In eukaryotes, nuclear DNA is packaged into chromatin by binding to histones and associated factors. Covalent modifications to histone tails are associated with specific transcriptional states of the associated DNA. Acetylation of lysine side-chains normally correlates with transcriptional activation, while deacetylation leads to transcriptional silencing. The regulatory roles of methylation of lysine and arginine residues appear to be more complex. Methylation of certain lysine residues is associated with active transcription, while methylation of others is associated with silencing and heterochromatin formation. Each methylation ‘mark’ is placed, removed and ‘interpreted’ in a site-specific manner by histone methyltransferases, demethylases and methyl-binding domains, respectively. The biological functions of the individual enzymes are largely undefined, and are the focus of current investigations (for review see [1, 2])
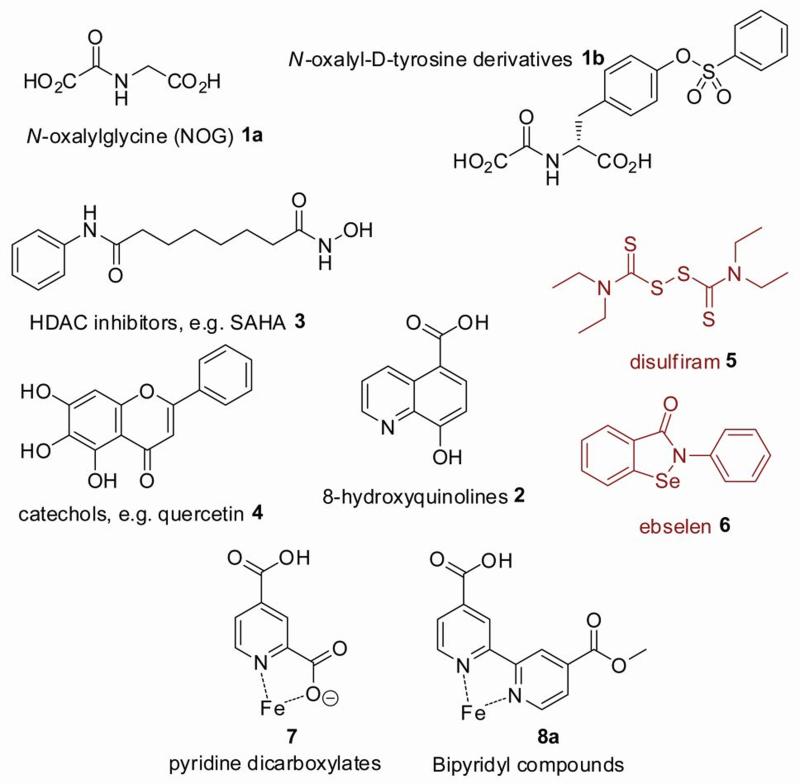
The JmjC histone demethylases are 2-oxoglutarate (2OG) dependent oxygenases that catalyse Nε-lysyl demethylation via hydroxylation of the methyl group in a 2-oxoglutarate and Fe(II)-dependent manner (Scheme 1). Human 2OG oxygenases catalyse a range of reactions, including hydroxylation of amino acids, DNA and small molecules, and demethylation of proteins and DNA.[3] 2OG oxygenases show promise as therapeutic targets. An inhibitor of γ-butyrobetaine hydroxylase (BBOX) is used for the treatment of cardiovascular disease[4, 5] and inhibitors of the hypoxia inducible factor (HIF) prolyl hydroxylases are in clinical trials for the treatment of anaemia.[6] Inhibitors of the collagen prolyl hydroxylases have also been evaluated as potential therapeutics for the treatment of liver fibrosis.[7, 8] The discovery of the JmjC domain histone demethylases, and the suggestions that some of them are potential therapeutic targets for cancer treatment,[9] has stimulated interest in their inhibition, but relatively few studies have been described. Reported inhibitors of the JmjC demethylases include N-oxalyl amino acids, 8-hydroxyquinolines, pyridine dicarboxylates, hydroxamic acids and catechol-type flavonoids (Figure 1).[10-13] Compounds which catalyse the ejection of a structural Zn(II) ion from the JMJD2 demethylases have also been reported (Figure 1). [14]
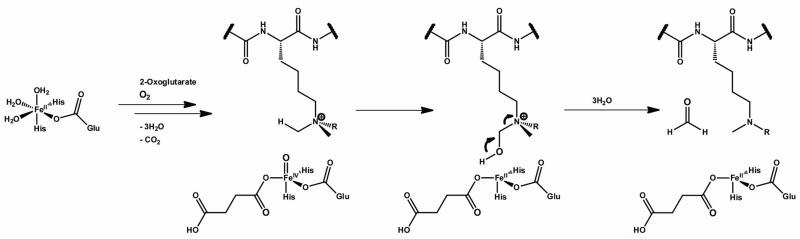
Scheme 1.
