Abstract
Background:
This study was designed to assess the microleakage of glass-ionomer (GI), mineral trioxide aggregate (MTA), and calcium-enriched mixture (CEM) cement as coronal orifice barrier during walking bleaching.
Materials and Methods:
In this experimental study, endodontic treatment was done for 70 extracted human incisors without canal calcification, caries, restoration, resorption, or cracks. The teeth were then divided into three experimental using “Simple randomization allocation” (n = 20) and two control groups (n = 5). The three cements were applied as 3-mm intra-orifice barrier in test groups, and bleaching process was then conducted using a mixture of sodium perborate powder and distilled water, for 9 days. For leakage evaluation, bovine serum albumin marker was traced in a dual-chamber technique with Bradford indicator. The Kruskal-Wallis and Mann-Whitney tests were used for statistical analysis.
Results:
The mean ± standard deviation leakage of samples from negative control, positive control, GI, MTA, and CEM cement groups were 0.0, 8.9 ± 0.03, 0.47 ± 0.02, 0.48 ± 0.02, and 0.49 ± 0.02 mg/mL, respectively. Statistical analysis showed no significant difference between three experimental groups (P > 0.05).
Conclusion:
It is concluded that GI, MTA, and CEM cements are considered as suitable intra-orifice barrier to provide coronal seal during walking bleaching.
Keywords: Calcium enriched mixture cement, coronal barrier, nonvital bleaching, RMGIC, WMTA
INTRODUCTION
Discolored anterior teeth are often considered as an esthetic detraction. Root canal therapy is known as the main etiologic factor for iatrogenic tooth discoloration (entombment of pulp tissue remnants or metal-including root canal sealers).[1] As a result, nonvital bleaching has gained so much attention, due to the growing demand for beautiful smiles and white teeth.[2]
Walking or internal bleaching technique was described by Spasser in 1961.[1] Effective bleaching agents used to lighten the tooth color in this technique include hydrogen peroxide (H2O2) and sodium perborate, the latter being effective by releasing hydrogen peroxide. Given the appropriate application, the walking bleaching is a relatively low-risk and minimally invasive intervention and bears only slight risks if performed correctly.[3] Nevertheless, concerns exist regarding decreasing the likelihood of cervical root resorption as an important complication. It is stated that the oxidative action of bleaching agent and releasing of nascent oxygen which is later transferred to the cervical periodontal ligament (PDL) through the dentinal tubules and cementum defects can act as a stimulus for inflammatory changes of the region and subsequent up-regulation of odontoclastic cells responsible for invasive cervical root resorption. This phenomenon is probably rendered by the high concentration of intra-coronal oxidizing agents reaching the root surface. Effective sealing of the canal orifice can eliminate the risk for this tubular transferring of the PDL irritants and thus can reduce the risk of this phenomenon.[4,5,6]
Due to decreased pH at the root surface, cementum necrosis happens, the periodontium is inflamed, and eventually root resorption emerges. This acidic environment is also known to enhance osteoclastic activity at the site. The incidence of cervical root resorption has been reported rather high, from 1% to 13%.[1]
The general recommendation today is:
Not to heat the bleaching agent in the access cavity, because heat can damage periodontal tissue and lead to an increased resorption rate at the root surface, and
To cover the root canal filling material with a base in order to eliminate or decrease the diffusion of oxidizing agents from the access cavity toward root surface.[7,8,9]
The importance of this cervical sealing has been extensively documented, for instance, over a 20-year period of follow-up of nonvital teeth bleached with 30% hydrogen peroxide with adequate cervical sealing, no root resorption was observed.[1,7,10]
A variety of dental materials such as hydrophilic filling materials (Cavit, Coltosol), intermediate restorative material (IRM), zinc oxide-eugenol cement, zinc phosphate cement, photo-activated temporary resin materials, and conventional or resin-modified glass-ionomer (GI) have been suggested as sealing agents to be laid on root-canal filling material during intra-coronal bleaching.[11,12] If temporary filling materials are used, their removal prior to final restoration of the bleached tooth is essential. Therefore, a 2-mm layer of GI cement has been recommended as a standard protective intra-orifice barrier during bleaching, which can be left in place to be a base for the final restoration.[1]
Microleakage tests can well demonstrate the sealing ability of root filling materials. In endodontics, this index is measured principally based on the penetration of trace agents through the filled canal; which include radioisotopes, dyes, bacterium and their products such as proteins.[13]
