Abstract
Erectile dysfunction (ED) is a common disorder that affects a quarter of US men, and has many causes, including endothelial impairment, low testosterone levels, prior surgical manipulation, and/or psychogenic components. Penile erection is a complex process requiring neurally mediated relaxation of arteriolar smooth muscle and engorgement of cavernosal tissues, mediated by nitric oxide (NO). Current medical therapies for ED largely seek to maximize endogenous NO signalling. Certain aetiologies, including diabetes, are difficult to treat with current modalities, emphasizing the need for new molecular targets. Research has demonstrated the importance of RhoA–Rho-associated protein kinase (ROCK) signalling in maintaining a flaccid penile state, and inhibition of RhoA–ROCK signalling potentiates smooth-muscle relaxation in an NO-independent manner. The mechanisms and effects of RhoA–ROCK signalling and inhibition suggest that the RhoA–ROCK pathway could prove to be a new therapeutic target for the treatment of ED.
Introduction
Erectile dysfunction (ED) is a common disorder with an estimated prevalence of 24% in men >40 years of age, according to the 2011 National Health and Wellness Survey.1 The aetiology of ED is multifactorial, and commonly includes endothelial impairment, structural alteration to the penile vasculature, low testosterone levels, prior surgical manipulation and/or psychogenic components.2,3 The sensitivity of erectile tissues and the erectile response to systemic disease is underscored by the use of ED onset as an early indicator of cardiovascular disease and diabetes mellitus.4,5 Erection is a complex neurovascular process involving sympathetic and parasympathetic signalling via the major pelvic ganglion and neurovascular bundles, as well as somatic signalling via the pudendal nerves.2,6 This signalling results in relaxation of tonically contracted smooth muscle in cavernosal sinusoids and arterioles, increasing blood inflow and causing venous occlusion, and subsequent penile engorgement.
Relaxation of erectile tissues
The neurotransmitter nitric oxide (NO) and the three nitric oxide synthase (NOS) isoforms have principal roles in mediating penile erection.7–9 The constitutively active NOS isoforms neuronal NOS (nNOS) and endothelial NOS (eNOS) are found in neurons and endothelial cells of the major pelvic ganglion, autonomic and somatic neurons and the endothelium of the erectile tissues. Additionally, an inducible form, iNOS, is upregulated in response to certain stimuli, including cytokines and inflammatory pathway activation.9 Once synthesized, NO diffuses out of its tissues of origin into smooth-muscle cells, where it binds to and stimulates guanylyl cyclase, which increases cGMP. cGMP activates protein kinase G (PKG), which phosphorylates several intracellular proteins and ion transporters, resulting in hyperpolarization and decreased cytoplasmic Ca2+, causing smooth-muscle relaxation.10–13 eNOS activity is enhanced by Ser1177 phosphorylation by protein kinase B (PKB, also known as Akt), increasing NO production.14 Conversely, phosphodiesterases (PDEs) catalyse hydrolysis of cGMP, causing cytoplasmic Ca2+ accumulation, smooth-muscle contraction, and detumescence.15 PDE type 5 (PDE5) is the most active of the thirteen PDEs found in cavernosal tissue, leading to its pharmacological targeting via PDE5 inhibitors, which have become the mainstay therapies for medical management of ED.15 However, even as the importance of NO-mediated erection was being discovered, studies using Nω-nitro-L-arginine methyl ester (L-NAME), an NOS inhibitor, demonstrated that increased intra cavernosal pressures could be achieved despite inhibition of the NO pathway, suggesting other pathways were also involved in penile erection.13
The penis is maintained in a flaccid state through chronic smooth-muscle contraction via binding of norepinephrine, endothelin-1 and angiotensin II to their respective smooth-muscle G-protein-coupled receptors (GPCRs). The GPCR signalling cascade increases the level of cytosolic Ca2+, which binds to calmodulin, causing a structural change that enables calmodulin to complex with and activate myosin light chain kinase (MLCK). Once activated, MLCK phosphorylates the regulatory myosin light chain (MLC), enabling the myosin filament heads to bind to actin and cause smooth-muscle contraction.12 This signalling cascade maintains the chronically contracted state of smooth muscle in flaccid tissues. However, the cytosolic Ca2+ concentration is not proportional to the extent of MLC phosphorylation and contraction.16,17 Early experiments using isolated vascular smooth muscle demonstrated that, despite keeping intracellular Ca2+ concentrations constant, phenylephrine stimulation causes an increase in contraction that is maintained when phenylephrine stimulation is withdrawn, suggesting a sensitizing mechanism to Ca2+.16
RhoA-mediated calcium sensitization