The JmjC-domain histone demethylases catalyse N-demethylation. For each demethylation reaction, 2OG is oxidised by molecular oxygen to generate succinate, CO2 and a reactive ferryl-oxo species which hydroxylates lysyl Nε-methyl groups; the unstable hemiaminal intermediate then fragments to release formaldehyde and the demethylated lysine residue.
Figure 1.
Structures of some previously reported histone demethylase inhibitors. Compounds 5 and 6 inhibit by ejecting Zn(II) from the enzyme.
In a study describing various template inhibitors of the JmjC demethylases, we found that 2,2′-bipyridyl compounds with at least one 4-carboxylate group inhibit the histone demethylase JMJD2E.[11] A related series of compounds, 5,5′-dicarboxylate-2,2′-bipyridyls, is reported to inhibit the collagen prolyl-4-hydroxylases.[15] 2,2′-Bipyridine and bipyridyl compounds have also been used as inhibitors of the HIF hydroxylases.[16] Although it is likely that in some cases the enzyme inhibition effects of bipyridyl compounds result from metal chelation in solution, they also have the potential to inhibit via active site binding, as is the case for some 2OG oxygenases; however, to date there is no structural information on their mechanism of action. Here we report structure-activity relationship studies and analyses on bipyridyl inhibitors of JMJD2E.
The bipyridyl compounds tested were synthesised according to Scheme 2. Thus, 4,4′-dicarboxy-2,2′-bipyridine 9 was esterified to give the dimethyl or diethyl esters, which were then hydrolysed to yield the mono-esters 8a or 8b respectively. 8a was coupled to a set of suitably protected primary amines to yield compounds 11a-b, 14a-e, 16, 18a-b, 20, 22, 24 and 27 which were then hydrolysed and deprotected to yield the free carboxylic acids 12a-b, 13a-b, 15a-e, 17, 19a-b, 21, 23, 25 and 28, respectively (Table 1). A derivative of 4-carboxy-2,2′-bipyridine, 30, was synthesised to evaluate the importance of a 4-carboxyl group (Scheme 2).
Scheme 2.
Reagents and conditions: a) SOCl2, MeOH, reflux, overnight, 90 %; b) KOH, MeOH/THF (1:1), overnight, reflux, 70 %; c) 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI), 1-hydroxybenzotriazole (HOBT), triethylamine, DMF, overnight, r.t.; d) KOH, MeOH/THF, reflux, 4 h; e) CF3COOH, 2% H2O, 4h, r.t.
