Abstract
Background
In the present study, the methanol extracts from the leaves, as well as compounds namely sigmoidin I (1), atalantoflavone (2), bidwillon A (3), neocyclomorusin (4), 6α-hydroxyphaseollidin (5) and neobavaisoflavone (6) (from the bark extract) were tested for their activities against a panel of Gram-negative bacteria including multi-drug resistant (MDR) phenotypes.
Methods
Broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) and the minimum bactericidal concentrations (MBCs) of the extracts as well as compounds 1–6.
Results
The MIC results indicated that the crude extracts from the leaves and bark of this plant were able to inhibit the growth of 96.3 % of the 27 tested bacteria. Compounds 2–6 displayed selective activities, their inhibitory effects being obtained on 8.3 %, 41.7 %, 58.3 %, 58.3 % and 66.7 % of tested bacteria respectively for 2, 3, 5, 6 and 4. The lowest MIC value of 8 μg/mL was obtained with 6 against Escherichia coli ATCC8739, Enterobacter cloacae ECCI69, Klebsiella pneumoniae KP55, Providencia stuartii NAE16 and Pseudomonas aeruginosa PA01.
Conclusion
The present study demonstrates that Erythrina sigmoidea is a potential source of antibacterial drugs to fight against MDR bacteria. Neobavaisoflavone (6) is the main antibacterial consituents of the bark crude extract.
Keywords: Antibacterial, Erythrina sigmoidea, Compounds, Multidrug resistance, Neobavaisoflavone
Background
Medicinal plants have been used since ancient times in the management of human including microbial infections. Approximately 60 % of world’s population still relies on medicinal plants for their primary healthcare [1]. The African mainland has between 40,000-60,000 plant species, of which approximately 35,000 are endemic [2, 3]. Cameroon has a rich biodiversity, with about 8,620 plants species [4]. Several Camerooninan medicinal plants were previously reported for their antibacterial activities against multi-drug resistant Gram-negative bacteria [5–8]. Some of the them include Beilschmiedia cinnamomea and Echinops giganteus [5], Beilschmiedia obscura, Pachypodanthium staudtii and Peperomia fernandopoiana [9] or Capsicum frutescens [10]. The antimicrobial activities of many secondary metabolites from Cameroonian plants were also reported [11, 12]. In our continuing search of new herbal drug from the Cameroon flora, the present study was designed to demonstrate the antibacterial activity of the extracts and compounds from Erythrina sigmoidea Hua (Fabaceae). Erythrina sigmoidea is a tree of up to 6 m high, with stems armed with stout found in Senegal, Nigeria, Cameroon, Chad and Central African Republic [13]. The plant is traditionally used as antidotes (venomous stings, bites, etc.), diuretic, febrifuge and to treat arthritis, rheumatism, pulmonary troubles, stomach troubles, infectious diseases and kidney diseases [13]. In the Western Region of Cameroon, the aqueous extracts from leaves, bark and roots are used to treat gastrointestinal infections, venereal diseases and leprosy [14]. Previously phytochemical study this plant led to the isolation of sigmoidin I (1), atalantoflavone (2), bidwillon A (3), neocyclomorusin (4), 6α-hydroxyphaseollidin (5), and neobavaisoflavone (6) [15]. They displayed good cytotoxicity towards drug-sensitive and drug resistant cancer cell line [15]. In addition, they showed low cytotoxicity against the normal AML12 hepatocytes [15].
Methods
Plant material and extraction
The leaves and bark of Erythrina sigmoidea (Fabaceae) were collected in April 2013 in Bangangté (West Region of Cameroon). The plant was identified by a botanist of the National Herbarium in Yaoundé, Cameroon and compared with voucher kept under the registration number N°24470/HNC.
