Abstract
Background
Chronic Lymphocytic Leukemia (CLL) is a lymphoproliferative disease characterized by multiple recurring clonal cytogenetic anomalies and is the most common leukemia in adults. Chromosomal abnormalities associated with CLL include trisomy 12 and IGH;BCL3 rearrangement [t(14;19)(q32;q13)] that juxtaposes a proto-oncogenic gene BCL3 and an immunoglobulin heavy chain, a translocation that may be associated with shorter survival. In addition to the IGH;BCL3 rearrangement, other translocations involving 14q32 locus are involved in various lymphoproliferative pathologies pointing toward the significance of IGH locus in oncogenic progression. Significantly, in the majority of B-cell neoplasms that carry an IGH;BCL3 rearrangement, it is a sole translocation involving an IGH locus.
Case Presentation
We report a patient who, in addition to trisomy 12, carried a rare double-hit translocation characterized by the IGH;BCL3 translocation and an additional clonal IGH;BCL2 translocation involving IGH and another proto-oncogene BCL2, t(14;18)(q32;q21), commonly found in follicular lymphoma. Further single nucleotide polymorphism (SNP) array-based analysis detected a duplication of the 58.8 kb region at 19q13.32 adjacent to the BCL3 translocation junction on chromosome 19q13. Interestingly, the duplicated region contained ERCC2 gene, which encodes a DNA excision repair protein involved in the cancer-prone syndrome, xeroderma pigmentosum.
Conclusions
Taken together our findings indicate the existence of double-translocation driven oncogenic events involving both IGH loci and proto-oncogenes BCL2 and BCL3. Importantly, the IGH;BCL3 translocation was characterized by the duplication of the genomic region adjacent to BCL3, containing a major DNA repair factor, ERCC2.
Electronic supplementary material
The online version of this article (doi:10.1186/s13039-015-0203-y) contains supplementary material, which is available to authorized users.
Keywords: B-cell lymphoma/leukemia, Double IGH;BCL2 and IGH;BCL3 translocation, ERCC2 duplication
Background
Chronic lymphocytic leukemia (CLL) is a genetically heterogeneous neoplasm characterized by the progressive accumulation of B cells in bone marrow, lymph nodes and blood. The progression of the disease is highly variable, ranging from the indolent state to the highly aggressive leukemia marked by short survival times. Numerous chromosomal abnormalities have been shown to contribute to CLL, including but not limited to trisomy 12, loss of 11q22-q23 containing the ATM gene, loss of 13q14.3 and 6q, loss of 17p13 containing TP53 gene, and others [1].
A specific translocation [t(14;19)(q32;q13)] which juxtaposes the immunoglobulin heavy chain locus (IGH) sequence (HUGO, 14q32.33) and the gene encoding an anti-apoptotic protein BCL3 resulting in overexpression of BCL3 [2], is of a particular interest because it does not occur frequently, and it is usually associated with shorter survival [1]. In addition, this translocation as well as other translocations involving the IGH locus, although found in CLL, have been also described in poorly clinicopathologically described B cell lymphomas, categorized as atypical CLL [3]. Another translocation involving IGH locus and an anti-apoptotic protein BCL2, t(14;18)(q32;q21), is considered a hallmark of aggressive lymphomas, such as follicular lymphomas (FL) [4, 5] and diffuse large B-cell lymphomas (DLBCL) [6–8].