Comparing with the other methods available for leakage assessment in the field of endodontic research, from our standpoint, protein leakage test is superior, particularly because it has more clinical relevancy, furthermore, there is no liability for sample destruction, and hence the leakage might be evaluated repeatedly, if needed.[14,15]
In case of mineral trioxide aggregate (MTA), numerous studies have shown its suitability for a wide variety of applications such as successful treatment of invasive cervical resorption.[16] An outstanding specification of MTA is its superior leakage resistance, which may be explained by its marginal adaptation. MTA has biological benefits due to high concentration of calcium hydroxide, in its formulation after mixing with water.[17,18] Upon high alkalinity, it is hypothesized that MTA may prevent or arrest tooth resorption. On the other hand, the effect of alkaline and acidic pH values on physical properties of WMTA (white MTA) has been well-documented.[17] The potential tooth discoloration can be the only reason that prevents MTA to be used as an effective intra-orifice barrier during tooth bleaching.[16]
Recently, calcium enriched mixture (CEM) cement has been introduced as a new root-end filling material. It is dominantly composed of calcium compounds. Due to being biocompatible, well-tolerable by oral tissues such as pulp and PDL and providing comparable seal to MTA[19] this new biomaterial is proved to be applicable for other purposes in the field of endodontics, such as perforations repair, coronal seal, root canal filling, and vital pulp therapies as well.[20] Besides all the aforementioned criteria, CEM cement offers the advantage of not causing tooth discoloration that is favorable in cases of intra-coronal bleaching.[21]
This interventional study was designated and conducted to compare the sealing ability of GI, MTA and CEM cement when used as intra-orifice barrier in nonvital bleaching.
MATERIALS AND METHODS
Preparation of specimens
Ethical Committee of Mazandaran University of Medical Sciences approved (Code: 91-175) to use the extracted teeth after the informed consent form would have been filled out by the volunteers. Seventy freshly extracted human maxillary incisors with standard root length (10-13 mm) were selected for this study. The teeth were extracted because of periodontal reasons. After cleaning the teeth, radiographies were taken in buccolingual and mesiodistal directions. Teeth with calcification, internal or external resorption, cracks (detectable under light stereomicroscope), severe coronal or root caries, large coronal restoration, root fractures, dilacerations, deep depression on root surfaces, and those with apical foramen larger than file size #40 were excluded, and if necessary, they were replaced with intact ones. All teeth had mature apices and straight patent canals. To control the cross infection and minimize soft tissues and periodontal remnants, all teeth were stored in normal saline then in a 5.25% solution of sodium hypochlorite (NaOCl) (Golrang, Tehran, Iran) for 6 h and were finally stored in 0.5% chloramine solution until experiment commencement.
The access cavity was prepared using a high-speed handpiece and #2 round diamond bur (Jota, Ruthi, Switzerland) under copious water irrigation. Then, the working length was determined by #15 K-file (Mailer, Ballaigues, Switzerland) inserted into the canal until the file tip got visible at the apex. One millimeter was subtracted from this measurement and then recorded as the working length. The coronal and mid segments of the canals were prepared with sizes 3-1 Gates-Glidden drills (Mailer, Ballaigues, Switzerland) and then complete preparation of the canals was conducted using Mtwo rotary system (VDW, Munich, Germany) in standard preparation technique up to file 40/0.04 as the master apical preparation. The canal was alternatively irrigated with 2 mL of 2.6% NaOCl during instrumentation between each file size. Final irrigation of the canal was secured with 17% EDTA (Diadent Inc., Chongchong Buch Do, Korea) for removal of the smear layer, followed by 5 mL of normal saline. The canals were then dried with absorbent paper points (AriaDent, Tehran, Iran) and obturated using Gutta-percha (Diadent Inc., Chongchong Buch Do, Korea) and AH-26 sealer (Dentsply, Tulsa Dental, Tulsa, OK, USA) with cold lateral condensation technique. Excess sealer of the pulp chamber and dentinal tubules was removed with alcohol-soaked cotton pellets and the final obturation radiography was taken [Figure 1a]. Thereafter, a Peeso reamer #3 (Maillefer, Ballaigues, Switzerland) was used to remove the intra canal Gutta-percha, 3 mm below the cementoenamel junction (CEJ) in palatal aspect. The cavity depth was confirmed by a periodontal probe and radiographed as well [Figure 1b]. The pulp chamber was irrigated with saline and dried. The teeth were randomly divided into three experimental groups (n = 20) and two “positive and negative” control groups, of five each using “simple randomization allocation.” It means that each tooth selected was allotted to one group by sequence.