In addition to the reduction of cytosolic Ca2+ resulting from the NO cascade, dephosphorylation of MLC by myosin light chain phosphatase (MLCP) facilitates release of myosin from actin and smooth-muscle relaxation in an NO-independent manner.18 RhoA, a small monomeric GTPase, activates Rho-associated protein kinase (ROCK), a serine/threonine kinase, which phosphorylates the myosin-binding subunit of MLCP, thereby deactivating it and promoting contraction.17 In addition to elevating Ca2+ levels, smooth-muscle GPCR ligand-binding activates guanine exchange factor, which converts RhoA–GDP to RhoA–GTP. RhoA–GTP dissociates from RhoA–GDP dissociation inhibitor (RhoGDI), enabling RhoA to bind to multiple downstream targets and to migrate to the cellular membrane, where it binds to ROCK, causing autophosphorylation of ROCK and increasing its activity and ability to phosphorylate MLCP (Figure 1).11,19–21 Thus, by inhibiting MLCP, ROCK and RhoA act to sensitize myosin–actin contraction to lower levels of cytosolic calcium in smooth muscles, facilitating chronic tonic contraction and maintenance of the flaccid state. ROCK has two isoforms, ROCK-1 and ROCK-2, which are differentially expressed throughout the body.22 Earlier ED studies did not differentiate between the two isoforms, but data suggest that their respective involvements in ED vary depending on the aetiology of the condition.23–26 For example, in cavernosal-nerve-crush rat models, ROCK-2 upregulation in the penis seems to have a more dominant role, whereas ROCK-1 overexpression is prominent in diabetes-associated ED.23,24,26,27
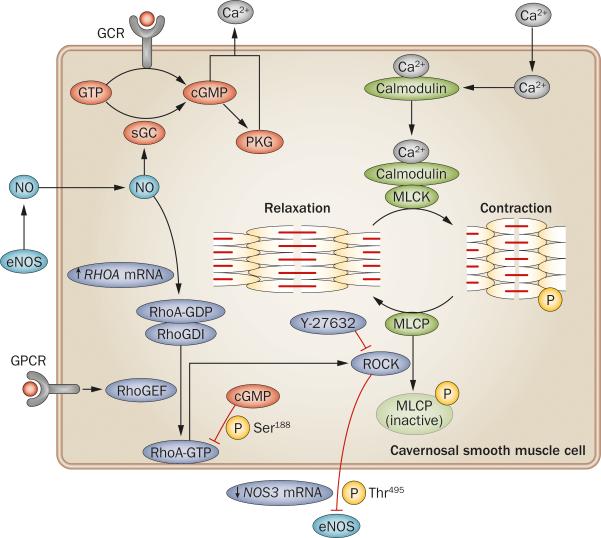
Figure 1.
Pathways of contraction and relaxation in cavernosal smooth-muscle cells. Ca2+ influx into cells increases intracellular Ca2+ that is available to bind to calmodulin. This binding causes a conformational change, enabling complexing with MLCK, with subsequent phosphorylation of the myosin–actin complex resulting in smooth muscle contraction and a flaccid penis. The Rho pathway is initiated by GPCR agonist binding, which activates RhoGEF, facilitating RhoA–GDP conversion to RhoA–GTP, and dissociation from inhibitory RhoGDI. RhoA–GTP relocates to the plasma membrane, where it binds to ROCK, facilitating autophosphorylation of ROCK that enhances its ability to phosphorylate and deactivate MLCP, augmenting the contractile response of the cell to Ca2+. Relaxation is largely mediated by cGMP. Conversion of GTP to cGMP is mediated by diffusion of NO into the cell and by GCR activation. cGMP facilitates Ca2+ efflux by activating PKG and cGMP-gated ion channels. Interactions between the pathways are complex. NO can increase expression of RhoA, but cGMP causes phosphorylation of RhoA, preventing interaction with ROCK. ROCK can phosphorylate and inhibit eNOS, and reduce eNOS expression. Abbreviations: cGMP, cyclic GMP; eNOS, endothelial nitric oxide synthase; GCR, guanylyl cyclase receptor; GPCR, G-protein-coupled receptor; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; NO, nitric oxide; PKG, protein kinase G; RhoGDI, RhoA–GDP dissociation inhibitor; RhoGEF, Rho guanine exchange factor; ROCK, Rho-associated protein kinase; sGC, soluble guanylyl cyclase; Y-27632, (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide.
Effects of RhoA–ROCK on erectile function
RhoA and ROCK are present in many tissues throughout the body and are involved in regulating various functions.28,29 Although they are present in the endothelial and neural tissues throughout the human corpora, the greatest effects of RhoA and ROCK are seen in penile erection, via modulation of the contractile state of the cavernous sinusoidal and arteriolar smooth-muscle cells. RhoA and ROCK concentrations are 17-fold greater in smooth muscle of rabbit corpus cavernosum than of ileum. Unlike other smooth-muscle types in which the contractile state varies more frequently, penile smooth muscle remains tonically contracted to maintain the flaccid state of the penis.30,31 Studies using the ROCK inhibitor, (R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide (Y-27632), a pyridine derivative initially investigated for antihypertensive properties, elegantly demonstrated the potential for ROCK inhibition to treat ED, and also showed that the effects of ROCK inhibition are independent of NO-mediated relaxation.30–32 Intracavernosal injection of Y-27632 increased intracavernosal pressure in a dose-dependent fashion, with the maximal effect of Y-27632 achieved at doses that did not significantly decrease mean arterial pressures.31 Additionally, the erectile response elicited by cavernous nerve stimulation was augmented by Y-27632 corporal injections.31 Furthermore, maximal smooth muscle relaxation in isolated cavernosal tissue was achieved via ROCK inhibi tion with Y-27632.31 These data show that inhibition of ROCK causes direct smooth muscle relaxation in the penis, thereby enhancing the penile erection.