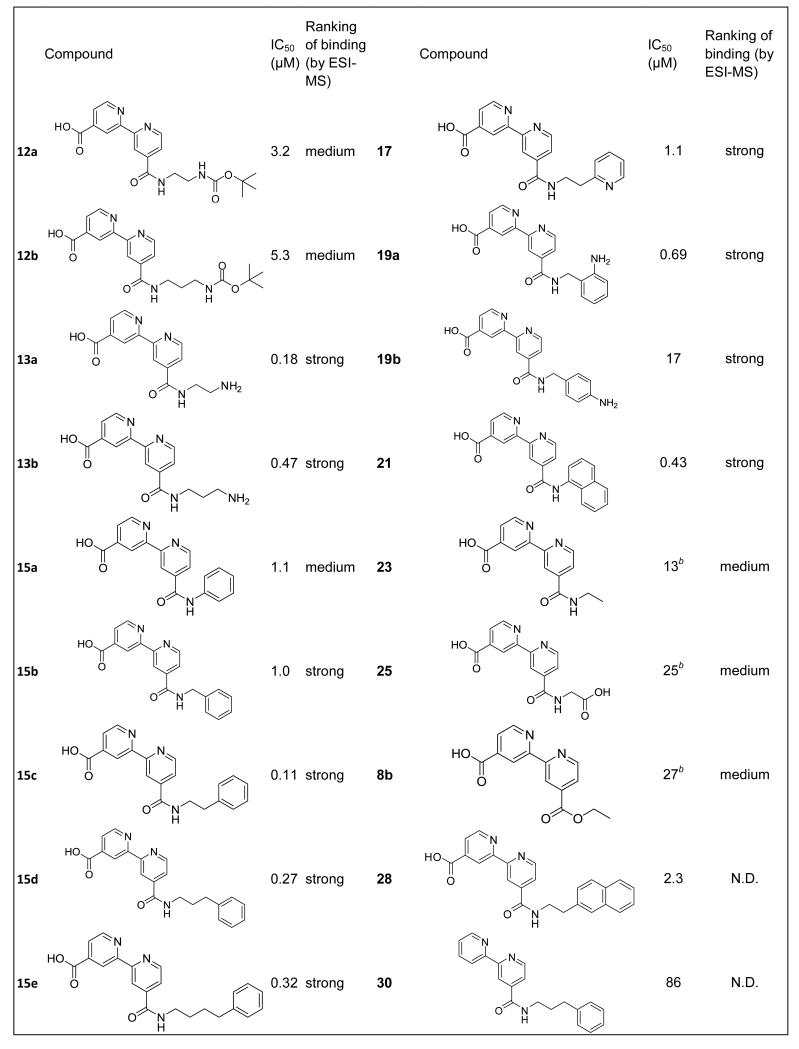
Table 1.
Inhibition of the histone demethylase JMJD2E (100 nM) by bipyridyl compounds. IC50s represent the result of three independent experiments, where standard errors in the log(IC50) are less than 10 %. Ranking of binding strength by ESI-MS is as shown in Figure 3. All compounds were counter-screened against FDH at the same concentrations used for IC50 determinations, and inhibition of FDH was not observed under these conditions, implying that the compounds only inhibited JMJD2E.
Inhibition assays for the histone demethylase JMJD2E were carried out using two complementary assay methods. Inhibition of histone demethylation was measured using a coupled enzyme assay, employing formaldehyde dehydrogenase (FDH) to convert formaldehyde to formate, with concomitant reduction of NAD+ to NADH, which was monitored spectrophotometrically.[11, 17, 18] Compounds were screened in 8-point serial dilutions against 100 nM JMJD2E, generating dose-response curves and IC50 data (Table 1); Each compound was also screened in a similar dose-response manner against FDH only, using 1μM formaldehyde as a substrate (to ensure that inhibitory effects were due to inhibition of JMJD2E and not FDH). To evaluate whether compounds were inhibiting by binding to the active site (as opposed to, or in addition to, chelation of iron in solution), non-denaturing electrospray ionisation protein mass spectrometry (ESI MS) (Figure 2) was used to detect binding of compounds to the intact protein complex.[11] Although there are caveats on the use of mass spectrometry for screening binding strength,[17] the results of both assay methods correlated well (Table 1), with more potent compounds showing a stronger binding interaction with the protein complex by ESI MS.