Figure 1.
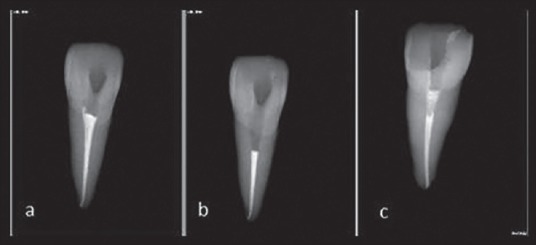
Processes for coronal barrier placement.
Experimental groups
In the experimental groups, the canal obturating material was covered as follows: Group 1-RMGIC-LC Fuji II (Fuji Corp. Hyogo, Japan), group 2-WMTA (Tooth-colored Formula, Anglus, Londrina, Brazil), and group 3-CEM Cement (BioniqueDent, Tehran, Iran). All the materials were prepared according to the manufacturer's instruction [Table 1], and were packed into the unfilled cervical portion of the canals up to the palatal and facial aspects of CEJ to provide a 3-mm thick barrier. At this step, all samples were radiographically evaluated for the length and density of the intra-orifice barrier [Figure 1c]. Wet cotton pellets were placed over WMTA and CEM cement to provide their setting hydration. All the teeth were temporized with Cavit (ESPE-Premier, Norristown, PA, USA) and incubated at 37°C for 24 h at a 90% relative humidity. The bleaching agent used in the study was a mixture of 0.15 g sodium perborate (Merck, Darmstadt, Germany and 0.05 mL distilled water. It was placed in the pulp chamber, and the teeth were restored again with cavit and then incubated…
Table 1.
Composition and usage instruction for material used in the study

The bleaching period was arranged for 9 days. The bleaching agent was being refreshed every 3 days. At the end of the 9th day, cavit was removed again, and pulp chamber was rinsed with distilled water and dried and was then filled with cavit for the last time.
Control groups
In positive control group, teeth received neither coronal barrier nor temporal restoration after canal obturation. External surfaces of teeth except for the 2-mm apical part were covered using two layers of nail polish.
In negative control group, all surfaces even the temporal restoration were covered and sealed with two layers of nail polish after filling the canals with sticky wax.
In case of specimens in three experimental groups, all surfaces were covered with two layers of nail polish except for access cavity margins and apical 2-mm portion.
All the procedures were standardized for all groups and conducted by one operator.
