig. 2b). The majority of respondents (714/777, 91.9 %) indicated familiarity with the recently published consensus guideline.
FIG. 2.
a Practice type of survey respondents. b Practice duration of respondents
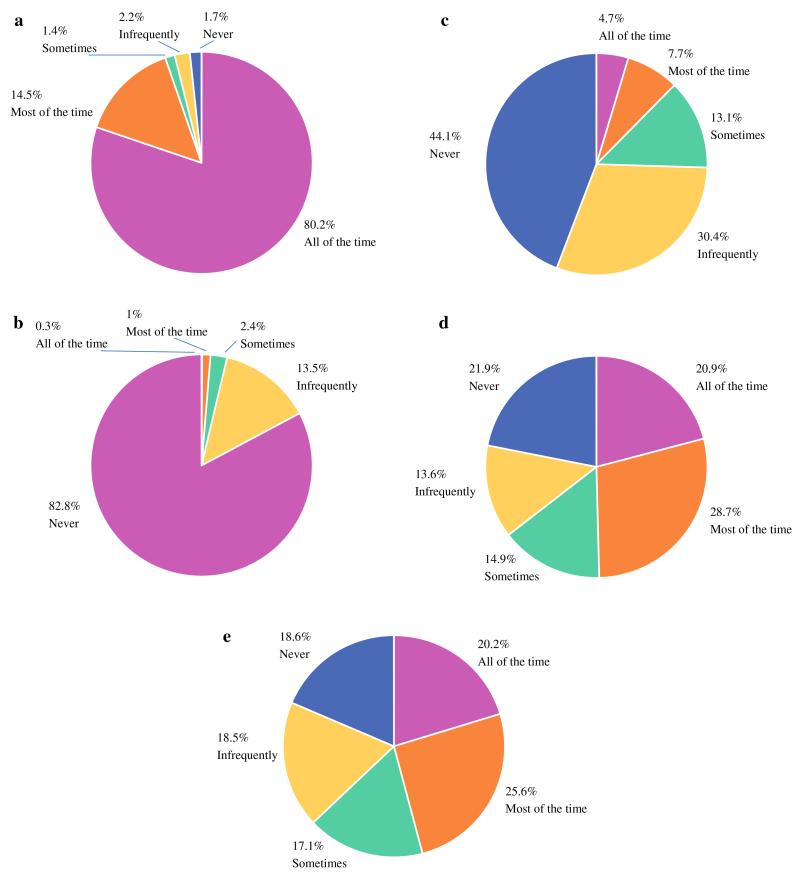
The case presented in scenario 1 was a woman with a 1.2-cm lesion with a small satellite lesion whose surgical pathology evaluation showed a 1.4-cm, high-grade invasive ductal carcinoma positive for estrogen receptor (ER) and progesterone receptor (PR), negative for HER2, with invasive tumor extending to the inked superior margin. In this scenario, 678 respondents (94.7 %) familiar with the guideline indicated they would perform re-excision most or all of the time, whereas 38 (5.3 %) indicated they would re-excise some of the time, infrequently, or never (Fig. 3a).
FIG. 3.
Surgeon preference for re-excision of margins. (a) In a patient with tumor extending to the inked margin; (b) in a patient with a favorable tumor subtype with tumor within 2 mm of the inked margin; (c) in a patient with triple negative subtype with tumor within 1 mm of the inked margin; (d) in a patient whose tumor was markedly larger on pathology than on preoperative imaging with multiple close margins; (e) in a patient with extensive ductal carcinoma in situ (DCIS) with multiple foci of DCIS extending to 1 mm of the inked margins and with ducts with cautery artifact at the margin
The case presented in scenario 2 was a woman with an 8-mm unifocal lesion on imaging whose surgical pathology evaluation showed an 8-mm, low-grade invasive ductal carcinoma positive for ER and PR, negative for HER2, with invasive tumor within 2 mm of the inked margin. In this scenario, only 9 respondents (1.3 %) indicated they would perform re-excision most or all of the time, 17 (2.4 %) indicated they would re-excise some of the time, and 691 (96.3 %) indicated they would perform re-excision infrequently or never (Fig. 3b).