Antimicrobial assays
Chemicals for antimicrobial assay
Compounds isolated from the bark of Erythrina sigmoidea included β- sigmoidin I (1), atalantoflavone (2), bidwillon A (3), neocyclomorusin (4), 6α-hydroxyphaseollidin (5) and neobavaisoflavone (6) (Fig. 1). Their isolation and identification were previously reported [15]. Chloramphenicol ≥ 98 % (Sigma-Aldrich, St. Quentin Fallavier, France) was used as reference antibiotics (RA) against Gram-negative bacteria. p-Iodonitrotetrazolium chloride ≥ 97 % (INT, Sigma-Aldrich) was used as microbial growth indicator [16, 17].
Fig. 1.
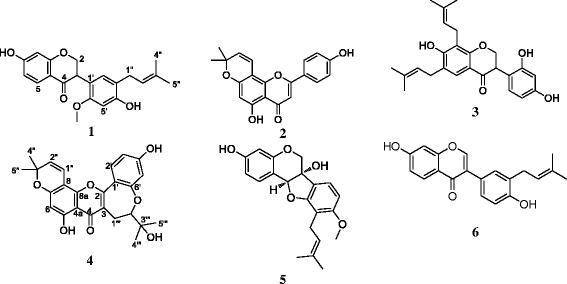
Chemical structures of the compounds isolated from Erythrina sigmoidea. sigmoidin I (1); atalantoflavone (2); bidwillon A (3); neocyclomorusin (4); 6α-hydroxyphaseollidin (5); neobavaisoflavone (6)
Microbial strains and culture media
The studied microorganisms included sensitive and resistant strains of Escherichia coli (ATCC8739, AG100, AG100A, AG100ATET, AG102, MC4100, W3110), Enterobacter aerogenes (ATCC13048, CM64, EA27, EA289, EA294, EA298), Enterobacter cloacae (ECCI69, BM47, BM67), Klebsiella pneumoniae (ATCC12296, KP55, KP63, K24, K2), Providencia stuartii (NEA16, ATCC29916, PS2636, PS299645) and Pseudemonas aeruginosa (PA01, PA124) obtained clinically or from the American Type Culture Collection. Their bacterial features are summarized in Table 1. Nutrient agar was used for the activation of the tested bacteria [18].
Table 1.
Bacterial strains used and their features
| Strains | Features and references | |
|---|---|---|
| Escherichia coli | ||
| ATCC8739 | Reference strain | |
| AG100 | Wild-type E. coli K-12 | [34] |
| AG100A | AG100 ΔacrAB::KANR | [11, 34, 35] |
| AG100ATET | ΔacrAB mutant AG100, with over-expressing acrF gene; TETR | [34] |
| AG102 | ΔacrAB mutant AG100, owing acrF gene markedly over-expressed; TETR | [12, 36] |
| MC4100 | Wild type E. coli | [37] |
| W3110 | Wild type E. coli | [37, 38] |
| Enterobacter aerogenes | ||
| ATCC13048 | Reference strains | |
| CM64 | CHLR resistant variant obtained from ATCC13048 over-expressing the AcrAB pump | [39] |
| EA27 | Clinical MDR isolate exhibiting energy-dependent norfloxacin and chloramphenicol efflux with KANR AMPR NALR STRR TETR | [40, 41] |
| EA289 | KAN sensitive derivative of EA27 | [42] |
| EA294 | EA289 acrA::KANR | [42] |
| EA298 | EA 289 tolC::KANR | [42] |
| Enterobacter cloacae | ||
| ECCI69 | Clinical MDR isolates, CHLR | [5] |
| BM47 | Clinical MDR isolates, CHLR | [5] |
| BM67 | Clinical MDR isolates, CHLR | [5] |
| Klebsiella pneumoniae | ||
| ATCC12296 | Reference strains | |
| KP55 | Clinical MDR isolate, TETR, AMPR, ATMR, CEFR | [43] |
| KP63 | Clinical MDR isolate, TETR, CHLR, AMPR, ATMR | [43] |