Protein leakage test
All the samples were mounted in a dual-chamber leakage apparatus as shown in schematic view [Figure 2]. First, the teeth were inserted from the cap end of a 3-mL plastic Eppendorf cylinder (Elkay, Shrewbury, MA, USA). The ending 3 mm of cylinders were cut previously, so the root tips passed through this part and were visible. Interfaces between the tubes and the teeth were sealed with sticky wax. The cylinders were placed in preautoclaved 10-mL glass vial tube with identical dimensions. The glass vials had been filled previously with 9 mL of distilled water. The junction line between microtube and vial was tightly covered and sealed with Parafilm (Supa Co., Tehran, Iran). The whole system was sterilized with ethylene oxide gas for 12 h. The plastic cylinders were filled with 1 mL of 22% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA). The assembly was incubated again at 37°C and a relative humidity of 90% for 30 days (test period). BSA was refreshed every day throughout the experiment. Bradford indicator was used to measure the concentration of leaked albumin form the upper chamber into the lower one at the end of the 30th day. Bradford protein reagent is an aqueous solution of Coomassie Brilliant Blue G (Sigma-Aldrich, St. Louis, MO, USA), ethanol, and phosphoric acid. The procedure is based on the formation of a complex between the dye, Brilliant Blue G, and proteins in solution. According to the manufacturer, albumin leakage into the solution and subsequent formation of the protein-dye complex would shift the wavelength of maximum absorption of Coomassie Brilliant Blue G from 465 to 596 nm. Color development is rapid. Only 5-min incubation is sufficient to read the samples at 596 nm. The amount of absorption is proportional to the protein present. The glass tubes were separated. Then 100 μL of test solution of the vials was pipetted into a new Eppendorf tube and 1 mL of Bradford protein reagent was added to the tube, and the contents were mixed.
Figure 2.
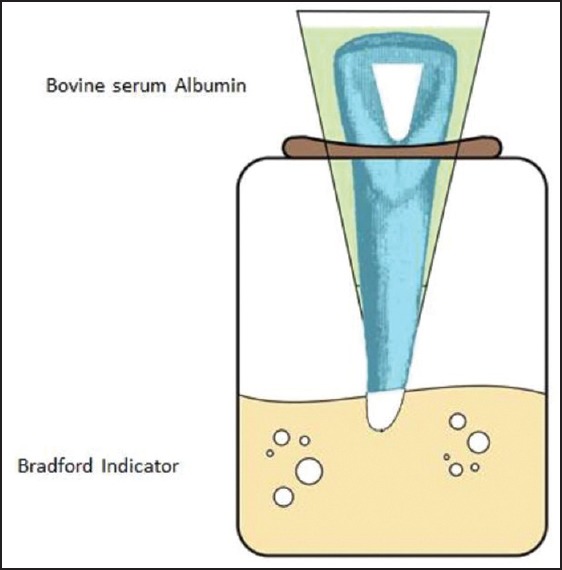
Schematic view of dual-chamber technique used for protein leakage analysis.
Using spectrophotometry, the maximum absorption was measured to calculate the microleakage. Statistical analysis of the data was conducted using the Kruskal-Wallis followed by the Mann-Whitney tests. The level of significance was set at 0.05 (17.0 SPSS for Windows, Chicago, IL, USA).
RESULTS
The mean ± standard deviation leakage of samples from negative control, positive control, GI, MTA, and CEM cement groups were 0.0, 8.9 ± 0.03, 0.47 ± 0.02, 0.48 ± 0.02, and 0.49 ± 0.02 mg/mL, respectively. The difference among the groups was not significant (P > 0.05).
DISCUSSION
The decrease of acidity on external surfaces of teeth during internal bleaching has been considered as an important causative factor for cervical root resorption.[22] For the very same reason, it is recommended to cover the canal filling materials with a barrier to occlude the dentinal tubules as a way of diffusion between pulp cavity and root surface. Considering the fact that root fillings per se are not leak proof, and that no sealer cement or obturation technique consistently prevents percolation through the canal, it is critical to take most care in creating bests of the seal and consider the quality of intra-orifice barrier in nonvital bleaching. The success of walking bleaching rests somehow upon the sealing quality of the cervical barrier, especially when the remaining dentin walls are very thin. It is strongly suggested to apply only low concentration of bleaching agents, or to mix sodium perborate with distilled water instead of hydrogen peroxide,[23] in order to govern the detrimental effects of the bleaching agents, although the importance of cervical barrier is not allowed to be ignored. Attempts are underway to introduce more qualified materials with the potential to provide a long-term seal. GI cement is the worldwide standard material supposed for this purpose. Recently MTA has been implemented to seal the root canal. To date, no study evaluated the intra-orifice sealing ability of CEM cement in bleaching.
