Abstract
Background:
Restless and violent behaviors are common in Emergency Departments (EDs), which need therapeutic interventions in most of the times. The first-generation anti-psychotic drugs are one of the most applicable therapeutic agents in the management of such patients, but their use has some limitations. Some studies suggest midazolam as an alternative medicine. Therefore, this study was performed with the aim of comparison of the efficacy and safety of haloperidol and midazolam in the restless management of referring patients to EDs.
Materials and Methods:
The present double-blinded trial was done on patients needed sedation and referred to the ED of Alzahra Hospital, Isfahan, Iran, in 2014. The patients were categorized into two random groups of haloperidol (5 mg) and midazolam receivers (2.5 mg for those weighing <50 kg and 5 mg in >50 kg), as intramuscular administration. The time to achieve sedation, need for rescue dose, need to resedation within the first 60 min, and adverse effects of drugs were compared among the groups.
Results:
Forty-eight patients were entered to the study. The mean age in the haloperidol and midazolam groups was 44.8 ± 4.1 years and 45.5 ± 4.7 years, respectively (P = 0.91). The mean time of sedation in the haloperidol and midazolam groups was 5.6 ± 0.3 min and 5.2 ± 0.1 min, respectively (P = 0.31). The mean time of full consciousness after sedation was 36.2 ± 4.5 min and 38.2 ± 3.4 min in the haloperidol and midazolam groups, respectively (P = 0.72). On average, time to arousal in the midazolam group was 10.33 min more than the haloperidol group, but it was not statistically significant.
Conclusion:
The results of the present study show that administration of midazolam and haloperidol have similar efficacy in the treatment of restless symptoms with the same recovery time from drug effects for referring patients to the ED. In addition, none of the adverse effects were observed in this study.
Keywords: Drug effects, drug prescription, emergency treatment, haloperidol, midazolam, movement disorders
INTRODUCTION
Restless and violent behaviors, which arise more from mental illnesses or poisoning with alcohol and drugs, are recognized as a common phenomenon in Emergency Departments (EDs) with about 10% prevalence.[1,2,3,4] In most of the cases, restlessness of patients prevents from performing necessary proceedings and increases the need for sedation. The first-generation anti-psychotic drugs such as haloperidol and droperidol have been used as acceptable agents for sedation of such patients since many years ago.[5,6] However, each of them has some special side effects and because of long half-life, they require long-term following. It causes that studies during recent 10 years have been more focused on alternative medicines such as second-generation anti-psychotic drugs and benzodiazepines.[7,8] Although new guidelines recommend second-generation anti-psychotic drugs as the first-line therapy, they are not accessible in many therapeutic centers. In addition, some of these drugs only have oral form which limits their use in restless patients.[6] Midazolam, a solvent benzodiazepine and short effect sedative agent, is also suggested as an alternative medicine to produce conscious sedation. The results of a study confirmed the efficacy and safety of midazolam in sedation of patients even more than haloperidol in EDs.[9] However, using this agent as a routine sedative drug for restlessness of patients is still under debate. Therefore, this study was performed with the aim of comparison of the efficacy and safety of haloperidol and midazolam in restless management of referring patients to EDs.
MATERIALS AND METHODS
Study design and participants
The present double-blinded, randomized clinical trial was done on patients referred to the ED of Alzahra Hospital, Isfahan, Iran, in 2014. The protocol of the study was confirmed by Ethical Committee of Isfahan University of Medical Sciences (grant number: 392318) and consent form was given by the patients. During the study, all researchers observed declaration of Helsinki.