| K24 | AcrAB-TolC, Laboratory collection of UNR-MD1, University of Marseille, France | [5] |
| K2 | AcrAB-TolC, Laboratory collection of UNR-MD1, University of Marseille, France | [5] |
| Providencia stuartii | [30] | |
| NEA16 | Clinical MDR isolate, AcrAB-TolC | |
| ATCC29916 | Clinical MDR isolate, AcrAB-TolC | |
| PS2636 | Clinical MDR isolate, AcrAB-TolC | |
| PS299645 | Clinical MDR isolate, AcrAB-TolC | |
| Pseudemonas aeruginosa | ||
| PA 01 | Reference strains | |
| PA 124 | MDR clinical isolate | [44] |
aAMP, ATMR, CEFR, CFTR, CHLR, FEPR, KANR, MOXR, STRR, TETR. Resistance to ampicillin, aztreonam, cephalothin, cefadroxil, chloramphenicol, cefepime, kanamycin, moxalactam, streptomycin, and tetracycline; MDR : Multidrug resistant
INT colorimetric assay for MIC and MBC determinations
MIC determinations on the tested bacteria were conducted using rapid p-iodonitrotetrazolium chloride (INT) colorimetric assay according to described methods [16] with some modifications [19, 20]. The test samples and chloramphenicol were first of all dissolved in DMSO/Mueller Hinton Broth (MHB) or DMSO/7H9 broth. The final concentration of DMSO was lower than 2.5 % and does not affect the microbial growth [21, 22]. The 96- wells microplate were used and the inoculum concentration was 1.5 × 106 CFU/mL [19, 20]. The plates were incubated at 37 °C for 18 h. The assay was repeated thrice. Wells containing adequate broth, bacterial inoculum and DMSO to a final concentration of 2.5 % served as negative control. The MIC of samples was detected after 18 h incubation at 37 °C, following addition (40 μL) of 0.2 mg/mL of INT and incubation at 37 °C for 30 min. Viable bacteria reduced the yellow dye to a pink. MIC was defined as the sample concentration that prevented the color change of the medium and exhibited complete inhibition of microbial growth [16]. The MBC was determined by adding 50 μL aliquots of the preparations, which did not show any growth after incubation during MIC assays, to 150 μL of adequate broth. These preparations were incubated at 37 °C for 48 h. The MBC was regarded as the lowest concentration of extract, which did not produce a color change after addition of INT as mentioned above [19, 20].
Results and discussion
Compounds tested in this study included five isoflavonoids: atalantoflavone (2), bidwillon A (3), neocyclomorusin (4), 6α-hydroxyphaseollidin (5), neobavaisoflavone (6) and one flavonoid: sigmoidin I (1) (Fig. 1). Their isolation and identification from the bark of Erythrina sigmoidea were previously reported [15]. These compounds as well as the crude extracts from the leaves and bark of Erythrina sigmoidea were tested for their antibacterial activities on a panel bacterial strains and the results are reported in Tables 2 and 3.
Table 2.