CEM cement has been recently proposed. Its formulation is composed of a different mixture of calcium compounds to provide a bioactive calcium-phosphate enriched material. Major components of CEM cement powder are CaO, SO3, and P2O3. Studies on CEM cement showed that it can release phosphorous and calcium ions which promotes the alkalinity and also leads to mineralization process, implying its hard tissue inductivity.[19] Hydroxyapatite formation is demonstrated to be resulted via the reaction between calcium and phosphorous ions released with endogenous and exogenous sources.[20] To date, CEM cement has not been studied as an intra-orifice plug to resist the microleakage in nonvital bleaching process while it has been implemented for treatment of furcal perforation, vital pulp therapies in permanent and primary teeth,[19,24,25,26,27,28,29,30,31,32] root end fillings,[14] management of root resorption and pathologic/iatrogenic perforations,[33,34,35,36] periradicular surgery,[35,37] and revascularization for necrotic immature permanent molars.[38]
Compared to MTA, CEM cement has a shorter setting time and also significantly superior results in film thickness and flow, easier handling, and enhanced antibacterial effect as well as better abilities to form hydroxyapatite in normal saline.[20,21] In addition to the mentioned properties, it has been shown that this material provides favorable apical/coronal sealing property similar to that of commercial types of MTA and superior to Intermediate Restorative Material (IRM).[35,39,40,41] It is shown that CEM cement has less microleakage compared to different types of MTA although not significant.[35] Moreover, CEM cement has rather lower cost.[20]
The sealing ability of endodontic materials has been evaluated using dye or bacterial penetration, electrical method, fluid filtration technique, human saliva leakage model, glucose leakage model, radioisotope tracing, and marginal adaptation.[13,42,43,44,45,46,47,48] Some advantages and also disadvantages have been reported for each of these techniques, for instance, bacterial leakage assesses the sealing ability of all portions while in dye leakage only the walls adjacent to material are observed. Bacterial leakage studies focus on the main pathogenic factors, but even this method cannot mirror the real clinical condition as special species or a limited number of bacteria are used and so, bacterial synergistic effect, influence of environment, thermal changes, salivary enzymes, buffering materials and antibodies are neglected. Of these techniques, some of them provide higher biological and clinical relevance. In this study, protein leakage approach was adopted since is met our answers more convincingly than the others.[14]
To show the importance of cervical barrier in leakage prevention, Valadares et al. stated that “the use of cervical barrier prevents the microleakage of Enterococcus faecalis.”[8]
According to Yavari et al., CEM and MTA are more effective than amalgam and composite resin, in case of coronal sealing in endodontically treated teeth.[49] Roghanizad and Jones revealed that amalgam, as an orifice plug, is more efficacious than cavit in preventing coronal microleakage.[50] Tselnik et al. reported no difference between gray MTA, white MTA, or a resin modified GI, in terms of bacterial penetration with human saliva model.[51] Their results are in agreement with ours. According to Ferk Luketic et al., MTA is considerably better than amalgam as an intra-orifice barrier.[52] Based on the findings of a recent study, using the glucose penetration model, Bailón-Sánchez et al. reported that “Cavit, and ProRoot MTA has similar abilities to resist leakage when used as intra-orifice barriers.”[53] Barrieshi-Nusair and Hammad compared GI and MTA as orifice plugs and reported that GI has more microleakage[54] which is not concurring with our findings. In their study, each group included 30 samples, and the leakage assessment method was dye leakage for 48 h, but we had 20 teeth in each experimental group which were analyzed in protein leakage approach for 30 days. The mentioned facts might be the reason of the different results obtained. In another study that was conducted by Vosoughhosseini et al. to compare MTA and GI as coronal barrier in nonvital bleaching, no significant difference was observed[11] which is in line with our findings.
The results of the present study revealed that CEM cement has sealing potential as an intra-orifice barrier against protein penetration that is comparable with that of MTA and GI. As mentioned before, this noticeable sealing ability can be probably the result of the reaction between calcium and phosphorous ions, although, in most part, can be related to hydrophilic nature, good antibacterial/fungal potential, high pH and formation of hydroxyapatite crystals as well.