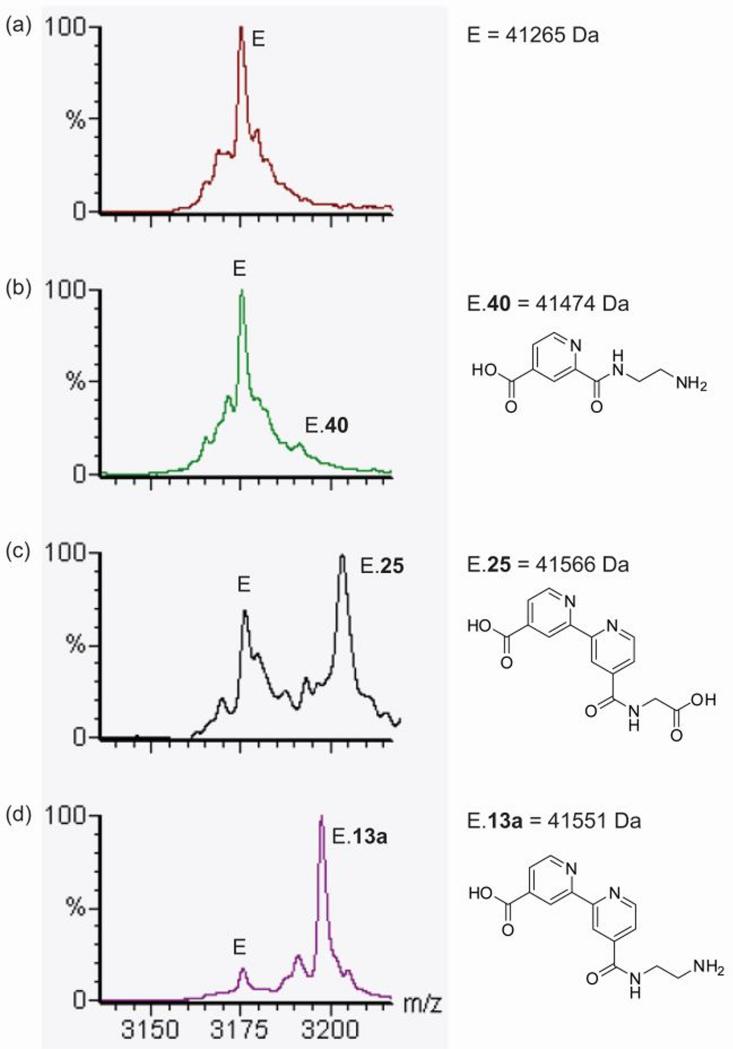
Figure 2.
Non-denaturing ESI-MS binding assay of inhibitors to JMJD2E. (a) JMJD2E.Fe(II) (labeled E) in the presence of compounds (b) 40, (c) 25 and (d) 13a, showing examples of relatively weak, medium and strong binding compounds, respectively.
Previous work on inhibitors of the JMJD2 demethylases demonstrated that the parent compound 2,4-pyridinedicarboxylate is a 2OG-competitive inhibitor. This compound was shown by crystallographic analyses (PDB ID 2VD7) to bind the active-site metal via the pyridine nitrogen and the 2-carboxylate, while the 4-carboxylate is positioned to interact with the 2OG-binding residues Lys206 and Tyr132, effectively ‘anchoring’ the compound in the active site (see Figure 4).[11] The bipyridyl compounds were, by analogy, predicted to bind in a similar manner, with the pyridinyl nitrogens chelating the active site metal, and the 4-carboxylate positioned to interact with Lys206 and Tyr132. In support of this proposal, all the bipyridyls tested were relatively potent inhibitors of JMJD2E (IC50s <30 μM), with the exception of that lacking the 4-carboxylate, compound 30, where the IC50 was 86 μM. This compound was 300-fold less potent than the equivalent compound with the 4-acid moiety, 15d.