We report a patient who was evaluated for leukocytosis with lymphocytosis, and who was found to have a marked bone marrow involvement by neoplastic lymphocytes showing a B-cell line of differentiation as determined by flow cytometry and immunohistochemistry. Morphologically, the lymphocytes were small to medium in size, and a subset showed knobby cytoplasmic blebbing. Further immunophenotypic studies ruled out involvement by typical CLL or mantle cell lymphoma in this patient, highlighting an atypical characteristic of this malignancy. Surprisingly, in addition to trisomy 12, which is commonly found in CLL, chromosome analysis at the haploid band resolution 300-400 revealed the presence of clones carrying a single t(14;19)(q32;q13) IGH;BCL3 translocation or both, t(14;19)(q32;q13) IGH;BCL3 and t(14;18)(q32;q21) IGH;BCL2 translocations. Whereas single translocation events involving IGH and BCL3 or BCL2 loci can be detected in lymphoid cancers, double-hit translocations involving both IGH;BCL2 and IGH;BCL3 rearrangements in the same patient are exceptionally rare. Additionally, using SNP array analysis we detected a local microduplication event at the BCL3 translocation junction on chromosome 19 involving a DNA repair factor ERCC2. An additional file describing experimental procedures is available (see Additional file 1). However, due to the nature of the SNP array platform we cannot exclude the possibility that ERCC2 duplication occurred on the non-rearranged chromosome 19. Given the absence of classical CLL and mantle cell lymphoma immunophenotype, size and morphology of the neoplastic lymphocytes, and the presence of B-cell lymphoproliferative genetic markers characteristic of aggressive B-cell lymphomas, we favor the diagnosis of an aggressive monoclonal B-cell lymphoma/leukemia, likely B-prolymphocytic leukemia in this patient driven by the IGH;BCL3 and IGH;BCL2 mediated mechanisms.
Case presentation
Our patient was an 82-year-old African-American female who presented to her oncologist for leukocytosis. Complete blood count (CBC) data showed a white blood cell (WBC) count of 24.5 K/ MicroL with relative and absolute lymphocytosis of 70 % and 17.2K/ MicroL respectively. A bone marrow biopsy and aspirate was performed and the specimen was sent to the hematopathologist for evaluation. Flow cytometric analysis showed 40 % monoclonal B-cells with lambda light chain restriction of moderate intensity with dim CD5 co-expression and the following immunophenotype: CD10-, CD19+, CD20+, CD200-, CD23 +/- (dim), FMC7 +/- (dim), CD38-, CD25-, CD103- and CD11c-.
By histology the bone marrow showed marked involvement by lymphocytes with interstitial pattern of distribution estimated at 80–90 % of the total marrow cellularity. H&E staining, CD20 and Pax5 immunohistochemistry confirmed the nature of the lymphocytes consistent with B-cell line of differentiation (Fig. 1a and b and data not shown). No lymphoid aggregate formation was seen. Morphologically the lymphocytes were small to medium in size and showed clumped nuclear chromatin with small but conspicuous nucleoli and no morphologic features suggestive of involvement by DLBCL, Burkitt lymphoma or other high-grade aggressive B-cell lymphomas.
Fig. 1.
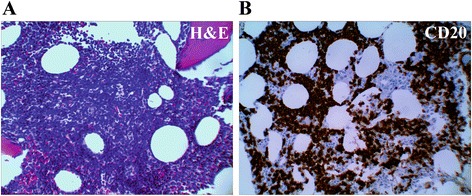
a H&E staining of the bone marrow showing marked interstitial involvement by medium sized cells with small but conspicuous nucleoli. b CD20 immunohistochemistry highlights marked involvement of the bone marrow by abnormal B-cells
By immunohistochemistry the B-cells were negative for Cyclin-D1 and, additionally, they were negative for Sox-11, arguing against the diagnosis of mantle cell lymphoma or Cyclin-D1 negative mantle cell lymphoma, respectively. Evaluation of the aspirate smear showed the lymphocytes with occasional small knobby cytoplasmic blebbing but no discernible villous hairy projections.
Overall, based on the immunophenotypic finding by flow cytometric analysis, it is unlikely that this lymphoma represents typical CLL, since it not only expresses monoclonal light chain with moderate intensity, it also shows weak CD23 expression and is negative for CD200. The possibility of mantle cell lymphoma and Cyclin D-1 negative mantle cell lymphoma was considered and further evaluated but ruled out by negative staining with Cyclin D1 and Sox-11 immunohistochemistry respectively. Given the morphologic observation of knobby cytoplasmic blebbing, the diagnosis of B-PLL (B- prolymphocytic leukemia) was considered a strong possibility.