The obtained results opened perspectives for use of this newly-introduced biomaterial in bleaching interventions on ground of sealing ability. Other experimental settings, particularly for leakage assessment are needed to simulate the clinical reality.
Among the limitations, technique sensitivity of protein leakage assessment method, and the cost of the materials were more prominent.
CONCLUSION
Considering the results of the present study, on ground of the leakage resistance, it is concluded that all materials used; GI, MTA, and CEM cement are able to convincingly seal the coronal portion of the canal during nonvital bleaching in order to prevent the cervical root resorption.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Zimmerli B, Jeger F, Lussi A. Bleaching of nonvital teeth. A clinically relevant literature review. Schweiz Monatsschr Zahnmed. 2010;120:306–20. [PubMed] [Google Scholar]
- 2.Elfallah HM, Swain MV. A review of the effect of vital teeth bleaching on the mechanical properties of tooth enamel. N Z Dent J. 2013;109:87–96. [PubMed] [Google Scholar]
- 3.Abbott P, Heah SY. Internal bleaching of teeth: An analysis of 255 teeth. Aust Dent J. 2009;54:326–33. doi: 10.1111/j.1834-7819.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 4.Hachmeister DR, Schindler WG, Walker WA, 3rd, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28:386–90. doi: 10.1097/00004770-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Heithersay GS. Invasive cervical resorption: An analysis of potential predisposing factors. Quintessence Int. 1999;30:83–95. [PubMed] [Google Scholar]
- 6.Heithersay GS, Dahlstrom SW, Marin PD. Incidence of invasive cervical resorption in bleached root-filled teeth. Aust Dent J. 1994;39:82–7. doi: 10.1111/j.1834-7819.1994.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 7.De Oliveira LD, Carvalho CA, Hilgert E, Bondioli IR, de Araújo MA, Valera MC. Sealing evaluation of the cervical base in intracoronal bleaching. Dent Traumatol. 2003;19:309–13. doi: 10.1046/j.1600-9657.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 8.Valadares MA, Soares JA, Nogueira CC, Cortes MI, Leite ME, Nunes E, et al. The efficacy of a cervical barrier in preventing microleakage of Enterococcus faecalis in endodontically treated teeth. Gen Dent. 2011;59:e32–7. [PubMed] [Google Scholar]
- 9.Khoroushi M, Feiz A, Ebadi M. Influence of intermediary filling material on microleakage of intracoronally bleached and restored teeth. Dent Res J (Isfahan) 2009;6:17–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JJ, Cunningham CJ, Montgomery S. Cervical canal leakage after internal bleaching. J Endod. 1992;18:476–81. doi: 10.1016/s0099-2399(06)81346-4. [DOI] [PubMed] [Google Scholar]
- 11.Vosoughhosseini S, Lotfi M, Shahmoradi K, Saghiri MA, Zand V, Mehdipour M, et al. Microleakage comparison of glass-ionomer and white mineral trioxide aggregate used as a coronal barrier in nonvital bleaching. Med Oral Patol Oral Cir Bucal. 2011;16:e1017–21. doi: 10.4317/medoral.17306. [DOI] [PubMed] [Google Scholar]
- 12.Azevedo RA, Silva-Sousa YT, Souza-Gabriel AE, Messias DC, Alfredo E, Silva RG. Fracture resistance of teeth subjected to internal bleaching and restored with different procedures. Braz Dent J. 2011;22:117–21. doi: 10.1590/s0103-64402011000200005. [DOI] [PubMed] [Google Scholar]
- 13.Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: Effects of blood contamination. J Endod. 1994;20:159–63. doi: 10.1016/S0099-2399(06)80326-2. [DOI] [PubMed] [Google Scholar]
- 14.Moradi S, Disfani R, Ghazvini K, Lomee M. Sealing ability of orthograde MTA and CEM cement in apically resected roots using bacterial leakage method. Iran Endod J. 2013;8:109–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Moradi S, Lomee M, Gharechahi M. Comparison of fluid filtration and bacterial leakage techniques for evaluation of microleakage in endodontics. Dent Res J (Isfahan) 2015;12:109–14. [PMC free article] [PubMed] [Google Scholar]