MICs and MBC (μg/mL) of the crude extracts from Erythrina sigmoidea and chlroramphenicol on the panel of tested bacteria
| Bacterial strains | Tested plant samples, MIC and MBC (μg/ml) and ratio MBC/MIC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Erythrina sigmoidea leaves extract | Erythrina sigmoidea bark extract | Chloramphenicol | |||||||
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| Escherichia coli | |||||||||
| ATCC8739 | 64 | 64 | 1 | 16 | 64 | 4 | 4 | 64 | 16 |
| AG100 | 32 | 256 | 8 | 32 | 128 | 4 | 8 | >512 | na |
| AG100A | 512 | 1024 | 2 | 256 | 1024 | 4 | 4 | >512 | na |
| AG100ATET | 1024 | 1024 | 1 | 256 | 512 | 2 | 32 | >512 | na |
| AG102 | 512 | 1024 | 2 | 128 | 1024 | 8 | 8 | >512 | na |
| MC4100 | 1024 | 1024 | 1 | 512 | 512 | 1 | 32 | >512 | na |
| W3110 | 512 | 512 | 1 | 512 | 512 | 1 | 8 | >512 | na |
| Enterobacter aerogenes | |||||||||
| ATCC13048 | 128 | 256 | 2 | 128 | 1024 | 8 | 16 | 128 | 8 |
| CM64 | 1024 | >1024 | na | 1024 | na | 512 | >512 | na | |
| EA27 | 256 | 256 | 1 | 64 | 128 | 2 | 128 | >512 | na |
| EA289 | 1024 | >1024 | na | 512 | >1024 | na | 512 | >512 | na |
| EA298 | 512 | 512 | 1 | 512 | 1024 | 2 | 256 | >512 | na |
| EA294 | 64 | 512 | 8 | 16 | 128 | 8 | 4 | 32 | 8 |
| Enterobacter cloacae | |||||||||
| ECCI69 | 1024 | >1024 | na | 1024 | >1024 | na | 256 | >512 | na |
| BM47 | 1024 | 1024 | 1 | 1024 | 1024 | 1 | 512 | >512 | na |
| BM67 | 1024 | >1024 | na | 1024 | 1024 | 1 | 256 | >512 | na |
| Klebsiella pneumoniae | |||||||||
| ATCC11296 | 256 | 256 | 1 | 64 | 512 | 8 | 16 | 128 | 8 |
| KP55 | 512 | >1024 | na | 256 | >1024 | na | 64 | 256 | 4 |
| KP63 | 128 | >1024 | na | 16 | 128 | 8 | 128 | >512 | na |
| K24 | 256 | 512 | 2 | 128 | >1024 | na | 16 | >512 | na |
| K2 | 128 | 1024 | 8 | 64 | 512 | 8 | 16 | 256 | |
| Providencia stuartii | |||||||||
| ATCC29916 | 128 | >1024 | na | 32 | 128 | 4 | 8 | 128 | 16 |
| NAE16 | 128 | 128 | 1 | 32 | >1024 | na | 8 | 256 | 32 |
| PS2636 | 1024 | >1024 | na | 1024 | 1024 | 1 | 64 | >512 | na |
| PS299645 | 512 | 1024 | 2 | 64 | 128 | 2 | 32 | >512 | na |
| Pseudomonas aeruginosa | |||||||||
| PA01 | 1024 | 1024 | 1 | 256 | 256 | 1 | 16 | 256 | 8 |
| PA124 | >1024 | >1024 | na | >1024 | >1024 | na | 64 | |256 | 4 |
na: not applicable
Table 3.
MICs and MBC of compounds from the bark of Erythrina sigmoidea on selected bacteria
| Bacterial strains | Tested compounds, MIC and MBC (μg/ml) and ratio MBC/MIC | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||||||||||
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| Escherichia coli | ||||||||||||||||||
| ATCC8739 | - | - | na | - | - | na | 512 | - | na | 256 | - | na | 512 | - | na | 8 | - | na |
| AG100ATET | - | - | na | 128 | - | na | - | - | na | 256 | - | na | 512 | - | na | 32 | - | na |
| AG102 | - | - | na | - | - | na | 512 | - | na | 128 | 512 | 4 | 512 | 512 | 1 | - | - | na |
| Enterobacter aerogenes | ||||||||||||||||||
| ATCC13048 | - | - | na | - | - | na | - | - | na | 512 | - | na | - | - | na | - | - | na |
| EA289 | - | - | na | - | - | na | - | - | na | - | - | na | - | - | na | - | - | na |
| Enterobacter cloacae | ||||||||||||||||||
| ECCI69 | - | - | na | - | - | na | - | - | na | - | - | na | - | - | na | 8 | 512 | 64 |
| Klebsiella pneumoniae | ||||||||||||||||||
| ATCC11296 | - | - | na | - | - | na | - | - | na | - | - | - | na | - | - | na | ||
| KP55 | - | - | na | - | - | na | 256 | - | na | 256 | 512 | 2 | 512 | - | na | 8 | - | na |
| Providencia stuartii | ||||||||||||||||||
| ATCC29916 | - | - | na | - | - | na | 256 | - | na | 256 | - | na | 512 | - | na | - | - | na |
| NAE16 | - | - | na | - | - | na | - | - | na | 256 | - | na | 512 | - | na | 8 | - | na |