The bipyridyl derivative resulting from coupling with ethylenediamine, 13a, inhibits JMJD2E with IC50 = 180 nM, showing a 36-fold improvement of inhibition compared to 4′-(methoxycarbonyl)-2,2′-bipyridine-4-carboxylic acid (IC50 = 6.6 μM). Analysis of a model of JMJD2A in complex with 13a suggested that this improvement might in part be due to formation of a salt-bridge between the amine of bipyridyl derivative 13a and Asp135 at the active site. To investigate the importance of the amine, the inhibition of JMJD2E activity by compound 23, with a hydrogen substituting for an amino group, was evaluated. Compound 23 was found to have an IC50 of 13 μM, representing 72-fold lower potency than 13a. Similarly, compounds 25 and 26 were significantly less potent than 13a, suggesting that the amine of 13a is important in binding.
The optimal position of the amine was then investigated. 13b (IC50 = 470 nM) exhibited a 2-fold potency decrease over 13a, which could reflect a sub-optimal length between the bipyridine core and Asp135. Consistent with a preference for the positive charge at this position, the IC50 values of 12a and 12b, where the amine is neutralized by a Boc group, were elevated (with IC50 = 3.2 μM and IC50 = 5.3 μM for 12a and 12b respectively). Introduction of unfunctionalised aromatic amides (15a with IC50 = 1.1 μM, and 15b, IC50 1.0 μM, resulted in a decrease in potency relative to 13a. The naphthalene derivative (compound 21) showed increased inhibition relative to its phenyl analogue (15a), suggesting that hydrophobic or π-π interactions in the active site might play a role in binding.
Based on the possibility that a combination of aromatic ring and amine might give improved potency, anilines 19a and 19b were synthesized and evaluated. The IC50s of 19a and 19b (690 nM and 17 μM respectively) showed no improvements relative to 13a, but demonstrated the importance of the regiochemistry of the position of the aromatic amine. Compounds 15c, 15d and 15e were synthesized with the aim of tuning the length between the bipyridine and phenyl group. Compounds 15c, 15d and 15e (with IC50s of 110 nM, 270 nM and 320 nM respectively) had similar activities to 13a, demonstrating that, at least for the compounds analysed, the chain lengths longer than one methylene all conferred similar potencies. These compounds might form π-π interactions between their phenyl ring and Tyr175 which is close to Asp135 in the substrate binding site. However, the addition of a second benzene ring in compound 28 caused a 20-fold loss of potency relative to its analogue 15c.
Although bipyridyl compounds are expected to chelate Fe(II) in solution, it is clear from the ESI-MS binding data that these compounds inhibit by binding to the active site. Further, the IC50s for most compounds are 10-fold to 100-fold less than the concentration of Fe(II) used in the assays (10 μM), also indicating that their inhibitory effects are not due predominantly to iron chelation in solution. Finally, the substantial differences (>100 fold) in IC50 values between compounds anticipated to chelate iron similarly in solution (e.g between compound with a 4-carboxylate (12a-28) and one without the 4-carboxylate (30)) implies that these compounds inhibit at least in part as a result of specific interactions in the active site. To confirm this proposal, we also determined IC50s for compounds 13a, 15c and 15d at 50 μM Fe(II). The results showed that IC50s did not shift with the increase in Fe(II) concentration (data not shown), indicating that these compounds inhibit JMJD2E predominantly by mechanisms other than Fe(II) chelation in solution.