The case in scenario 3 was a woman with a 2.3-cm lesion whose surgical pathology evaluation showed a 2.5-cm, high-grade, triple-negative, invasive ductal carcinoma with a single focus of invasive tumor within 1 mm of the inked margin. In this scenario, 90 respondents (12.4 %) indicated they would perform re-excision all or most of the time, 95 (13.1 %) indicated they would re-excise some of the time, and 540 (74.5 %) indicated they would perform re-excision infrequently or never (Fig. 3c).
In scenario 4 was a woman with a 2.4-cm unifocal lesion on imaging whose surgical pathology evaluation showed a 7-cm invasive lobular carcinoma positive for ER and PR, negative for HER2, with invasive tumor within 1 mm of the inferior, anterior, and superior margins. In this scenario, 353 respondents (49.6 %) indicated they would perform re-excision all or most of the time, 106 (14.9 %) indicated they would re-excise some of the time, and 253 (35.5 %) indicated they would perform re-excision infrequently or never (Fig. 3d).
The case in scenario 5 was a woman with a 1.6-cm unifocal lesion whose surgical pathology evaluation showed a 1.9-cm invasive ductal carcinoma positive for ER, negative for PR, and positive for HER2 with extensive DCIS, multiple foci of invasive carcinoma within 2 mm of the inked margin, a focus of DCIS extending to 1 mm of the inked margin, and ducts with cautery artifact at the inked margin. In this scenario, 330 respondents (45.8 %) indicated they would perform re-excision all or most of the time, 123 (17.1 %) indicated they would re-excise some of the time, and 267 (37.1 %) indicated they would perform re-excision infrequently or never (Fig. 3e).
Comparison by practice type revealed that surgeons in an academic practice were only slightly more likely to be familiar with the recently published guideline than surgeons in private practice (95 vs. 90.7 %, P = 0.046). Breast-only surgeons were more likely to be familiar with the guideline than general or oncologic surgeons who perform breast surgery (97.4 vs. 85.6 %, P < 0.001). However, responses to the five clinical scenarios did not differ by practice type. Analysis of responses by clinical practice duration showed that surgeons in practice for more than 25 years were more likely than surgeons with 25 or fewer years in practice to indicate they would perform margin re-excision for the patient with triple-negative breast cancer and a close margin (scenario 3) (P = 0.016).
DISCUSSION
Results of this survey of ASBrS members showed the vast majority of respondents were familiar with the SSO–ASTRO consensus guideline on margins for BCS with WBI in patients with stage I and II invasive breast cancer, and most would appropriately re-excise margins when there was ink on tumor. Furthermore, most responded they would not re-excise margins when tumor was close to (but not touching) the inked margin, in agreement with the guideline. However, for more complex scenarios, respondents utilized their individual clinical judgment to determine whether re-excision was needed.
These data suggest a change in perceptions on the definitions of an adequate margin from prior to publication of the SSO–ASTRO guideline on margins. For example, in 2005 Taghian and colleagues published results of a survey of radiation oncologists revealing that nearly 46 % of North American radiation oncologists and 27.6 % of European radiation oncologists considered “no tumor cells on the ink” an adequate margin, whereas others required margins of 1 mm (7.4 % of North American and 11.2 % of European radiation oncologists) or 2 mm (21.8 % of North American and 8.8 % of European radiation oncologists).7 More recently, Azu et al. utilized the Los Angeles and Detroit Surveillance, Epidemiology, and End Results registries to demonstrate that wide variations existed amongst surgeons with respect to consideration of an adequate margin width.8 These authors found that 11 % of surgeons used a standard of “tumor not touching ink,” whereas 42 % used a margin width of 1–2 mm, 28 % used a width of C5 mm, and 19 % used a width of [1 cm. These marked variations in clinical practice prior to publication of the SSO-ASTRO guideline prompted us to question if surgical practice has changed since the guideline was published.