NO and RhoA–ROCK pathway relationships
The importance of NO-independent pathways for erection has been known for well over a decade.13 L-NAME and methylene blue are potent NOS inhibitors that prevent and reverse erection initiated by NO-mediated stimulation.13,31,33 ROCK inhibition by Y-27632 treatment restored erectile responses in rats pretreated with L-NAME or methylene blue. However, although erectile responses can be achieved by inhibiting RhoA–ROCK despite concomitant NO inhibition, several studies suggest crosstalk exists between the pathways.
Early studies investigating the role of RhoA regulation by NO in hypertension demonstrated that activation of the NO pathway inhibited RhoA-mediated smooth-muscle contraction.10,34,35 Activation of PKG in rat aortal smooth-muscle cells by treatment with the cGMP analogue bromo-cGMP, or nitroprusside, resulted in RhoA phosphorylation at Ser188, and translocation from the cellular membrane to the cytosol. Translocation away from the membrane prevents RhoA from binding to and activating ROCK.35 HeLa cells undergo stress-fibre development when the RhoA pathway is activated. This stress-fibre development was prevented in cells with constitutive expression of PKG, but was restored by the additional constitutive expression of RhoA with a mutation at Ser188, preventing phosphorylation.34 These data suggest that effectors of the activated NO pathway inhibit the RhoA pathway, which would augment the erectile response.
The homeostatic interactions between the NO and RhoA–ROCK pathways are more complicated than reciprocal negative regulation. Chronic NO inhibition is associated with decreased RhoA activity.36 Arteries isolated from hypertensive rats treated with the NOS inhibitor L-NAME in their drinking water have a greater relaxation response when exposed to RhoA inhibition via Y-27632 compared with animals without chronic L-NAME treatment.36 Aortas from rats with hypertension induced by L-NAME treatment also have an enhanced contractile response when stimulated with α2-adrenergic receptor agonist UK-14304, which potentiates RhoA activation.36 Inhibition of NO by L-NAME leads to decreased RHOA mRNA and RhoA protein expression in rat aortas, which is prevented by treatment with the PDE5 inhibitor sildenafil.37 Stimulation of the NO pathway via nitroprusside results in increased RHOA mRNA production and stabilization, leading to greater RhoA protein expression.37 Together, these studies suggest that NO expression is required for, and related to, RhoA expression. The positive correlation between NO and RhoA could help to maintain vascular homeostasis between the relaxed and contractile states by ensuring that one pathway does not become predominant.
RhoA–ROCK activity inhibits NO-mediated relaxation by two independent mechanisms: decreasing eNOS expression and directly inhibiting eNOS activation.14,38–40 Human umbilical vein endothelial cells (HUVECs) that have been transfected with vectors that constitutively express active RhoA or ROCK demonstrate decreased eNOS protein expression, which is associated with post-transcriptional regulation linked to destabilization of eNOS-encoding NOS3 mRNA.14,40 Additionally, ROCK inhibits eNOS by phosphorylating PKB, an upstream regulator of eNOS, at Ser473, resulting in decreased eNOS activation. In thrombin-activated HUVECs, ROCK also directly phosphorylates eNOS at Thr495, which is located in a well-studied inhibitor subunit of eNOS, preventing eNOS binding to calmodulin and subsequent eNOS activation.38
NO and RhoA–ROCK in ED pathology
The NO and RhoA–ROCK pathways are important in maintenance of erectile homeostasis, and show complex cross-talk. The relationships between these pathways can be altered in various disease processes leading to ED. Inhibition of the RhoA–ROCK pathway, in common with potentiation of the NO pathway by PDE5 inhibitors, has a role in ED therapies (Table 1).
Table 1.