Cytogenetic studies of bone marrow preparations from this patient revealed two related abnormal clones in eight of twenty-two metaphase spreads examined. The first clone (stemline [sl]), six cells, contained a translocation between the long (q) arm of chromosomes 14 and 19 (Fig. 2a, arrows), and gain of one copy of chromosome 12 (underlined). The second clone, a composite of two cells, was a doubling of the stemline clone (the first clone) with two copies of an additional translocation between 14q and 18q (Fig. 3a, red arrows, six copies of chromosome 12 are underlined). The remaining fourteen cells contained a normal karyotype. Additionally, FISH studies using BCL3 and IGH break-apart probes confirmed BCL3 and IGH translocations in the first clone (Fig. 2b and c, arrows). With respect to the second clone, IGH;BCL2 dual-fusion probe picked up eight signals for IGH (Fig. 3b, black and white IGH image) which, in combination with karyotype data, was indicative of the presence of eight IGH derivative translocation products. This suggests that all IGH loci are rearranged in the second clone in addition to the near duplication of the chromosome content. BCL2 probe picked up six BCL2 signals (Fig. 3b, black and white BCL2 image) indicating on the presence of four translocated BCL2 loci and two intact BCL2 loci from the non-translocated chromosome 18. Importantly, IGH and BCL2 signals formed four fusions (Fig. 3b, “merge” panel, arrows) confirming two derivative IGH;BCL2 loci on chromosomes 14 and chromosome 18. In addition, IGH break-apart probe confirmed the presence of four translocated IGH loci (Fig. 3b, right panel, four red and four green signals). In summary, first clone was characterized by the IGH;BCL3 translocation event resulting in derivative chromosomes, 14 and 19 (Fig. 4a) and second clone was characterized by the presence of IGH;BCL2 translocation in addition to IGH;BCL3 translocation and near tetraploid chromosome content resulting in four derivative chromosomes 14 and two derivative chromosomes 18 and 19 (Fig. 4b).
Fig. 2.
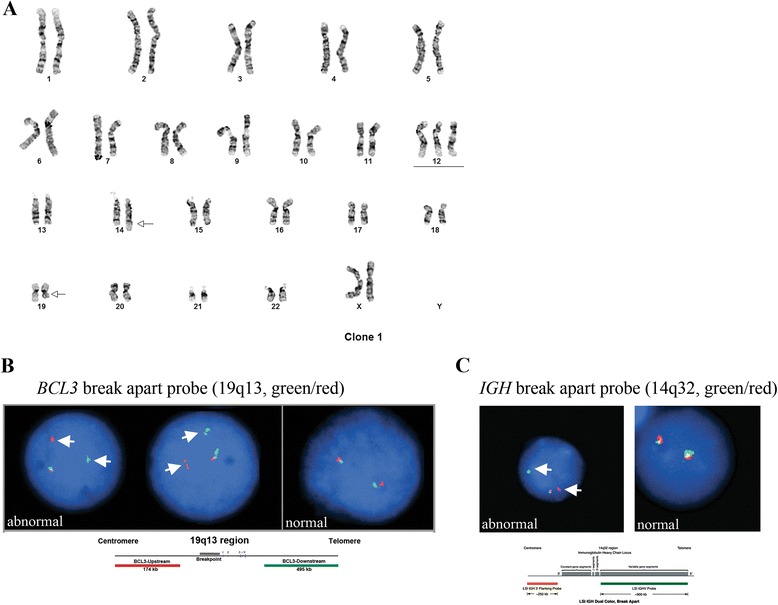
a Karyotype of the first clone. The first clone (stemline [sl]), six cells, contained a translocation between the long (q) arms of chromosomes 14 and 19 (arrows), and gain of one copy of chromosome 12 (underlined). b BCL3 break-apart FISH probe (green/red) indicates rearrangement of a BCL3 locus in the first clone (arrows). Normal FISH signal is shown on the right side of the panel for comparison. c IGH break-apart FISH probe (green/red) indicates rearrangement of an IGH locus in the first clone (arrows). Normal FISH signal is shown on the right side of the panel for comparison
Fig. 3.