| Pseudomonas aeruginosa | ||||||||||||||||||
| PA01 | - | - | na | - | - | na | 256 | - | na | 256 | - | na | 512 | - | na | 8 | - | na |
| PA124 | - | - | na | - | - | na | - | - | na | - | - | na | - | - | na | 256 | - | na |
sigmoidin I (1); atalantoflavone (2); bidwillon A (3); neocyclomorusin (4); 6α-hydroxyphaseollidin (5); neobavaisoflavone (6); (−): MIC or MBC >512 μg/mL; nt: not tested as MIC was >512 μg/mL
Results of the MIC determinations indicate that crude extracts from leaves and bark of this plants were able to inhibit the growth of 26 of the 27 (96.3 %) tested Gram-negative bacteria, and the obtained MIC values ranged from 16 to 1024 μg/mL (Table 2). Compound 1 was not active whilst 2–6 displayed selective activities (Table 3), the MIC values below or equal to 512 μg/mL being noted on 1/12 (8.3 %), 5/12 (41.7 %), 7/12 (58.3 %), 7/12 (58.3 %) and 8/12 (66.7 %) tested bacteria respectively for 2, 3, 5, 6 and 4. The lowest MIC value of 16 μg/mL for crude extracts was obtained with the bark extract against Escherichia coli ATCC8739, Enterobacter aerogenes EA294 and Klebsiella pneumoniae KP63. The corresponding value for the tested compounds (8 μg/mL) was obtained with 6 against E. coli ATCC8739, Enterobacter cloacae ECCI69, K. pneumoniae KP55, Providencia stuartii NAE16 and Pseudomonas aeruginosa PA01. The antimicrobial activity of a phytochemical (crude extract) has been defined as significant when MIC is below 100 μg/mL, moderate when 100 μg/mL < MIC < 625 μg/mL or low when MIC > 625 μg/mL [4, 23]. On this basis, the crude extracts from Erythrina sigmoidea could be considered as promising herbal drug > In fact, MIC values below 100 μg/mL were obtained with leaves and bark extracts respectively against 3/27 (11.1 %) and 10/27 (37.0 %) tested bacteria. Compound 6 can also be considered as a good antimicrobial agent, as MIC values below 10 μg/mL were obtained on 5/12 (41.7 %) tested bacteria. Interestingly, the bark extract was more active (lower MIC value) than chloramphenicol on some MDR strains such as E. aerogenes EA27, K. pneumoniae KP63, highlighting its good antimicrobial potency. Minimal bactericidal concentration (MBC) values below or equal to 1024 μg/mL were also obtained on 18/27 (66.7 %) and 20/27 (74.1 %) tested bacterial strains respectively for leaves and bark extracts. Data from Tables 2 and 3 indicated that some MBC/MIC ratios were below 4, indicating that the studied extracts exerted bactericidal effects on certain Gram negative bacteria [24–26]. However, a keen look of the MICs and MBCs of compounds indicated that they rather exerted bacteriostatic effects (MBC/MIC > 4) [24–26]. It should be noted that the antibacterial spectra of compounds were lower than that of the bark extract. This suggested that a possible synergistic effect between the constituents of this extract could be expected. It should also be noted that the bark extract was not active on the resistant P. aeruginosa PA124 strains contrary to the isolated compound 6. This can either be due to the fact that this active compound (6) is less concentrated in the initial crude extract or to the possible interactions with other constituent. Regarding the clinical involvement of MDR bacteria in treatment failures [11, 12, 27, 28], the antibacterial activity of the crude extracts as well as that of compound 6 could be considered promising. Pseudomonas aeruginosa is an important nosocomial pathogen, highly resistant to clinically used antibiotics, leading to substantial morbidity and mortality [29]. MDR Enterobacteriaceae, including K. pneumoniae, E. aerogenes, E.cloacae and P. stuartii and E. coli have also been classified as antimicrobial-resistant organisms of concern in healthcare facilities [11, 12, 30].