Assessment of ROCK inhibitors in animal models of disease states associated with ED
| Disease state | Effects on RhoA/ROCK | ROCK inhibitor | Effects of ROCK inhibition | Animal model | Reference |
|---|---|---|---|---|---|
| Normal physiology | NA | Y-27632 | ICI increased ICP in dose-dependent fashion, independent of NO inhibition | Rat Rabbit |
30 – 32 |
| Hypertension | Increased RhoA expression in cavernosal tissues | Y-27632 Fasudil Azaindole-1 |
Systemic treatment improved systemic hypertension via systemic arterial relaxation ICI improved ED, augmented PDE5 inhibitor effects |
DOCA rat SHRSP |
41,43–45 |
| Diabetes | Increased RhoA activity with decreased eNOS expression Increased apoptosis Increased ROCK-2 activity Increased MAPK Increased ROCK-2 activity and apoptosis in the MPG |
Y-27632 RhoA−Adenovirus Fasudil SAR407899 Atorvastatin Testosterone |
Improved relaxation independent of exogenous NO inhibition Normalized RhoA expression and erectile response, improved eNOS expression Normalized PTEN, caspase-3 and PKB activities, decreased apoptosis |
STZ rabbit STZ rat STZ mouse Rock1−/+ mouse Rock2−/+ mouse Human |
26,47,48, 54–56 |
| Ageing | Increased activity with normal expression | RhoA−Adenovirus | Decreased ROCK activity with increased ICP, with improved response to concomitant PDE5 inhibitor treatment | Aged versus young rat | 21,58 |
| Prostatectomy | Cavernosal tissue apoptosis and remodelling Increased RhoA and ROCK-2 expression | Y-27632 | Increased ICP in dose-dependent manner | Cavernosal nerve-crush rat | 24,61,64 |
Abbreviations: DOCA, deoxycorticosterone acetate–salt; ED, erectile dysfunction; eNOS, endothelial nitric oxide synthase; ICI, intracorporal injection; ICP, intracavernosal pressure; MAPK, mitogen-activated protein kinase; MPG, major pelvic ganglion; NA, not applicable; RhoA−adenovirus, adenovirus encoding dominant-negative RhoA mutant; ROCK, Rho-associated protein kinase; SHRSP, stroke prone-spontaneously hypertensive rats; STZ, streptozotocin-induced diabetic; Y-27632, (R)-(+Hrans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide.
Hypertension
Hypertension is a well-known risk factor for ED, and approximately 30% of hypertensive individuals have decreased erectile function.41,42 The RhoA–ROCK signalling pathway is involved in systemic hypertension, support ing its investigation in hypertension-induced ED.32 In hypertensive rats, erectile tissue undergoes pathological collagen remodelling and altered relaxation response earlier in the disease process than aortic tissues, emphasizing the value of ED as an early indicator of hypertension.5 Aortic and erectile tissues from spontaneously hypertensive rats undergo modifications to collagen I, III, and V distributions and have impaired acetylcholine-induced relaxation, and these modifications occur earlier in erectile tissues.5 Additionally, spontaneously hypertensive rats have decreased cGMP levels and decreased NOS3 mRNA expression, and although total eNOS protein levels are unchanged, levels of activated eNOS are decreased compared with the levels in normotensive rats.41 The changes correlate with increased smooth-muscle contraction and decreased relaxation in response to phenylephrine and acetylcholine, respectively, in hypertensive animals,41 and are mitigated in a dose-dependent fashion by the ROCK inhibitor hydroxyfasudil.41 RhoA protein expression is elevated in both deoxycorticosterone acetate (DOCA)-salt rats and spontaneously hypertensive rats. Intracorporal injection of Y-27632 improves their erectile function, which is augmented by co-treatment with systemic PDE5 inhibitors, supporting the need to target multiple pathways for effective ED treatment.43,44 Thus, in the setting of hypertension, both impaired relaxation and increased contraction contribute to ED. More recently developed ROCK inhibitors, including azaindole-1 and SAR407899, have shown promise in recovering erections in preclinical rat studies.45–47 Azaindole-1, a more selective ROCK inhibitor with less secondary kinase inhibition than Y-27632 and fasudil, demonstrated both antihypertensive and proerectile effects.45,46
Diabetes
The pathophysiology of ED in diabetes is multifactorial, involving endothelial dysfunction, decreased eNOS activity, increased contractile sensitivity and decreased nitrergic nerve signalling. Several studies have shown that diabetes substantially affects the NO-mediated relaxation pathway in the penis. Animal models of diabetes have demonstrated decreased NOS gene and protein expression in corporal endothelium and nerves with concomitantly decreased cGMP levels and smooth-muscle relaxation.27,48–50 In these models, type 1 diabetes was induced in rats by injection with streptozotocin alone and type 2 diabetes was induced with a high-fat diet and low-dose streptozotocin injection.27,48 Cavernosal tissues in diabetic-model rats had decreased protein levels of eNOS, nNOS and PKG, with decreased erectile response to electrical stimulation compared with nondiabetic animals.27,48 Transfection with adenovirus carrying a dominant-negative RHOA gene mutation that inhibits RhoA rescued erectile response, constitutive NOS activity, eNOS and cGMP levels.48 In a similar study, PDE5 levels were similar in diabetic and nondiabetic rats, which could explain the muted erectile response to intracavernosal injections of PDE5 inhibitors in diabetic men with ED.27,47,51 Additionally, RhoA, ROCK-1 and type A endothelin receptor levels were elevated in diabetic rats, suggesting an enhanced contractile response in addition to impaired relaxation in diabetic ED.27