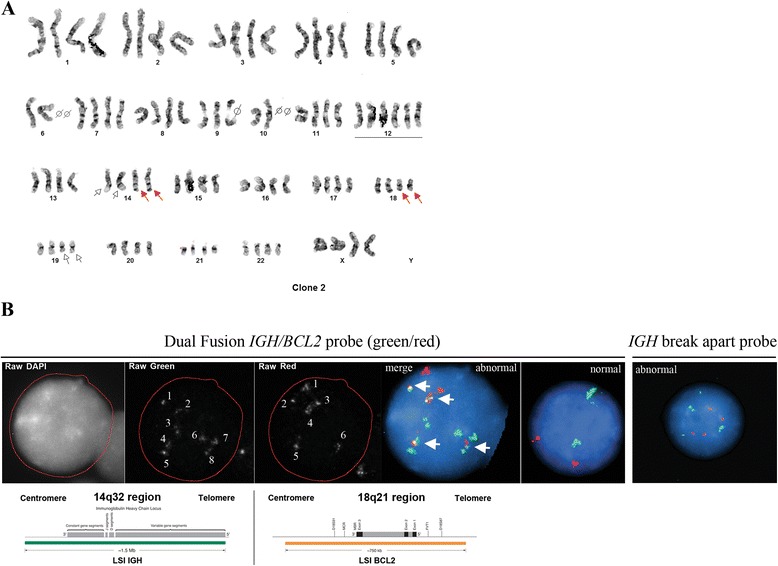
a Representative karyotype of the second clone. The second clone, a composite of two cells, was a doubling of the stemline clone (the first clone) with two copies of a translocation between 14q and 18q (IGH;BCL2, red arrows), in addition to a 14q and 19q translocation (IGH;BCL3, white arrows). Six copies of chromosome 12 are underlined. b FISH analysis of the second clone using dual fusion IGH;BCL2 probe (green/red). Black and white image for IGH signal shows 8 loci indicating that all IGH sequences are rearranged and present on derivative chromosomes 14, 18, and 19. Black and white image for BCL2 signal shows 6 loci which represent two intact and two derivative chromosomes 18, and two derivative chromosomes 14. Merged image shows green IGH signals and red BCL2 signals which overlap in four loci, arrows (two copies of derivative chromosome 14 and two copies of derivative chromosome 18). Right panel, IGH break-apart probe (green/red) signal showing four green and four red foci indicative of the presence of four rearranged IGH loci
Fig. 4.
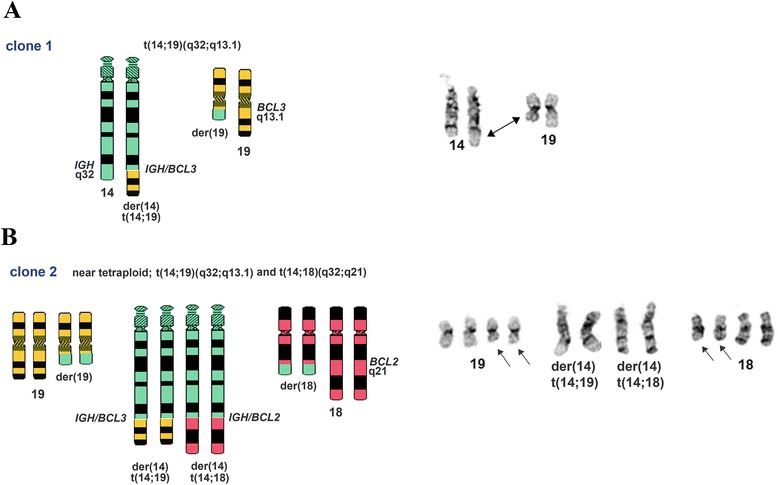
Schematic representation of the rearrangements described in clone 1 and clone 2. Chromosomes involved in rearrangements are shown on the right. a Rearrangements in clone 1 involving IGH (14q32) and BCL3 (19q13.1) which results in two derivative chromosomes (der(19) and der(14). Rearranged chromosomes 14 and 19 are indicated by an arrow on the right. b Rearrangements in clone 2 which includes chromosome duplication event and IGH (14q32) and BCL2 (18q21) translocation in addition to IGH;BCL3 rearrangements. Therefore clone 2 contains 8 derivative chromosomes (a pair of each der(19), der(18), der(14)t(14;19), and der(14)t(14;18)). Rearranged chromosomes 18 and 19 are indicated by the arrows on the right
The patient’s ISCN was 47,XX,+12,t(14;19)(q32;q13.1)[6]/89 ~ 90,slx2,t(14;18)(q32;q21)x2[cp2]/46,XX[14].nuc ish(D11Z1,ATM)x2[198],(CCND1x2,IGHx3)[78/200],(D12Z3x3,D13S319x2)[117/200],(IGHx2)(5'IGH sep 3'IGHx1)[153/300]/(IGHx4)(5'IGH sep 3'IGHx4)[1/300],(IGHx3,BCL2x2)[123/300]/(IGHx8,BCL2x6)(IGH con BCL2x4)[1/300],(TP53,D17Z1)x2[189],(BCL3x2)(5'BCL3 sep 3'BCL3x1)[99/200].