The studied population included patients with 18-65 years of age who referred to the ED because of medical diseases, drug poisoning, or trauma, and need the sedative agent to sedation. The need for sedation was diagnosed by an emergency specialist. Sensitivity to haloperidol or midazolam, contraindications to these drugs such as severe intoxication with alcohol, sympathomimetic agents, agitation because of reversible factors (such as hypotension, hypoxia, and hypoglycemia), tachycardia or bradycardia, respiratory distress, pregnancy, symptoms of withdrawal syndrome, and receiving sedative agents within the past 12 h were the exclusion criteria. Sampling was performed consecutively. Twenty-three samples were considered as the sample volume for each group; it was achieved by 95% confidence coefficient (α = 0.05), power of 80% (β = 0.2), and standard error of 14 min for the time to achieve sedation of midazolam and 25 min for haloperidol, with 13.9% difference between the two groups.[10]
Randomization was done using computerized block randomization (blocks of six) by an independent physician. All emergency staff included physicians, nurses, and researchers were blind to the therapeutic groups. To ensure from blinded status of the study, drugs were prepared as clear solutions in dark packs by the researcher, who was the only person informed from their contents. These packs were coded and delivered to drug prescribers.
Therapeutic intervention
The patients were categorized into two random groups of haloperidol (5 mg, single-dose, intramuscular (IM) administration) and midazolam receivers (2.5 mg for those weighing <50 kg and 5 mg in >50 kg, single-dose, IM administration) [Figure 5]. Induction of sedation was evaluated by a valid criterion with three scores Table 1 that from three (turbulence intensity, need to full harness, and require constant care) to one (without restless, without need to permanent supervision, and dormant), the appropriate sedation was considered as the score of three.[11] Co-researchers were trained for using this criterion and finding appropriate condition to use rescue dose that was detected only by a trained physician. The maximum permissible dose for midazolam was 20 mg and if the patient needed more, the physician was informed from the prescribed drug and initiated other treatments. In such cases, the patient was excluded from the study and this considered as a treatment failure. All the patients were consistently monitored and their vital signs (body temperature, blood pressure, respiratory rate, and pulse rate), arterial oxygen saturation level, blood sugar level, and side effects were recorded.
Figure 5.
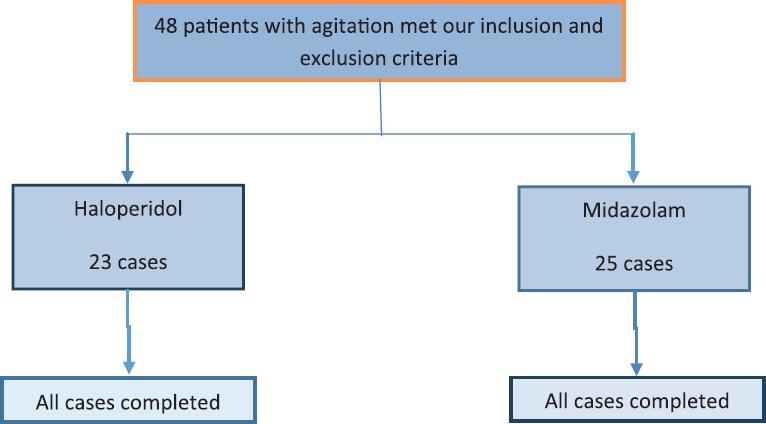
Consort diagram of participant flow
Table 1.
Restless score

Evaluated outcomes
In this project, the primary outcomes were considered as the time to achieve sedation and full consciousness. The achievement time was defined as the duration between drug prescription and proper induction of sedation (Score = 1). The full consciousness was also the time from drug prescription to full consciousness of the patient (Score = 3). The secondary outcomes were need rescue dose to produce primary sedation, need for resedation within the first 60 min and side effects included the need for management of ventilator and airways, arterial oxygen level drop below 90%, systolic hypotension below 90 mmHg, dystonic reactions, seizure, vomiting or aspiration, and movement disorders.
Statistical analysis
Data were analyzed by SPSS statistical software program, version 20 (SPSS Inc., Chicago, IL, USA). Results were presented as mean ± standard error (SE) for quantitative variables and number (percent) for qualitative variables. To compare the quantitative variables between two groups, independent Student's t-test (or Mann-Whitney test, as appropriate) was used. Distribution of study participants in terms of categorical variables was compared between two groups using the Chi-square test (or Fisher's exact test, as appropriate). Multiple linear regression analysis was used to examine factors associated with time to arousal. P < 0.05 was considered as statistically significant level.