To the best of our knowledge, the antibacterial activity of the crude extracts from the Erythrina sigmoidea as well as compounds 2–6 against MDR bacteria is being reported for the first time. However, the antibacterial activities of compounds belonging to the classes flavonoids and isoflavonoids are well known [31]. In addition, a preliminary antibacterial study of flavonoids from the stem bark of Erythrina burttii showed that bidwillon A was active against E. coli and Staphylococcus aureus [32]. Neobavaisoflavone also displayed antifungal activity against Aspergillus fumigatus and Cryptococcus neoformans [33]. The present study provides additional information on the antimicrobial potency of neobavaisoflavone (6).
Conclusions
The results of the present study are interesting, taking in account the medical importance of the studied microorganisms. These data provided evidence that the crude extracts from Erythrina sigmoidea as well as some of its constituents, and mostly neobavaisoflavone (6) could be potential antimicrobial drugs to fight MDR bacterial infections.
Acknowledgements
Authors are thankful to the Cameroon National Herbarium for identification of the plant.
Footnotes
Competing interests
The authors declare that there are no competing interest.
Authors’ contributions
DEJ, JAKN, LPS and LKO carried out the study; VK designed the experiments, wrote the manuscript, and provided the bacterial strains; BTN and VK supervised the work; all authors read and approved the final manuscript.
References
- 1.WHO WHO. Tuberculosis, Fact sheet No. 104. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 2.United Nation Environment Programme. Biodiversity in Africa; 2011. http://www.eoearth.org/view/article/150570. Accessed on February 02, 2012.
- 3.Kuete V, Efferth T. African flora has the potential to fight multidrug resistance of cancer. BioMed Research International. 2015;2015:914813. doi: 10.1155/2015/914813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 2010;1:123. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fankam AG, Kuete V, Voukeng IK, Kuiate JR, Pages JM. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement Altern Med. 2011;11:104. doi: 10.1186/1472-6882-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voukeng IK, Kuete V, Dzoyem JP, Fankam AG, Noumedem JA, Kuiate JR, et al. Antibacterial and antibiotic-potentiation activities of the methanol extract of some cameroonian spices against Gram-negative multi-drug resistant phenotypes. BMC Res Notes. 2012;5:299. doi: 10.1186/1756-0500-5-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement Altern Med. 2013;13(1):164. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seukep JA, Fankam AG, Djeussi DE, Voukeng IK, Tankeo SB, Noumdem JA, et al. Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. Springerplus. 2013;2:363. doi: 10.1186/2193-1801-2-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fankam AG, Kuiate JR, Kuete V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant Gram-negative phenotypes. BMC Complement Altern Med. 2014;14:241. doi: 10.1186/1472-6882-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touani FK, Seukep AJ, Djeussi DE, Fankam AG, Noumedem JA, Kuete V. Antibiotic-potentiation activities of four Cameroonian dietary plants against multidrug-resistant Gram-negative bacteria expressing efflux pumps. BMC Complement Altern Med. 2014;14:258. doi: 10.1186/1472-6882-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuete V, Ngameni B, Tangmouo JG, Bolla JM, Alibert-Franco S, Ngadjui BT, et al. Efflux pumps are involved in the defense of Gram-negative bacteria against the natural products isobavachalcone and diospyrone. Antimicrob Agents Chemother. 2010;54(5):1749–1752. doi: 10.1128/AAC.01533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuete V, Alibert-Franco S, Eyong KO, Ngameni B, Folefoc GN, Nguemeving JR, et al. Antibacterial activity of some natural products against bacteria expressing a multidrug-resistant phenotype. Int J Antimicrob Agents. 2011;37(2):156–161. doi: 10.1016/j.ijantimicag.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Burkill H. The useful plants of West Tropical Africa. London: Royal Botanic Garden Kew; 1985. [Google Scholar]