Further studies using single nucleotide polymorphism arrays on the patient’s DNA sample detected the presence of an extra chromosome 12, confirming our karyotype analysis (Fig. 5a). Interestingly, we also detected a microduplication event at the cytogenetically defined BCL3 translocation junction (19q13.1) containing a major nucleotide excision repair gene ERCC2 (ISCN arr[hg19](12)X3,19q13.32(45,835,983-45,894,768)x3), (Fig. 5b-d). Polymorphisms in this gene are associated with xeroderma pigmentosum (XP), XP associated with Cockayne syndrome (XP/CS), and trichothiodystrophy (TTD) [9]. However, we do not exclude the possibility that ERCC2 duplication occurred on the intact chromosome 19.
Fig. 5.
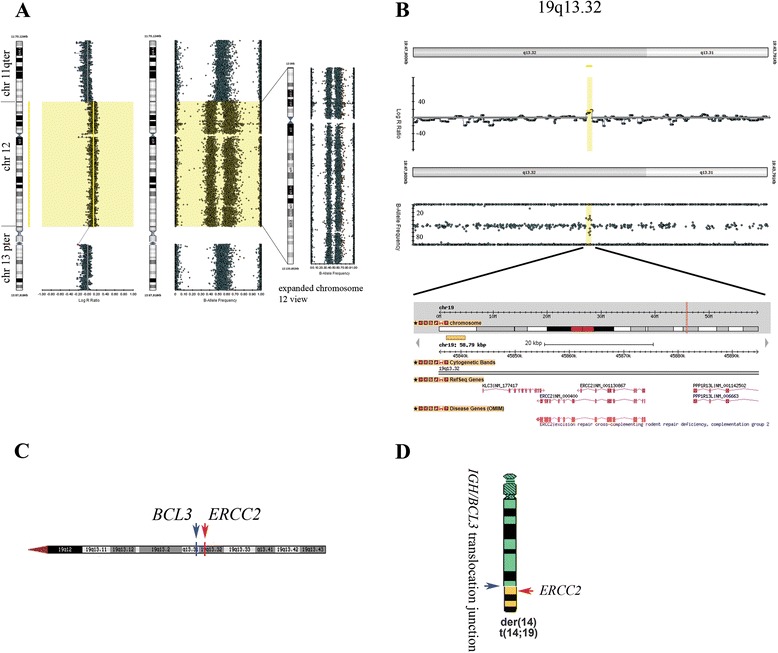
Single Nucleotide Polymorphism (SNP) array analysis. a Confirmation of an extra copy of chromosome 12. In the Log R ratio panel (left) chromosomal boundaries between the q terminal of chromosome 11, chromosome 12, and the p terminal of chromosome 13 are demarcated by lines. The expanded chromosome 12 view (right), shows an isolated view of chromosome 12 (B-Allele frequency). b Microduplication of the genomic region containing ERCC2 gene. c Genomic mapping of ERCC2 gene located in the 19q13.32 cytogenetic band, proximal to the BCL3 gene locus (19q13.31). d Placement of the ERCC2 gene relative to the hypothesized IGH;BCL3 rearrangement junction depicted in the schematics (the possibility exists that ERCC2 duplication occurred on the non-translocated chromosome 19 as stated in text)
Conclusions
In this report we describe a case of a monoclonal B-cell lymphoma/leukemia with extensive bone marrow involvement and CLL-like immunophenotype showing CD5 expression by flow cytometric analysis and furthermore displaying a double translocation event between IGH loci and both BCL3 and BCL2 gene loci. Additionally, we detected a microduplication event in the genomic locus adjacent to BCL3 gene involving a nucleotide excision repair protein ERCC2. Our karyotype analysis identified two abnormal clones in this patient. The first clone contained, in addition to trisomy 12, a single cytogenetically defined translocation t(14;19)(q32;q13) involving IGH;BCL3 loci. The second clone represented a near duplication of the chromosome content of the first clone and the presence of two copies of an additional translocation involving IGH locus and BCL2 gene t(14;18)(q32;q21). Therefore, all IGH loci located on the chromosome 14 were rearranged in the second clone which was confirmed by our FISH studies.