- 16.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review — Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review — Part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Torabinejad M, Parirokh M. Mineral trioxide aggregate: A comprehensive literature review — part II: Leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Asgary S, Ahmadyar M. Vital pulp therapy using calcium-enriched mixture: An evidence-based review. J Conserv Dent. 2013;16:92–8. doi: 10.4103/0972-0707.108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009;35:243–50. doi: 10.1016/j.joen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Sharma DS, Sharma S, Natu SM, Chandra S. An in vitro evaluation of radicular penetration of hydrogen peroxide from bleaching agents during intra-coronal tooth bleaching with an insight of biologic response. J Clin Pediatr Dent. 2011;35:289–94. doi: 10.17796/jcpd.35.3.q83063560x866037. [DOI] [PubMed] [Google Scholar]
- 23.Palo RM, Valera MC, Camargo SE, Camargo CH, Cardoso PE, Mancini MN, et al. Peroxide penetration from the pulp chamber to the external root surface after internal bleaching. Am J Dent. 2010;23:171–4. [PubMed] [Google Scholar]
- 24.Nosrat A, Asgary S. Apexogenesis treatment with a new endodontic cement: A case report. J Endod. 2010;36:912–4. doi: 10.1016/j.joen.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010;43:565–71. doi: 10.1111/j.1365-2591.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 26.Malekafzali B, Shekarchi F, Asgary S. Treatment outcomes of pulpotomy in primary molars using two endodontic biomaterials. A 2-year randomised clinical trial. Eur J Paediatr Dent. 2011;12:189–93. [PubMed] [Google Scholar]
- 27.Asgary S, Nosrat A, Homayounfar N. Periapical healing after direct pulp capping with calcium-enriched mixture cement: A case report. Oper Dent. 2012;37:571–5. doi: 10.2341/11-417-S. [DOI] [PubMed] [Google Scholar]
- 28.Fallahinejad Ghajari M, Asgharian Jeddi T, Iri S, Asgary S. Treatment outcomes of primary molars direct pulp capping after 20 months: A randomized controlled trial. Iran Endod J. 2013;8:149–52. [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrdad L, Malekafzali B, Shekarchi F, Safi Y, Asgary S. Histological and CBCT evaluation of a pulpotomised primary molar using calcium enriched mixture cement. Eur Arch Paediatr Dent. 2013;14:191–4. doi: 10.1007/s40368-013-0038-3. [DOI] [PubMed] [Google Scholar]
- 30.Nosrat A, Seifi A, Asgary S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: A randomized clinical trial. Int J Paediatr Dent. 2013;23:56–63. doi: 10.1111/j.1365-263X.2012.01224.x. [DOI] [PubMed] [Google Scholar]
- 31.Torabzadeh H, Asgary S. Indirect pulp therapy in a symptomatic mature molar using calcium enriched mixture cement. J Conserv Dent. 2013;16:83–6. doi: 10.4103/0972-0707.105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asgary S, Eghbal MJ, Ghoddusi J. Two-year results of vital pulp therapy in permanent molars with irreversible pulpitis: An ongoing multicenter randomized clinical trial. Clin Oral Investig. 2014;18:635–41. doi: 10.1007/s00784-013-1003-6. [DOI] [PubMed] [Google Scholar]
- 33.Samiee M, Eghbal MJ, Parirokh M, Abbas FM, Asgary S. Repair of furcal perforation using a new endodontic cement. Clin Oral Investig. 2010;14:653–8. doi: 10.1007/s00784-009-0351-8. [DOI] [PubMed] [Google Scholar]
- 34.Asgary S, Ahmadyar M. One-visit endodontic retreatment of combined external/internal root resorption using a calcium-enriched mixture. Gen Dent. 2012;60:e244–8. [PubMed] [Google Scholar]