RESULTS
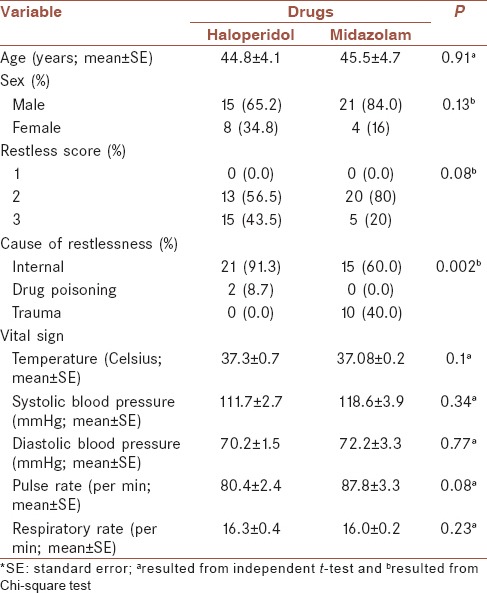
Baseline characteristics of the studied population in the two groups are presented in Table 2. The mean age of patients was 44.8 ± 4.1 and 45.5 ± 4.7 years, in haloperidol and midazolam groups, respectively. The reason of restlessness in 91.3% of patients taken haloperidol and 60.0% of those received midazolam was internal (P = 0.002). None of the basic vital signs had significant difference between two groups [Table 2].
Table 2.
Baseline characteristics of two studied groups
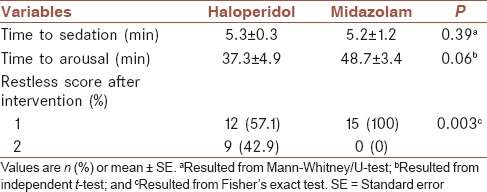
The mean ± SE time to sedation was 5.6 ± 03 and 5.2 ± 0.1 min, in haloperidol and midazolam groups, respectively [Table 3]. There was no significant difference between time to arousal in two groups [Table 3]. In addition, mean and 95% CI for time to sedation and time to arousal has been shown in Figure 1, by groups. Restless scores before and after sedation had no significant difference between two groups [Table 3].
Table 3.
Comparison of time to sedation, time to arousal, and restless score before and after intervention in two groups, for the total sample
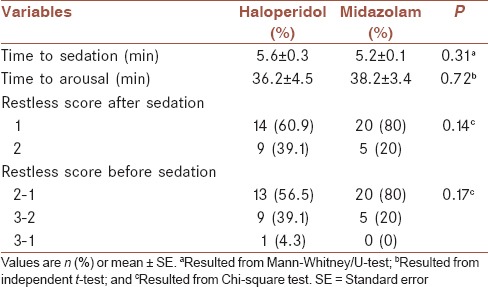
Figure 1.
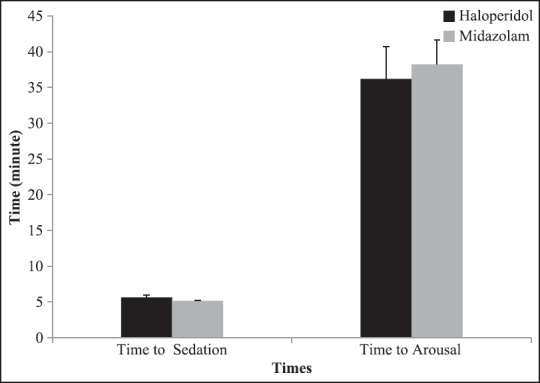
Mean and 95% CI of time to sedation and time to arousal by groups
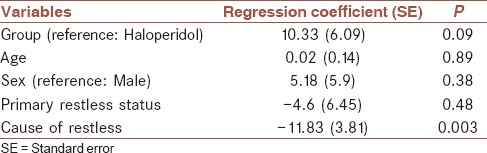
The results of multiple linear regression analysis of factors associated with the time to arousal for the total sample are presented in Table 4. There was an inverse significant relationship between cause of restless and time to arousal (β = −11.83, P < 0.01). The associations among age, sex, and primary restless status with time to arousal were not significant. On average, time to arousal in midazolam group was 10.33 min more than haloperidol group, but it was not statistically significant.