Chronic diabetes leads to adverse erectile tissue remodelling, involving increased apoptosis, collagen deposition and decreased endothelial cell proliferation, according to human and animal studies.9,47,51 Studies in myocardium and erectile tissues demonstrate RhoA–ROCK pathway involvement in the upregulation of apoptosis, through increased activation of caspase-3 and PTEN, and decreased PKB activity.52,53 In a mouse model of heart failure, ROCK-1 was found to be a substrate of activated caspase-3, and cleavage resulted in a 130 kDa ROCK-1 fragment. This ROCK-1 fragment acted in a feed-forward manner, further activating caspase-3, which was associated with increased PTEN activation, inhibition of PKB and increased apoptosis. This process was inhibited by a small interfering RNA directed towards ROCK-1 expression and was attenuated in Rock1−/− null animals, suggesting that activated ROCK-1 plays a critical role in a poptotic pathways.52
The mediators of RhoA upregulation in diabetes have not all been identified, but disruption of the homeostatic mechanisms observed in nondiabetic animal models might lead to an excess of RhoA–ROCK signalling. Human fetal penile smooth-muscle cells exposed to elevated glucose media demonstrate increased RhoA membrane translocation and ROCK activity.54 Cavernosal tissues from streptozotocin-induced diabetic mice have elevated levels of membrane-bound, activated RhoA and increased protein levels of ROCK-2, but equivalent levels of ROCK-1 when compared with nondiabetic mice.55 The diabetic mice have impaired nitrergic corpus cavernosum relaxation and impaired acetylcholine-induced endothelial relaxation.55 These findings are associated with increased corpus cavernosum levels of active p38 mitogen-activated protein kinase (MAPK), myosin phosphatase target subunit 1 (MYPT-1) and arginase.55 RhoA is an upstream regulator of p38 MAPK, and increased activities of both are associated with enhanced arginase activity, which is believed to have a role in ED. Arginase and NOS act on the same substrate, L-arginine, suggesting that increased arginase activity results in decreased L-arginine availability for NO production. Diabetic heterozygous Rock2−/+ knockout mice have improved corpus cavernosum and endothelial relaxation as well as decreased p38 MAPK, MYPT-1 and arginase levels compared with wild-type diabetic mice.55
Elevated levels of RhoA, ROCK-2 and pro-apoptotic phosphorylated PTEN, and decreased levels of anti-apoptotic phosphorylated PKB were also seen in the major pelvic ganglion of diabetic rats, highlighting the importance of neuronal ROCK and apoptosis in diabetic ED.56 Four weeks of treatment with intraperitoneal injection of hydroxyfasudil, the active metabolite of fasudil, rescued erectile function and normalized levels of phosphorylated PTEN and phosphorylated PKB, but did not reduce expression of RhoA or ROCK-2 in the major pelvic ganglion.56 The erectogenic effects of hydroxyfasudil were observed following a 48-hour washout period, suggesting that this agent causes long-term molecular changes, such as decreasing apoptosis.56 Similarly, chronic inhibition of ROCK with oral fasudil in diabetic rats normalized PTEN, caspase-3 and PKB activities and subsequently decreased tissue apoptosis in corpus cavernosum.57
These data help to explain the clinical findings of decreased PDE5 inhibitor efficacy in the treatment of ED in men with diabetes. Precontracted cavernosal tissue from diabetic rabbits treated with Y-27632 achieved levels of relaxation similar to Y-27632-treated tissue from nondiabetic animals, but PDE5 inhibitor treatment induced less relaxation in the tissue from diabetic rabbits.47 Additional NOS inhibition by administration of exogenous L-NAME further diminished the relaxation response to PDE5 inhibition, but did not attenuate Y-27632-initiated relaxation, underlining the limitations of current medical therapies.47 A newer ROCK inhibitor, SAR407899, was shown to be more potent and to have longer duration of action than either Y-27632 or sildenafil treatment of isolated cavernosal strips from diabetic rats, rabbits and humans.47
The beneficial effects of several other pro-erectile agents have also been linked to ROCK inhibition. Improved erectile response with testosterone supplementation in diabetic rats is linked to ROCK expression normalization.26 Similarly, atorvastatin improves nerve stimulation, induces erection and augments the effects of concomitant sildenafil or Y-27632 treatment in diabetic rats and rabbits by preventing RhoA membrane translocation and activation without affecting glycaemia, plasma lipid levels or the hypogonadal state.54
Ageing
Increasing age is associated with higher risks of diabetes and hypertension, but is also an independent risk factor for ED, with an estimated increase in ED of 1.2% per year between the ages of 40 and 49 years and 4.6% per year between the ages of 60 and 69 years.58,59 Mechanisms believed to be involved in the association between ageing and ED include reduction of testosterone levels and decreasing NO availability as a function of lower NOS expression, and increased levels of reactive oxygen species in cavernosal tissues.21 RhoA–ROCK signalling seems to have an important role. Unlike other disease states, which demonstrate increased expression of RhoA and its downstream targets, baseline RhoA expression seems to be unchanged in ageing, but signalling is apparently increased, with elevated membrane-bound RhoA in old rats compared with young rats.58 In aged animals, RhoA pathway inhibition via cavernosal injection of Y-27632, or of adenovirus encoding a dominant-negative mutated RhoA, decreases ROCK activity and improves intracavernosal pressures at baseline and following electrical stimulation.58 As with other aetiologies of ED, ROCK inhibition potentiates PDE5 inhibitor treatment in aged animals.60