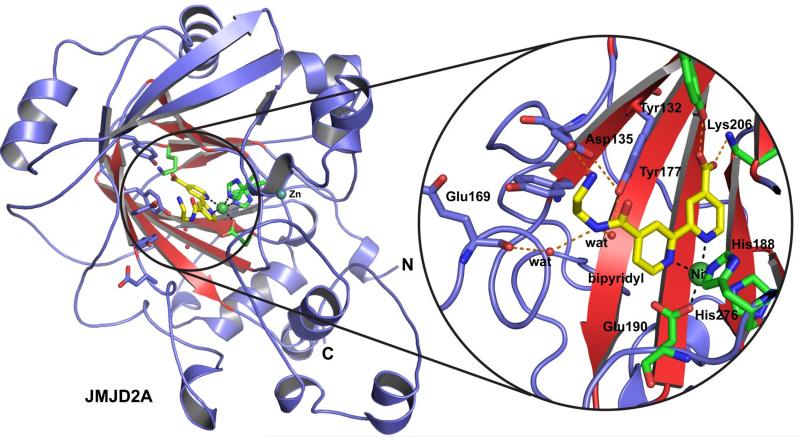
We then worked to obtain a crystal structure for JMJD2A in complex with one of the more potent bipyridyl inhibitors, 13a. A structure was solved for JMJD2A in complex with zinc, nickel (substituting for iron) and 13a to 2.0 Å resolution using a reported structure (PDB ID: 2OX0) as a search model (Figure 3). The structure reveals that 13a binds the active site metal by bidentate chelation through both pyridinyl nitrogens, verifying the proposed overall mode of iron binding of bipyridyl compounds to JMJD2A and likely other 2OG oxygenases including the HIF hydroxylases. Notably, the carboxylate of 13a is positioned to interact with Lys206 and Tyr132 in a manner analogous to that observed for 2OG, so rationalising the difference in potency resulting from addition of a C-4 carboxylate to one of the pyridinyl rings. The amide nitrogen of 13a is positioned to form two hydrogen bonds with water molecules which in turn form hydrogen bonds to the phenol oxygen of Tyr177 and to the backbone carbonyl oxygen of Glu169, and an electrostatic interaction with Asp135 (2.7 Å). For other potent inhibitors such as 15c-e it is likely that the lack of the salt bridge with Asp135, and the flexibility of the aliphatic chain, would allow the inhibitors to adopt conformations placing the aliphatic/aromatic sidechains in the substrate binding region of the active site. This general mode of binding would also explain the relatively similar potencies of compounds 15c-e, if the sidechains are projecting into the solvent-accessible region of the substrate binding groove.
Figure 3.
Views from the crystal structure of JMJD2A in complex with compound 13a (yellow sticks). The double-stranded β-helix (conserved in 2OG oxygenases) is in red. Residues that bind Fe(II) and 2OG are shown as green sticks, Ni(II), which replaces Fe(II) for crystallography, is shown as a green sphere. Other residues likely interacting with 13a are shown as blue sticks.
Comparison of the crystal structure of the JMJD2A.13a complex with structures of JMJD2A in complex with fragments of its histone H3K9 and K36 substrates (PDB IDs 2OQ6 and 2OS2) reveals that, although 13a does not occupy the Nε-methyllysine binding groove, it is likely to block binding of the peptide substrate by occupying part of the binding site for the peptide backbone.
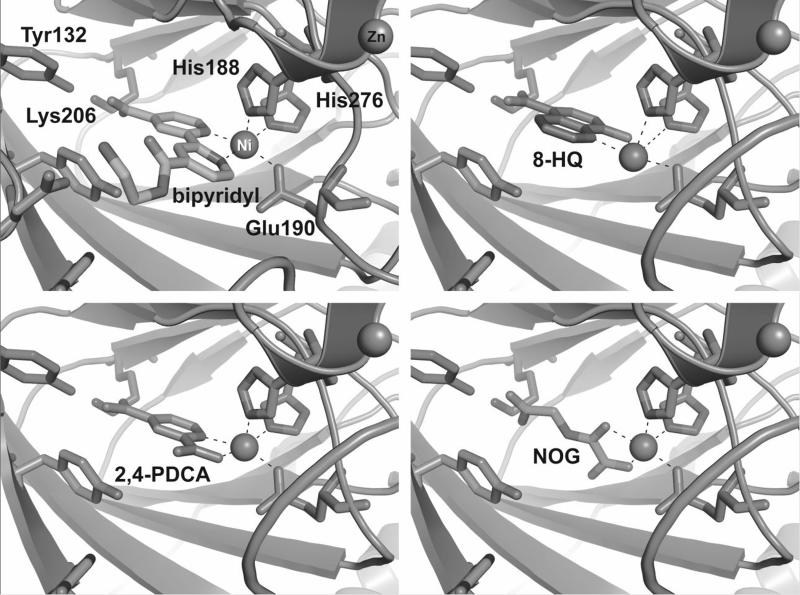