In the current study, undertaken 5 months following publication of the guideline, surgeons were in agreement about the need to return to the operating room for re-excision of margins when tumor was at the inked margin. This is not surprising, because data suggest that when tumor is present at the inked margin (positive margin), patients have a greater than two times increase of IBTR compared with patients with close or negative margins.13,16,17 Furthermore, this increased recurrence risk with positive margins is not reversed (or negated) by the addition of a radiation boost or endocrine therapy.13,14,18 Thus, it is not surprising that surgeons stated they would perform re-excision when there was ink on tumor (positive margin).
Surgeons in our current study were also in agreement about the lack of necessity of re-excision when a unifocal invasive tumor with favorable biological features was close to the margin but had no ink on tumor. In our survey, only 9 respondents (1.3 %) indicated they would perform re-excision all of the time or most of the time in this scenario. This finding suggests a marked shift in alignment of the definition of a negative margin from that described in the above-aforementioned studies undertaken prior to publication of the guideline.7,8
Our survey revealed less consensus among respondents regarding need for re-excision in a patient with a unifocal tumor of the more aggressive triple-negative subtype close to the margin. In this scenario, 90 surgeons (12.4 %) indicated they would return to the operating room for re-excision all of the time or most of the time. Although themeta-analysis on which the SSO–ASTRO guideline was based produced no clear evidence that unfavorable biology was mitigated by wider negative margins, our survey indicates that surgeons continue to consider tumor subtype in decision making regarding the need to perform re-excision.13
There was much less agreement among respondents regarding need for re-excision in more complex scenarios, including the scenario of a patient with significant discordance between tumor size on preoperative imaging and pathologic size and tumor close to multiple margins (scenario 4) and the scenario of a woman with extensive DCIS with multiple foci of DCIS extending to within 1 mm of the margins and ducts with cautery artifact at the margin (scenario 5). It is not surprising that surgeon practice would vary widely in these complex scenarios. Patients whose tumors have an extensive intraductal component have been found to have a higher likelihood of significant residual DCIS in re-excised breast tissue and a higher likelihood of additional foci of DCIS located 2 cm or more from the index cancer.19,20 Although the consensus panel that developed the SSO–ASTRO guideline did not support consistently requiring a margin greater than no ink on tumor for patients with extensive intraductal component, the panel did recommend consideration of postoperative mammography in such patients to assess for residual calcifications.13 The panel did note that patients with an extensive intraductal component may be selected for re-excision based on high-risk features, including young age and multiple close margins, consistent with many responses from this survey. However, the consensus panel was convened to specifically focus on margins for patients with invasive cancer receiving WBI. Another panel has been convened to provide a guideline for DCIS.
The endorsement of the SSO–ASTRO guideline on margins by ASCO emphasized the importance of performing postoperative mammography to assess for microcalcifications and called for flexibility in application of the guideline based on clinical judgment.21 In addition, Hunt and colleagues emphasized that each case should be evaluated by the multidisciplinary team including the surgeon, pathologist, breast imager, and radiation oncologist to ensure that clinical, pathologic, and treatment variables are considered in determining adequate margin width.15 Our study confirms that clinicians are employing clinical judgment to determine need for re-excision in more complex scenarios.
Our study has several potential limitations. First, our survey was brief and the response rate was low (<30 %). The survey was intentionally meant to be brief to maximize likelihood that busy surgeons targeted for participation would respond. It is possible that the response rate may have been higher if the survey was kept open for longer. Second, the scenarios presented were simple. Patient age, option to perform postoperative mammography, and options other than re-excision (i.e., mastectomy) were not included to save time needed to complete the survey. These details and options also were omitted because our goal was to obtain overall data on national surgical practice patterns for margin re-excision following publication of the guideline. Third, there is potential for bias in that there is no way to know if those who did not respond were a group unfamiliar with the recently published guideline or a group choosing not to follow the guideline. Lastly, though we did not include questions about practice patterns prior to publication of the guideline to ascertain whether actual changes were put into practice following publication of the guideline, historic data indicate that there has been a marked change in the alignment of the definition of margins and need for re-excision.7,8
CONCLUSIONS
Our study documents that the majority of surgeons who responded were familiar with the recently published SSO–ASTRO guideline on margins, and most of these surgeons would re-excise margins when there is ink on tumor (positive margin). Most would not re-excise margins when ink did not touch the tumor, in agreement with the SSO–ASTRO guideline. However, for more complex scenarios, surgeons utilized their individual clinical judgment to determine whether re-excision was needed.