RhoA–ROCK in postprostatectomy ED
Over 60,000 prostatectomies are performed each year to treat prostate cancer, resulting in a 10–100% prevalence of ED, depending on comorbidities, preoperative erectile function and the extent of the cancer.61 ED results from trauma to the neurovascular bundle that provides the parasympathetic signalling required for cavernosal relaxation, and occurs despite careful dissection and bilateral nerve-sparing techniques.61 Consequently, detrimental penile remodelling occurs, with increased smooth-muscle apoptosis, collagen deposition and fibrosis, which is associated with penile hypoxia. The use of PDE5 inhibitors to enhance penile rehabilitation in patients follow ing radical prostatectomy remains inconclusive.62,63 Alteration of several pathways has been implicated in postprostatectomy ED, including decreases in prostaglandin E1 and nNOS, and increases in transforming growth factor beta-1, endothelin-1 and RhoA.61,64 In a model of bilateral cavernous nerve injury used to assess prostatectomy-induced ED, protein expression of RhoA and ROCK-2, but not ROCK-1, increase in the penis,64 corresponding to increased RhoA GTPase, total ROCK activity and membrane-bound RhoA, with decreased erectile responses.24 Impairment of cavernosal pressures was prevented in animals with cavernous nerve injuries that were treated in vivo with Y-27632, suggesting a role for ROCK inhibitors in penile rehabilitation following prostatectomy.24,64
Current clinical use of ROCK inhibitors
The prevalence of the RhoA pathway in ED has made it a logical pharmacological target for clinical application. Despite its widespread use in the lab, Y-27632 is a preclinical ROCK inhibitor with no current use in clinical trials, or FDA approval. By contrast, fasudil, which can be taken orally and which is currently approved for cerebral vasospasm, is being evaluated in five clinical trials, although none of them are investigating ED.28 SAR407899, which is eightfold more active than fasudil, is being compared with placebo and sildenafil in a crossover, randomized, double-blind phase II trial sponsored by Sanofi-Aventis, which has completed enrolment of 24 patients, but to our knowledge has produced no published results thus far.65 Additionally, SAR407899 is being evaluated in a phase I trial on hypertension in patients with chronic kidney disease, again with no results reported to date. Azaindole-1, a newer, selective ROCK inhibitor, improves ED in rats after nerve-crush injury and has antihypertensive effects when given systemically, but it is not currently being evaluated for ED in a clinical trial.45,46
Conclusions
RhoA and ROCK inhibition provides a unique avenue to augment current medical therapies by targeting new pathways both in the corpus cavernosum and in the major pelvic ganglion that have not yet been exploited pharmacologically for the treatment of ED. Importantly, inhibition of ROCK targets neurological deficits in ED and induces improved erectile haemodynamics, independent of NO synthesis in the penis, and could be a more efficacious ED treatment alternative to the use of PDE5 inhibitors in hard-to-treat populations of patients. Additional clinical trials are necessary to better understand how ROCK inhibitors will perform in the complex and variable setting of clinical ED, and which aetiologies will lead to the best response. More preclinical studies are needed to further investigate the role of the RhoA–ROCK pathway in the many aetiologies of ED, and the relative importance of the different ROCK isotypes, and to improve our understanding of other RhoA targets and how they affect erectile function. With greater knowledge of the role of the RhoA–ROCK pathway in ED, and its interaction with NO signalling, we will be better placed to bring much-needed therapeutic targeting of this pathway to the clinical arena, and so to improve the treatment of ED.
Key points.
■ RhoA–ROCK signalling is important for erectile homeostasis, and acts by potentiating Ca2+-dependent contraction to maintain penile flaccidity
■ Dysregulation of the RhoA–ROCK pathway has an important role in the aetiology of erectile dysfunction
■ RhoA–ROCK inhibition improves erectile dysfunction, even for hard-to-treat causes, such as diabetes
■ Several RhoA–ROCK inhibitors are being investigated in clinical trials and could provide new treatment options
Review criteria.
A search was performed for original articles published and catalogued within MEDLINE and PubMed up to the time of this article. The search terms used were “RhoA”, “ROCK”, “erectile dysfunction”, “nitric oxide” and “smooth muscle relaxation”, alone and in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for further relevant papers.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed to researching data for the article, discussion of content, writing the article and reviewing and editing the manuscript before submission.