Table 4.
Regression analysis of factors associated with time to arousal for the total sample
The results of multiple linear regression analysis of factors associated with the time to arousal in internal patients are presented in Table 5. There were positive associations among age, sex, and restless score with time to arousal, but they were not statistically significant. On average, time to arousal in midazolam group was 8.3 min more than haloperidol group, but it was not statistically significant.
Table 5.
Regression analysis of factors associated with time to arousal in internal group

Comparison of time to sedation, time to arousal, and restless score after intervention between two groups for internal patients is presented in Table 6. The mean ± SE time to sedation was 5.3 ± 0.3 and 5.2 ± 1.2 min, in haloperidol and midazolam groups, respectively. The mean time to arousal was higher in midazolam group (48.7 ± 3.4), but statistically was not significant. Restless scores after sedation had significant difference between two groups (P < 0.01) [Table 6].
Table 6.
Comparison of time to sedation, time to arousal, and restless score after intervention between two groups for internal patients
Mean and 95% CI for time to arousal has been showed in Figure 2, by groups and grading of restless before intervention. In patients with decreased restless, the mean time to arousal was 38.70 ± 6.75 and 42.20 ± 3.70 min, in haloperidol and midazolam groups, respectively. In addition, mean and 95% CI for time to arousal based on grading of restless after intervention and changes in grading of restless after intervention has been shown in Figures 3 and 4, respectively. According to Figure 4, mean time to arousal in patients that grade of restless after intervention changed from two to one was not significantly different between two groups, but in patients that grade of restless after intervention changed from three to one was significantly higher in haloperidol group.
Figure 2.
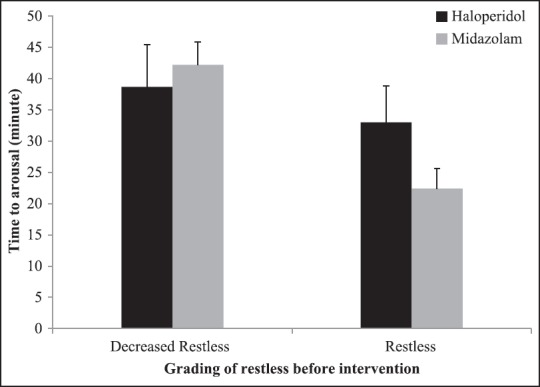
Mean and 95% CI for time to arousal based on grading of restless before intervention by groups
Figure 3.
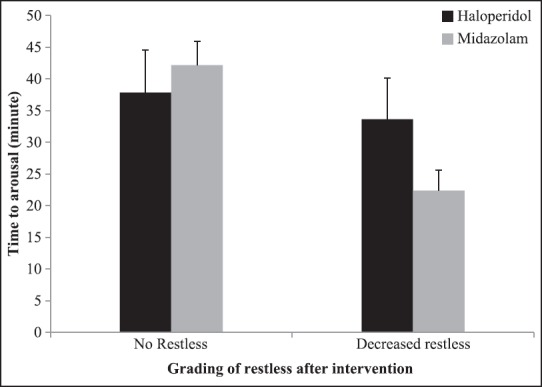
Mean and 95% CI for time to arousal based on grading of restless after intervention by groups
Figure 4.
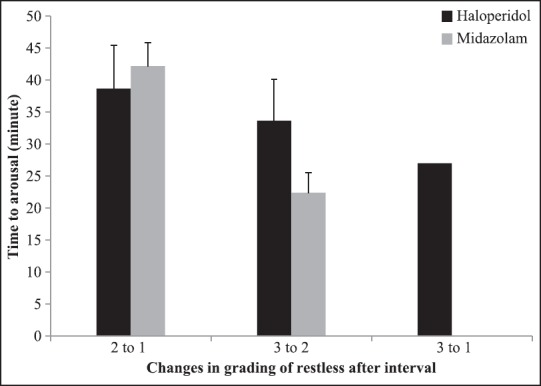
Mean and 95% CI for time to arousal based on changes in grading of restless after intervention by groups
All the patients were sedated in the first 10 min and became full consciousness at a maximum time of 80 min after injection. There was no need for rescue dose to the induction of sedation. None of the side effects was seen in these patients and vital signs were in normal range during this period. In each group, 18 patients became full consciousness before 60 min, and analyses did not show any difference among groups in this area (P = 0.08). Since none of the patients have agitation after full consciousness, there was no need to resedation for them.