ACKNOWLEDGMENT
The authors thank Lisheng Yi and Bryan Fellman for help with statistical analyses, Stephanie Deming for editorial assistance, and Antoinette Smithson for help with preparation of this manuscript.
Footnotes
DISCLOSURE Suzanne Klimberg, Ascendant Diagnostics, Medical Director, Stock Ownership
REFERENCES
- 1.Fisher B, Rn R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320(13):822–8. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year followup of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Blichert-Toft M, Nielsen M, During M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47(4):672–81. doi: 10.1080/02841860801971439. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332(14):907–11. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 5.Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol. 1989;14(3):177–184. doi: 10.1016/0167-8140(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 6.van Dongen JA, Bartelink H, Fentiman IS, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Instit Monographs. 1992;(11):15–18. [PubMed] [Google Scholar]
- 7.Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241(4):629–39. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azu M, Abrahamse P, Katz SJ, Jagsi R, Morrow M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010;17(2):558–63. doi: 10.1245/s10434-009-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467–75. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 10.Olsen MA, Nickel KB, Margenthaler JA, et al. Increased risk of surgical site infection among breast-conserving surgery re-excisions. Ann Surg Oncol. 2015;22(6):2003–9. doi: 10.1245/s10434-014-4200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenup RA, Peppercorn J, Worni M, Hwang ES. Cost implications of the SSO-ASTRO consensus guideline on margins for breast-conserving surgery with whole breast irradiation in stage I and II invasive breast cancer. Ann Surg Oncol. 2014;21(5):1512–4. doi: 10.1245/s10434-014-3605-x. [DOI] [PubMed] [Google Scholar]
- 12.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–64. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 13.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507–15. doi: 10.1200/JCO.2013.53.3935. [DOI] [PubMed] [Google Scholar]
- 14.Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol. 2014;21(3):717–30. doi: 10.1245/s10434-014-3480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt KK, Smith BD, Mittendorf EA. The controversy regarding margin width in breast cancer: enough is enough. Ann Surg Oncol. 2014;21(3):701–3. doi: 10.1245/s10434-014-3497-9. [DOI] [PubMed] [Google Scholar]
- 16.Wazer DE, Schmidt-Ullrich RK, Ruthazer R, et al. Factors determining outcome for breast-conserving irradiation with margin-directed dose escalation to the tumor bed. Int J Radiat Oncol Biol Phys. 1998;40(4):851–8. doi: 10.1016/s0360-3016(97)00861-4. [DOI] [PubMed] [Google Scholar]
- 17.Mansfield CM, Komarnicky LT, Schwartz GF, et al. Ten-year results in 1070 patients with stages I and II breast cancer treated by conservative surgery and radiation therapy. Cancer. 1995;75(9):2328–36. doi: 10.1002/1097-0142(19950501)75:9<2328::aid-cncr2820750923>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Jones HA, Antonini N, Hart AA, et al. Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol. 2009;27(30):4939–47. doi: 10.1200/JCO.2008.21.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnitt SJ, Connolly JL, Khettry U, et al. Pathologic findings on re-excision of the primary site in breast cancer patients considered for treatment by primary radiation therapy. Cancer. 1987;59(4):675–81. doi: 10.1002/1097-0142(19870215)59:4<675::aid-cncr2820590402>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol. 1990;8(1):113–8. doi: 10.1200/JCO.1990.8.1.113. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz TA, Somerfield MR, Griggs JJ, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32(14):1502–6. doi: 10.1200/JCO.2014.55.1572. [DOI] [PubMed] [Google Scholar]