References
- 1.Foster SA, Annunziata K, Shortridge EF, Freedman D, Viktrup L. Erectile dysfunction with or without coexisting benign prostatic hyperplasia in the general US population: analysis of US National Health and Wellness Survey. Curr. Med. Res. Opin. doi: 10.1185/03007995.2013.837385. http://dx.doi.org/10.1185/03007995.2013.837385. [DOI] [PubMed]
- 2.Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol. Clin. North Am. 2005;32:379–395. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J. Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 4.Yao F, et al. Erectile dysfunction may be the first clinical sign of insulin resistance and endothelial dysfunction in young men. Clin. Res. Cardiol. 2013;102:645–651. doi: 10.1007/s00392-013-0577-y. [DOI] [PubMed] [Google Scholar]
- 5.Behr-Roussel D, et al. Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R276–R283. doi: 10.1152/ajpregu.00040.2004. [DOI] [PubMed] [Google Scholar]
- 6.Akingba AG, Burnett AL. Endothelial nitric oxide synthase protein expression, localization, and activity in the penis of the alloxan-induced diabetic rat. Mol. Urol. 2001;5:189–197. doi: 10.1089/10915360152745885. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro LJ, et al. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 8.Burnett AL, et al. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J. Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- 9.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J. Androl. 2003;24:S17–S37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 10.Chitaley K, Webb RC. Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension. 2002;39:438–442. doi: 10.1161/hy02t2.102960. [DOI] [PubMed] [Google Scholar]
- 11.Lin C-S, Xin Z-C, Wang Z, Lin G, Lue TF. Molecular Yin and Yang of erectile function and dysfunction. Asian J. Androl. 2008;10:433–440. doi: 10.1111/j.1745-7262.2008.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 13.Reilly CM, Lewis RW, Stopper VS, Mills TM. Androgenic maintenance of the rat erectile response via a non-nitric-oxide-dependent pathway. J. Androl. 1997;18:588–594. [PubMed] [Google Scholar]
- 14.Ming X-F, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol. Cell. Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Küthe A, et al. Expression of different phosphodiesterase genes in human cavernous smooth muscle. J. Urol. 2001;165:280–283. doi: 10.1097/00005392-200101000-00079. [DOI] [PubMed] [Google Scholar]
- 16.DeFeo TT, Morgan KG. Calcium-force relationships as detected with aequorin in two different vascular smooth muscles of the ferret. J. Physiol. 1985;369:269–282. doi: 10.1113/jphysiol.1985.sp015900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 18.Rembold CM. Regulation of contraction and relaxation in arterial smooth muscle. Hypertension. 1992;20:129–137. doi: 10.1161/01.hyp.20.2.129. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki T, Kato M, Takai Y. Consequences of weak interaction of rho GDI with the GTP-bound forms of rho p21 and rac p21. J. Biol. Chem. 1993;268:23959–23963. [PubMed] [Google Scholar]
- 20.Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J. Biochem. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- 21.Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin. Sci. (Lond.) 2006;110:153–165. doi: 10.1042/CS20050255. [DOI] [PubMed] [Google Scholar]
- 22.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 23.Bivalacqua TJ, et al. Attenuated RhoA/Rhokinase signaling in penis of transgenic sickle cell mice. Urology. 2010;76:510.e7–510.e12. doi: 10.1016/j.urology.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannan JL, et al. Inhibition of Rho-kinase improves erectile function, increases nitric oxide signaling and decreases penile apoptosis in a rat model of cavernous nerve injury. J. Urol. 2013;189:1155–1161. doi: 10.1016/j.juro.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SY, Park K, Paick J-S, Kim SW. Change of erectile function and responsiveness to phosphodiesterase type 5 inhibitors at different stages of streptozotocin-induced diabetes in rats. J. Sex. Med. 2011;8:1352–1361. doi: 10.1111/j.1743-6109.2010.02099.x. [DOI] [PubMed] [Google Scholar]
- 26.Vignozzi L, et al. Testosterone regulates RhoA/ Rho-kinase signaling in two distinct animal models of chemical diabetes. J. Sex. Med. 2007;4:620–630. doi: 10.1111/j.1743-6109.2007.00440.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiou W-F, Liu H-K, Juan C-W. Abnormal protein expression in the corpus cavernosum impairs erectile function in type 2 diabetes. BJU Int. 2010;105:674–680. doi: 10.1111/j.1464-410X.2009.08852.x. [DOI] [PubMed] [Google Scholar]
- 28.Breitenlechner C, et al. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, Fasudil, and H-1152P. Structure. 2003;11:1595–1607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Priviero FB, Jin L-M, Ying Z, Teixeira CE, Webb RC. Up-regulation of the RhoA/Rhokinase signaling pathway in corpus cavernosum from endothelial nitric-oxide synthase (NOS), but not neuronal NOS, null mice. J. Pharmacol. Exp. Ther. 2010;333:184–192. doi: 10.1124/jpet.109.160606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2+ sensitization in erectile function. J. Biol. Chem. 2002;277:30614–30621. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 31.Chitaley K, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat. Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 32.Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 33.Moody JA, Vernet D, Laidlaw S, Rajfer J, Gonzalez-Cadavid NF. Effects of long-term oral administration of L-arginine on the rat erectile response. J. Urol. 1997;158:942–947. doi: 10.1097/00005392-199709000-00076. [DOI] [PubMed] [Google Scholar]
- 34.Sawada N, et al. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem. Biophys. Res. Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- 35.Sauzeau V, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 36.Carter RW, Begaye M, Kanagy NL. Acute and chronic NOS inhibition enhances alpha(2)-adrenoreceptor-stimulated RhoA and Rho kinase in rat aorta. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1361–H1369. doi: 10.1152/ajpheart.01101.2001. [DOI] [PubMed] [Google Scholar]