DISCUSSION
The findings of the present study showed that midazolam and haloperidol have similar efficacy and safety for the improvement of restless symptoms and also the same recovery time from drug effects in patients referred to the ED. In this study, none of the drug side effects was seen in different doses. The present protocols have more emphasis on calming agitated patients that have more effect on the primary sedation and turns it to be the main goal in the initial management of agitated patients in EDs.[12,13] These guidelines stress on using the second-generation anti-psychotic drugs such as zuclopenthixol acetate and ziprasidone.[13] As American Association of Emergency Psychiatry Best practices in Evaluation and Treatment of Agitation project stated that the second-generation anti-psychotic drugs are the first-line treatment to calm patients,[12,14,15] in many conditions, these drugs have not yet been used.[13] It may be because there is no strong evidence available on their priority to the first-generation anti-psychotic drugs.[16] Even in the recent studies, it has been declared that both drug generations have the similar efficacy and safety on calming agitated patients referred to the ED.[17] On the other hand, in many situations, the infusion form of second-generation anti-psychotic drugs are not available and most of the emergency specialists prefer to use infusion drugs in patients who have severe agitation and do not collaborate to take drugs orally. Haloperidol, as a first-generation anti-psychotic drug, has a high potency to calm patients. Studies have shown that the best administration route in such cases is IM injection. Although effects such as dystonic reactions and neuroleptic malignant syndrome and akathisia may happen even with using single-dose of this drug, it does not make limitation to use it in emergency conditions.[18] Haloperidol reaches to the peak level in plasma within 20 min and has about 20 h half-life. Thus, beside rapidly acting to improve symptoms, it can make long-acting, too, which is the positive point of this drug based on recent protocols. The findings of the present study showed that haloperidol is a safe, rapidly-acting, and long-acting drug in the improvement of restless symptoms for emergency cases. These findings are along with the results of other researches stated that using haloperidol diminished violent behavior in 83% of patients after 30 min from drug administration.[10]
Midazolam has been introduced as an alternative medicine instead of haloperidol in the recent years. Several studies have shown that midazolam is a safe and effective drug in EDs and in some cases, has superior acting even than haloperidol. Say, Wyant et al. showed that this drug has better efficacy than haloperidol in controlling the agitation.[9] While, Knott et al. declared there is no difference in the onset of action between droperidol and midazolam. Nevertheless, patients sedated with midazolam may be needed active management of airways.[19] Another study presented in using midazolam alone, the sedation induction time is longer than combination therapy with olanzapine and droperidol. Yet, no difference was seen in side effects and duration of stay at hospital among three mentioned groups.[20] While, it was expected that in combination therapy, because of using fewer doses of each drug, lesser side effects were be presented. The finding of the present study is representative on the high safety of midazolam in relieving restless symptoms. Although no side effects were seen in taking midazolam, IM administration effects of it should be considered, which included respiratory depression, amnesia, paradoxical reactions, and confusion. Sampling was done consecutively, but in the absence of researcher at ED, referring patients were not evaluated; subsequently, selection bias is probable. However, demographic variables of referring patients in this time were similar to subjects of the present project, the possibility of such bias is nearly low. Lack of evaluating the second-generation anti-psychotic drugs is another limitation of this study. Choosing haloperidol had a theoretical base that included limited access to second-generation drugs in hospitals and showing similar efficacy of haloperidol with these drugs in recent researches, based on them, haloperidol is used as a treatment arm. Moreover, lack of placebo (control group) was another limitation that because of ethical issues, it was not possible to cancel treatment for agitated patients.
CONCLUSION
The results of the present study show that administration of midazolam and haloperidol have similar efficacy in the treatment of restless symptoms with the same recovery time from drug effects for referring patients to the ED. In addition, none of the drug effect was observed in this study.