- 35.Asgary S. Furcal perforation repair using calcium enriched mixture cement. J Conserv Dent. 2010;13:156–8. doi: 10.4103/0972-0707.71650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asgary S, Nosrat A, Seifi A. Management of inflammatory external root resorption by using calcium-enriched mixture cement: A case report. J Endod. 2011;37:411–3. doi: 10.1016/j.joen.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Asgary S, Ehsani S. Periradicular surgery of human permanent teeth with calcium-enriched mixture cement. Iran Endod J. 2013;8:140–4. [PMC free article] [PubMed] [Google Scholar]
- 38.Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: A review and report of two cases with a new biomaterial. J Endod. 2011;37:562–7. doi: 10.1016/j.joen.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Haghgoo R, Arfa S, Asgary S. Microleakage of CEM cement and ProRoot MTA as furcal perforation repair materials in primary teeth. Iran Endod J. 2013;8:187–90. [PMC free article] [PubMed] [Google Scholar]
- 40.Ghorbani Z, Kheirieh S, Shadman B, Eghbal MJ, Asgary S. Microleakage of CEM cement in two different media. Iran Endod J. 2009;4:87–90. [PMC free article] [PubMed] [Google Scholar]
- 41.Asgary S, Eghbal MJ, Parirokh M. Sealing ability of a novel endodontic cement as a root-end filling material. J Biomed Mater Res A. 2008;87:706–9. doi: 10.1002/jbm.a.31678. [DOI] [PubMed] [Google Scholar]
- 42.Tang HM, Torabinejad M, Kettering JD. Leakage evaluation of root end filling materials using endotoxin. J Endod. 2002;28:5–7. doi: 10.1097/00004770-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21:109–12. doi: 10.1016/s0099-2399(06)80433-4. [DOI] [PubMed] [Google Scholar]
- 44.Torabinejad M, Lee SJ, Hong CU. Apical marginal adaptation of orthograde and retrograde root end fillings: A dye leakage and scanning electron microscopic study. J Endod. 1994;20:402–7. doi: 10.1016/S0099-2399(06)80300-6. [DOI] [PubMed] [Google Scholar]
- 45.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–5. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 46.Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19:541–4. doi: 10.1016/S0099-2399(06)81282-3. [DOI] [PubMed] [Google Scholar]
- 47.Torabinejad M, Ung B, Kettering JD. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J Endod. 1990;16:566–9. doi: 10.1016/S0099-2399(07)80198-1. [DOI] [PubMed] [Google Scholar]
- 48.Brosco VH, Bernardineli N, Torres SA, Consolaro A, Bramante CM, de Moraes IG, et al. Bacterial leakage in root canals obturated by different techniques. Part 1: Microbiologic evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e48–53. doi: 10.1016/j.tripleo.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Yavari HR, Samiei M, Shahi S, Aghazadeh M, Jafari F, Abdolrahimi M, et al. Microleakage comparison of four dental materials as intra-orifice barriers in endodontically treated teeth. Iran Endod J. 2012;7:25–30. [PMC free article] [PubMed] [Google Scholar]
- 50.Roghanizad N, Jones JJ. Evaluation of coronal microleakage after endodontic treatment. J Endod. 1996;22:471–3. doi: 10.1016/S0099-2399(96)80080-X. [DOI] [PubMed] [Google Scholar]
- 51.Tselnik M, Baumgartner JC, Marshall JG. Bacterial leakage with mineral trioxide aggregate or a resin-modified glass ionomer used as a coronal barrier. J Endod. 2004;30:782–4. doi: 10.1097/00004770-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Ferk Luketic S, Malcic A, Jukic S, Anic I, Segovic S, Kalenic S. Coronal microleakage of two root-end filling materials using a polymicrobial marker. J Endod. 2008;34:201–3. doi: 10.1016/j.joen.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Bailón-Sánchez ME, González-Castillo S, González-Rodríguez MP, Poyatos-Martínez R, Ferrer-Luque CM. Intraorifice sealing ability of different materials in endodontically treated teeth. Med Oral Patol Oral Cir Bucal. 2011;16:e105–9. [PubMed] [Google Scholar]
- 54.Barrieshi-Nusair KM, Hammad HM. Intracoronal sealing comparison of mineral trioxide aggregate and glass ionomer. Quintessence Int. 2005;36:539–45. [PubMed] [Google Scholar]