Comparison of the binding mode of compound 13a with crystal structures of other inhibitors in complex with JMJD2A (2,4-PDCA, 5-carboxy-8-hydroxyquinoline, N-oxalyl-D-(O-benzyl)tyrosine and N-oxalylglycine) reveals that the bipyridyl compound binds in the same plane as the other two aromatic inhibitors, 2,4-PDCA and 5-carboxy-8-hydroxyquinoline, occupying the two coordination sites opposite His276 and Glu190 (Figure 4).[11, 12, 17, 19, 20] In contrast, 2OG, N-oxalylglycine and N-oxalyl-D-(O-benzyl)tyrosine coordinate opposite Glu190 and His188. In the case of 2OG this presumably leaves the site opposite His276 available for the binding of molecular oxygen. Although the situation with iron may be different to that for nickel (used for crystallography), these observations suggest that compounds such as 13a, 2,4-PDCA and 5-carboxy-8-hydroxyquinoline may inhibit JMJD2 demethylases not only by competition with 2OG, but also by occupation of the oxygen binding site.
Figure 4.
Comparison of metal coordination by various JMJD2 demethylase inhibitors. The bipyridyl compound 13a, 8-hydroxyquinoline (8HQ) and 2,4-pyridine-dicarboxylate inhibitors coordinate the active site metal (Ni(II) substitutes for Fe(II) for crystallography) by occupying the two coordination sites opposite His276 and Glu190. In contrast, N-oxalylglycine (NOG), a 2OG analogue, coordinates by occupying the coordination sites opposite Glu190 and His188.
Overall we have shown that modifications to the 4,4′-dicarboxy-2,2′-bipyridine template can result in substantial increases in potency against JMDJ2E (IC50 of 6.6 μM for the lead compound 8a, while the most potent compound identified, 15c, had an IC50 of 110 nM, which represents a 66-fold improvement in potency).[11] These increases in potency are, at least in part, likely mediated by both hydrophobic/π-π interactions and, in the case of some compounds (e.g. 13a), by electrostatic interactions. It is possible that potency of the bipyridyl compounds can be increased further by using the crystal structure of 13a in complex with JMJD2A, including by further derivatisation of the 4-carboxylate to occupy the Nε-methyllysine side chain binding groove. Notably, bipyridyl compounds have been found to inhibit both the collagen prolyl-4-hydroxylases[15, 21] and the hypoxia inducible factor prolyl-4-hydroxylases,[16] suggesting that these compounds might be relatively generic inhibitors of 2OG oxygenases. Our work could thus enable the rational structure-based functionalisation of the bipyridyl template to enable the production of inhibitors with different selectivities for use in biological research and medicinal applications.
Experimental Section
Experimental details, including compound synthesis, inhibition assays, protein purification and crystallography, are contained in the electronic supporting information available online.
Supplementary Material
Acknowledgements
We thank the Wellcome Trust, the Biotechnology and Biological Sciences Research Council and the European Union for funding.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author.