- 14.Mabeku L, Kuiate J, Oyono E. Screening of some plants used in the cameroonian folk medicine for the treatment of infectious diseases. Int J Biol. 2011;3:13–21. [Google Scholar]
- 15.Kuete V, Sandjo LP, Djeussi DE, Zeino M, Kwamou GM, Ngadjui B, et al. Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Invest New Drugs. 2014;32:1053–1062. doi: 10.1007/s10637-014-0137-y. [DOI] [PubMed] [Google Scholar]
- 16.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64(8):711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 17.Mativandlela SPN, Lall N, Meyer JJM. Antibacterial, antifungal and antitubercular activity of (the roots of) Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root extracts. S Afr J Bot. 2006;72(2):232–237. doi: 10.1016/j.sajb.2005.08.002. [DOI] [Google Scholar]
- 18.Kuete V, Kamga J, Sandjo LP, Ngameni B, Poumale HM, Ambassa P. et al. Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae). BMC Complement Altern Med. 2011;11:6. [DOI] [PMC free article] [PubMed]
- 19.Kuete V, Nana F, Ngameni B, Mbaveng AT, Keumedjio F, Ngadjui BT. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae) J Ethnopharmacol. 2009;124(3):556–561. doi: 10.1016/j.jep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Kuete V, Wansi JD, Mbaveng AT, Kana Sop MM, Tadjong AT, Beng VP, et al. Antimicrobial activity of the methanolic extract and compounds from Teclea afzelii (Rutaceae) S Afr J Bot. 2008;74(4):572–576. doi: 10.1016/j.sajb.2008.02.004. [DOI] [Google Scholar]
- 21.Kuete V, Ngameni B, Simo CC, Tankeu RK, Ngadjui BT, Meyer JJ, et al. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae) J Ethnopharmacol. 2008;120(1):17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Kuete V, Wabo GF, Ngameni B, Mbaveng AT, Metuno R, Etoa FX, et al. Antimicrobial activity of the methanolic extract, fractions and compounds from the stem bark of Irvingia gabonensis (Ixonanthaceae) J Ethnopharmacol. 2007;114(1):54–60. doi: 10.1016/j.jep.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76(14):1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 24.Mims C, Playfair J, Roitt I, Wakelin D, Williams R. Antimicrobials and chemotherapy. In: Mims CA, et al. Eds. Med Microbiol Rev. 1993;35:1–34. [Google Scholar]
- 25.Mbaveng AT, Kuete V, Mapunya BM, Beng VP, Nkengfack AE, Meyer JJ, et al. Evaluation of four Cameroonian medicinal plants for anticancer, antigonorrheal and antireverse transcriptase activities. Environ Toxicol Pharmacol. 2011;32(2):162–167. doi: 10.1016/j.etap.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Mbaveng AT, Ngameni B, Kuete V, Simo IK, Ambassa P, Roy R, et al. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae) J Ethnopharmacol. 2008;116(3):483–489. doi: 10.1016/j.jep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Blot S, Depuydt P, Vandewoude K, De Bacquer D. Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis. 2007;20(4):391–396. doi: 10.1097/QCO.0b013e32818be6f7. [DOI] [PubMed] [Google Scholar]
- 28.Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29(6):630–636. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso O, Alves AF, Leitao R. Surveillance of antimicrobial susceptibility of Pseudomonas aeruginosa clinical isolates from a central hospital in Portugal. J Antimicrob Chemother. 2007;60(2):452–454. doi: 10.1093/jac/dkm214. [DOI] [PubMed] [Google Scholar]