These results are consistent with a neoplastic process in this patient’s bone marrow specimen. Importantly, the translocation t(14;19)(q32;q13) involving IGH and BCL3 loci, which results in altered BCL3 expression, has been described in aggressive forms of lymphomas and atypical CLLs [3, 10]. The translocation t(14;18)(q32;q21) is a recurring abnormality in B-cell lymphomas. This translocation places the oncogene BCL2 on 18q21 within the immunoglobulin heavy chain locus on 14q32 and therefore deregulates the BCL2 function. Juxtaposition of both BCL3 and BCL2 genes next to the active IGH locus leads to their altered expression, decreased apoptosis, and leukemia progression [10, 11]. Concurrent rearrangements of BCL2(18q21) and BCL3(19q13) have been reported in atypical chronic lymphocytic leukemia at Richter's transformation [12]. Both BCL3 and BCL2 are proto-oncogenes, however mechanistically, BCL2 functions as a key regulator of apoptosis through the mitochondrial pathways [11], whereas BCL3 is a predominantly nuclear protein recruited to the NFκB-responsive promoters where it regulates apoptotic program [13], suggesting that rearrangements of BCL2 and BCL3 may differentially modulate leukemic progression through distinct molecular pathways. The appearance of the IGH;BCL3 clone followed by the genome near duplication event and the appearance of the second clone containing both IGH;BCL3 and IGH;BCL2 rearrangements in our patient might, therefore, have an additive effect on the development of this neoplasm.
ERCC2 duplication in this patient is an intriguing finding, because of the role of ERCC2 in DNA nucleotide excision repair process. Specific ERCC2 polymorphisms are implicated in susceptibility to melanoma [14] as well as triple negative breast cancer [15]. However, according to the Database of Genomic Variants (http://dgv.tcag.ca/dgv/app/home), duplications of ERCC2 genomic region are also found in healthy individuals. Therefore, the significance of ERCC2 duplication in context of IGH;BCL3 and IGH;BCL2 rearrangements requires further investigation. Additionally, the existence of the microduplication event at the major translocation junction supports the previously reported genomic instability events at the translocation breakpoints and emphasizes the significance of the molecular analysis of sequences adjacent to the cytogenetically defined translocation junctions including balanced rearrangements [16].
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgements
We would like to extend our gratitude to the patient and patient’s family for allowing the publication of the clinical data. We would also like to thank Heidi Zuniga form the Anschutz Medical Campus Health Sciences Library at the University of Colorado-Denver for the administrative support.
Additional file
Experimental procedures. (PDF 269 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RA planned and participated in SNP array experiments, co-wrote the manuscript and participated in data analysis, BC organized the data and participated in data analysis, KH, CJ, SB, JL and LM performed FISH analysis, CJ, YG, AOM, and VM performed karyotype analysis, PB and JL performed SNP array and analyzed SNP array data, DM analyzed and signed out the case, MP is an attending physician overseeing this clinical case, CM performed pathological examination of the patient specimen and contributed to the manuscript preparation, KS directed and supervised the laboratory procedures and co-wrote the manuscript.
Contributor Information
Roman Alpatov, Email: Roman.Alpatov@ucdenver.edu.
Cyrus Manavi, Email: Cyrus.Manavi@wilmed.org.
Karen Swisshelm, Email: karen.swisshelm@ucdenver.edu.