- 37.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J. Biol. Chem. 2003;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto M, et al. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem. Biophys. Res. Commun. 2007;361:462–467. doi: 10.1016/j.bbrc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Mita S, Kobayashi N, Yoshida K, Nakano S, Matsuoka H. Cardioprotective mechanisms of Rho-kinase inhibition associated with eNOS and oxidative stress-LOX-1 pathway in Dahl salt-sensitive hypertensive rats. J. Hypertens. 2005;23:87–96. doi: 10.1097/00004872-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J. Biol. Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 41.Saito M, et al. Hydroxyfasudil ameliorates penile dysfunction in the male spontaneously hypertensive rat. Pharmacol. Res. 2012;66:325–331. doi: 10.1016/j.phrs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Burchardt M, et al. Hypertension is associated with severe erectile dysfunction. J. Urol. 2000;164:1188–1191. [PubMed] [Google Scholar]
- 43.Wilkes N, White S, Stein P, Bernie J, Rajasekaran M. Phosphodiesterase-5 inhibition synergizes rho-kinase antagonism and enhances erectile response in male hypertensive rats. Int. J. Impot. Res. 2004;16:187–194. doi: 10.1038/sj.ijir.3901149. [DOI] [PubMed] [Google Scholar]
- 44.Chitaley K, Webb RC, Dorrance AM, Mills TM. Decreased penile erection in DOCA-salt and stroke prone-spontaneously hypertensive rats. Int. J. Impot. Res. 2001;13(Suppl. 5):S16–S20. doi: 10.1038/sj.ijir.3900773. [DOI] [PubMed] [Google Scholar]
- 45.Lasker GF, et al. The selective Rho-kinase inhibitor azaindole-1 has long-lasting erectile activity in the rat. Urology. 2013;81:465.e7–465.e14. doi: 10.1016/j.urology.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pankey EA, et al. The Rho kinase inhibitor azaindole-1 has long-acting vasodilator activity in the pulmonary vascular bed of the intact chest rat. Can. J. Physiol. Pharmacol. 2012;90:825–835. doi: 10.1139/y2012-061. [DOI] [PubMed] [Google Scholar]
- 47.Guagnini F, Ferazzini M, Grasso M, Blanco S, Croci T. Erectile properties of the Rho-kinase inhibitor SAR407899 in diabetic animals and human isolated corpora cavernosa. J. Transl. Med. 2012;10:59. doi: 10.1186/1479-5876-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bivalacqua TJ, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc. Natl Acad. Sci. USA. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem. Biophys. Res. Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 50.Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int. J. Impot. Res. 2007;19:129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- 51.Costa C, et al. Increased endothelial apoptotic cell density in human diabetic erectile tissue —comparison with clinical data. J. Sex. Med. 2009;6:826–835. doi: 10.1111/j.1743-6109.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 52.Chang J, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc. Natl Acad. Sci. USA. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li WJ, Park K, Paick J-S, Kim SW. Chronic treatment with an oral rho-kinase inhibitor restores erectile function by suppressing corporal apoptosis in diabetic rats. J. Sex. Med. 2011;8:400–410. doi: 10.1111/j.1743-6109.2010.01724.x. [DOI] [PubMed] [Google Scholar]
- 54.Morelli A, et al. Atorvastatin ameliorates sildenafil-induced penile erections in experimental diabetes by inhibiting diabetes-induced RhoA/Rho-kinase signaling hyperactivation. J. Sex. Med. 2009;6:91–106. doi: 10.1111/j.1743-6109.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- 55.Toque HA, et al. Activated Rho kinase mediates diabetes-induced elevation of vascular arginase activation and contributes to impaired corpora cavernosa relaxation: possible involvement of p38 MAPK activation. J. Sex. Med. 2013;10:1502–1515. doi: 10.1111/jsm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sezen SF, Lagoda G, Musicki B, Burnett AL. Hydroxyl Fasudil, an inhibitor of Rho signaling, improves erectile function in diabetic rats: a role for neuronal ROCK. J. Sex. Med. 2014;11:2164–2171. doi: 10.1111/jsm.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park K, Kim SW, Rhu KS, Paick J-S. Chronic administration of an oral Rho kinase inhibitor prevents the development of vasculogenic erectile dysfunction in a rat model. J. Sex. Med. 2006;3:996–1003. doi: 10.1111/j.1743-6109.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 58.Jin L, et al. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006;20:536–538. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- 59.Johannes CB, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J. Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- 60.Rajasekaran M, White S, Baquir A, Wilkes N. Rho-kinase inhibition improves erectile function in aging male Brown-Norway rats. J. Androl. 2005;26:182–188. doi: 10.1002/j.1939-4640.2005.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 61.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat. Rev. Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 62.Hatzimouratidis K, et al. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur. Urol. 2009;55:334–347. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 63.Pavlovich CP, et al. Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: results of a randomized double-blind trial with placebo. BJU Int. 2013;112:844–851. doi: 10.1111/bju.12253. [DOI] [PubMed] [Google Scholar]
- 64.Gratzke C, et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J. Urol. 2010;184:2197–2204. doi: 10.1016/j.juro.2010.06.094. [DOI] [PubMed] [Google Scholar]
- 65.Löhn M, et al. Pharmacological characterization of SAR407899, a novel rho-kinase inhibitor. Hypertension. 2009;54:676–683. doi: 10.1161/HYPERTENSIONAHA.109.134353. [DOI] [PubMed] [Google Scholar]