References
- [1].Cloos PA, Christensen J, Agger K, Helin K. Genes Dev. 2008;22:1115. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lim S, Metzger E, Schule R, Kirfel J, Buettner R. Int J Cancer. 2010;127:1991. doi: 10.1002/ijc.25538. [DOI] [PubMed] [Google Scholar]
- [3].Loenarz C, Schofield CJ. Trends Biochem Sci. 2011;36:7. doi: 10.1016/j.tibs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [4].Dambrova M, Liepinsh E, Kalvinsh I. Trends Cardiovasc Med. 2002;12:275. doi: 10.1016/s1050-1738(02)00175-5. [DOI] [PubMed] [Google Scholar]
- [5].Simkhovich BZ, Shutenko ZV, Meirena DV, Khagi KB, Mezapuke RJ, Molodchina TN, Kalvlns IJ, Lukevics E. Biochem Pharmacol. 1988;37:195. doi: 10.1016/0006-2952(88)90717-4. [DOI] [PubMed] [Google Scholar]
- [6].Yan L, Colandrea VJ, Hale JJ. Expert Opin Ther Pat. 20:1219. doi: 10.1517/13543776.2010.510836. [DOI] [PubMed] [Google Scholar]
- [7].Franklin TJ. Int J Biochem Cell B. 1997;29:79. doi: 10.1016/s1357-2725(96)00121-5. [DOI] [PubMed] [Google Scholar]
- [8].Myllyharju J. Ann of Med. 2008;40:402. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]
- [9].Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. Nature. 2006;442:307. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- [10].Hamada S, Kim TD, Suzuki T, Itoh Y, Tsumoto H, Nakagawa H, Janknecht R, Miyata N. Bioorg Med Chem Lett. 2009;19:2852. doi: 10.1016/j.bmcl.2009.03.098. [DOI] [PubMed] [Google Scholar]
- [11].Rose NR, Ng SS, Mecinović J, Liénard BMR, Bello SH, Sun Z, McDonough MA, Oppermann U, Schofield CJ. J Med Chem. 2008;51:7053. doi: 10.1021/jm800936s. [DOI] [PubMed] [Google Scholar]
- [12].King ONF, Li XS, Sakurai M, Kawamura A, Rose NR, Ng SS, Quinn AM, Rai G, Mott BT, Beswick P, Klose RJ, Oppermann U, Jadhav A, Heightman TD, Maloney DJ, Schofield CJ, Simeonov A. PLoS ONE. 2010;5:e15535. doi: 10.1371/journal.pone.0015535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hamada S, Suzuki T, Mino K, Koseki K, Oehme F, Flamme I, Ozasa H, Itoh Y, Ogasawara D, Komaarashi H, Kato A, Tsumoto H, Nakagawa H, Hasegawa M, Sasaki R, Mizukami T, Miyata N. J Med Chem. 2010;53:5629. doi: 10.1021/jm1003655. [DOI] [PubMed] [Google Scholar]
- [14].Sekirnik R, Rose NR, Thalhammer A, Seden PT, Mecinovic J, Schofield CJ. Chem Commun (Camb) 2009:6376. doi: 10.1039/b916357c. [DOI] [PubMed] [Google Scholar]
- [15].Hales NJ, Beattie JF. Journal of Medicinal Chemistry. 1993;36:3853. doi: 10.1021/jm00076a014. [DOI] [PubMed] [Google Scholar]
- [16].Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr. Proc Natl Acad Sci U S A. 2002;99:13459. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rose NR, Woon EC, Kingham GL, King ON, Mecinovic J, Clifton IJ, Ng SS, Talib-Hardy J, Oppermann U, McDonough MA, Schofield CJ. J Med Chem. 2010;53:1810. doi: 10.1021/jm901680b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sakurai M, Rose NR, Schultz L, Quinn AM, Jadhav A, Ng SS, Oppermann U, Schofield CJ, Simeonov A. Mol Biosyst. 2010;6:357. doi: 10.1039/b912993f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Couture JF, Collazo E, Ortiz-Tello PA, Brunzelle JS, Trievel RC. Nat Struct Mol Biol. 2007;14:689. doi: 10.1038/nsmb1273. [DOI] [PubMed] [Google Scholar]
- [20].Ng SS, Kavanagh KL, McDonough MA, Butler D, Pilka ES, Lienard BMR, Bray JE, Savitsky P, Gileadi O, von Delft F, Rose NR, Offer J, Scheinost JC, Borowski T, Sundstrom M, Schofield CJ, Oppermann U. Nature. 2007;448:87. doi: 10.1038/nature05971. [DOI] [PubMed] [Google Scholar]
- [21].Franklin TJ, Morris WP, Edwards PN, Large MS, Stephenson R. Biochem J. 2001;353:333. doi: 10.1042/0264-6021:3530333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.