References
- 1.Huret JL, Ahmad M, Arsaban M, Bernheim A, Cigna J, Desangles F, et al. Atlas of genetics and cytogenetics in oncology and haematology in 2013. Nucleic Acids Res. 2013;41(Database issue):D920–4. doi:10.1093/nar/gks1082. http://atlasgeneticsoncology.org/. [DOI] [PMC free article] [PubMed]
- 2.Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60(6):991–7. doi: 10.1016/0092-8674(90)90347-H. [DOI] [PubMed] [Google Scholar]
- 3.Soma LA, Gollin SM, Remstein ED, Ketterling RP, Flynn HC, Rajasenan KK, et al. Splenic small B-cell lymphoma with IGH/BCL3 translocation. Hum Pathol. 2006;37(2):218–30. doi: 10.1016/j.humpath.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Masir N, Campbell LJ, Goff LK, Jones M, Marafioti T, Cordell J, et al. BCL2 protein expression in follicular lymphomas with t(14;18) chromosomal translocations. Br J Haematol. 2009;144(5):716–25. doi: 10.1111/j.1365-2141.2008.07528.x. [DOI] [PubMed] [Google Scholar]
- 5.Masir N, Jones M, Abdul-Rahman F, Florence CS, Mason DY. Variation in BCL2 protein expression in follicular lymphomas without t(14;18) chromosomal translocations. Pathology. 2012;44(3):228–33. doi: 10.1097/PAT.0b013e3283513fb2. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal J, Neppalli VT, Wright G, Dave BJ, Horsman DE, Rosenwald A, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(6):961–8. doi: 10.1200/JCO.2005.03.4264. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal J, Meyer PN, Smith LM, Johnson NA, Vose JM, Greiner TC, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17(24):7785–95. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165(1):159–66. doi: 10.1016/S0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Compe E, Le May N, Egly JM. TFIIH subunit alterations causing xeroderma pigmentosum and trichothiodystrophy specifically disturb several steps during transcription. Am J Hum Genet. 2015;96(2):194–207. doi: 10.1016/j.ajhg.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapiro E, Radford-Weiss I, Bastard C, Luquet I, Lefebvre C, Callet-Bauchu E, et al. The most frequent t(14;19)(q32;q13)-positive B-cell malignancy corresponds to an aggressive subgroup of atypical chronic lymphocytic leukemia. Leukemia. 2008;22(11):2123–7. doi: 10.1038/leu.2008.102. [DOI] [PubMed] [Google Scholar]
- 11.Scarfo L, Ghia P. Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol Lett. 2013;155(1-2):36–9. doi: 10.1016/j.imlet.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Podgornik H, Pretnar J, Skopec B, Andoljsek D, Cernelc P. Concurrent rearrangements of BCL2, BCL3, and BCL11A genes in atypical chronic lymphocytic leukemia. Hematology. 2014;19(1):45–8. doi: 10.1179/1607845413Y.0000000078. [DOI] [PubMed] [Google Scholar]
- 13.Chang TP, Vancurova I. Bcl3 regulates pro-survival and pro-inflammatory gene expression in cutaneous T-cell lymphoma. Biochim Biophys Acta. 2014;1843(11):2620–30. doi: 10.1016/j.bbamcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Yin M, Wang LE, Amos CI, Zhu D, Lee JE, et al. Polymorphisms of nucleotide excision repair genes predict melanoma survival. J Invest Dermatol. 2013;133(7):1813–21. doi: 10.1038/jid.2012.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolarz B, Makowska M, Samulak D, Michalska MM, Mojs E, Wilczak M, et al. Single nucleotide polymorphisms (SNPs) of ERCC2, hOGG1, and XRCC1 DNA repair genes and the risk of triple-negative breast cancer in Polish women. Tumour Biol. 2014;35(4):3495–502. doi: 10.1007/s13277-013-1461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ordulu Z, Wong KE, Currall BB, Ivanov AR, Pereira S, Althari S, et al. Describing sequencing results of structural chromosome rearrangements with a suggested next-generation cytogenetic nomenclature. Am J Hum Genet. 2014;94(5):695–709. doi: 10.1016/j.ajhg.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