- 30.Tran QT, Mahendran KR, Hajjar E, Ceccarelli M, Davin-Regli A, Winterhalter M, et al. Implication of porins in beta-lactam resistance of Providencia stuartii. J Biol Chem. 2010;285(42):32273–32281. doi: 10.1074/jbc.M110.143305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndhlala AR, Amoo SO, Ncube B, Moyo M, Nair JJ, Van Staden J. 16 - Antibacterial, antifungal, and antiviral activities of African medicinal plants. In: Kuete V, editor. Medicinal Plant Research in Africa. Oxford: Elsevier; 2013. pp. 621–659. [Google Scholar]
- 32.Yenesew A, Derese S, Midiwo JO, Bii CC, Heydenreich M, Peter MG. Antimicrobial flavonoids from the stem bark of Erythrina burttii. Fitoterapia. 2005;76(5):469–472. doi: 10.1016/j.fitote.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Nkengfack AE, Vouffo TW, Fomum ZT, Meyer M, Bergendorff O, Sterner O. Prenylated isoflavanone from the roots of Erythrina sigmoidea. Phytochemistry. 1994;36(4):1047–1051. doi: 10.1016/S0031-9422(00)90489-8. [DOI] [PubMed] [Google Scholar]
- 34.Viveiros M, Jesus A, Brito M, Leandro C, Martins M, Ordway D, et al. Inducement and reversal of tetracycline resistance in Escherichia coli K-12 and expression of proton gradient-dependent multidrug efflux pump genes. Antimicrob Agents Chemother. 2005;49(8):3578–3582. doi: 10.1128/AAC.49.8.3578-3582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178(1):306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elkins CA, Mullis LB. Substrate competition studies using whole-cell accumulation assays with the major tripartite multidrug efflux pumps of Escherichia coli. Antimicrob Agents Chemother. 2007;51(3):923–929. doi: 10.1128/AAC.01048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baglioni P, Bini L, Liberatori S, Pallini V, Marri L. Proteome analysis of Escherichia coli W3110 expressing an heterologous sigma factor. Proteomics. 2003;3(6):1060–1065. doi: 10.1002/pmic.200300403. [DOI] [PubMed] [Google Scholar]
- 38.Sar C, Mwenya B, Santoso B, Takaura K, Morikawa R, Isogai N, et al. Effect of Escherichia coli wild type or its derivative with high nitrite reductase activity on in vitro ruminal methanogenesis and nitrate/nitrite reduction. J Anim Sci. 2005;83(3):644–652. doi: 10.2527/2005.833644x. [DOI] [PubMed] [Google Scholar]
- 39.Ghisalberti D, Masi M, Pages JM, Chevalier J. Chloramphenicol and expression of multidrug efflux pump in Enterobacter aerogenes. Biochem Biophys Res Commun. 2005;328(4):1113–1118. doi: 10.1016/j.bbrc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 40.Mallea M, Chevalier J, Bornet C, Eyraud A, Davin-Regli A, Bollet C, et al. Porin alteration and active efflux: two in vivo drug resistance strategies used by Enterobacter aerogenes. Microbiology. 1998;144(Pt 11):3003–3009. doi: 10.1099/00221287-144-11-3003. [DOI] [PubMed] [Google Scholar]
- 41.Mallea M, Mahamoud A, Chevalier J, Alibert-Franco S, Brouant P, Barbe J, et al. Alkylaminoquinolines inhibit the bacterial antibiotic efflux pump in multidrug-resistant clinical isolates. Biochem J. 2003;376(Pt 3):801–805. doi: 10.1042/bj20030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pradel E, Pages JM. The AcrAB-TolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob Agents Chemother. 2002;46(8):2640–2643. doi: 10.1128/AAC.46.8.2640-2643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chevalier J, Pages JM, Eyraud A, Mallea M. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem Biophys Res Commun. 2000;274(2):496–499. doi: 10.1006/bbrc.2000.3159. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzi V, Muselli A, Bernardini AF, Berti L, Pages JM, Amaral L, et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob Agents Chemother. 2009;53(5):2209–2211. doi: 10.1128/AAC.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