Financial support and sponsorship
This study was supported by grants from the Isfahan University of Medical Sciences, Iran (grant number: 392318).
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
MS contributed in the conception and design of the work, conducting the study, drafting and revising the draft, data analysis, approval of the final version of the manuscript, and agreed for all aspects of the work. MT contributed in the conception of the work, conducting the study, drafting and revising the draft, data collection, approval of the final version of the manuscript, and agreed for all aspects of the work. OA contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MZ contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This study was supported by grants from the Isfahan University of Medical Sciences, Iran (grant number: 392318).
REFERENCES
- 1.Karceski S, Morrell M, Carpenter D. The expert consensus guideline series: Treatment of epilepsy. Epilepsy Behav. 2001;2:A1–50. [Google Scholar]
- 2.TREC Collaborative Group. Rapid tranquillisation for agitated patients in emergency psychiatric rooms: A randomised trial of midazolam versus haloperidol plus promethazine. BMJ. 2003;327:708–13. doi: 10.1136/bmj.327.7417.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zun LS. An issue of equity of care: Psychiatric patients must be treated “on par” with medical patients. Am J Psychiatry. 2014;171:716–9. doi: 10.1176/appi.ajp.2014.14010002. [DOI] [PubMed] [Google Scholar]
- 4.Chun TH, Katz ER, Duffy SJ, Gerson RS. Challenges of managing pediatric mental health crises in the emergency department. Child Adolesc Psychiatr Clin N Am. 2015;24:21–40. doi: 10.1016/j.chc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Macht M, Mull AC, McVaney KE, Caruso EH, Johnston JB, Gaither JB, et al. Comparison of droperidol and haloperidol for use by paramedics: Assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18:375–80. doi: 10.3109/10903127.2013.864353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MP, Pepper D, Currier GW, Holloman GH, Jr, Feifel D. The psychopharmacology of agitation: Consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13:26. doi: 10.5811/westjem.2011.9.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson MP, MacDonald K, Vilke GM, Ronquillo L, Feifel D. Intramuscular ziprasidone: Influence of alcohol and benzodiazepines on vital signs in the emergency setting. J Emerg Med. 2013;45:901–8. doi: 10.1016/j.jemermed.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Walther S, Moggi F, Horn H, Moskvitin K, Abderhalden C, Maier N, et al. Rapid tranquilization of severely agitated patients with schizophrenia spectrum disorders: A naturalistic, rater-blinded, randomized, controlled study with oral haloperidol, risperidone, and olanzapine. J Clin Psychopharmacol. 2014;34:124–8. doi: 10.1097/JCP.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 9.Wyant M, Diamond BI, O’Neal E, Sloan A, Borison RL. The use of midazolam in acutely agitated psychiatric patients. Psychopharmacol Bull. 1990;26:126–9. [PubMed] [Google Scholar]
- 10.Nobay F, Simon BC, Levitt MA, Dresden GM. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11:744–9. doi: 10.1197/j.aem.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Daniel J, Chamberlain J, Castle D. The pharmacological management of behavioural disturbance in psychosis: A naturalistic study. Australas Psychiatry. 2007;15:380–4. doi: 10.1080/10398560701435754. [DOI] [PubMed] [Google Scholar]
- 12.Allen MH, Currier GW, Carpenter D, Ross RW, Docherty JP. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(Suppl 1):5–108. doi: 10.1097/00131746-200511001-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MP, Minassian A, Bahramzi M, Campillo A, Vilke GM. Despite expert recommendations, second-generation antipsychotics are not often prescribed in the emergency department. J Emerg Med. 2014;46:808–13. doi: 10.1016/j.jemermed.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Holloman GH, Jr, Zeller SL. Overview of Project BETA: Best practices in evaluation and treatment of agitation. West J Emerg Med. 2012;13:1–2. doi: 10.5811/westjem.2011.9.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilke G, Wilson M. Agitation: What every emergency physician should know. Emerg Med Rep. 2009;30:233–44. [Google Scholar]
- 16.Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation) Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Chan HY, Ree SC, Su LW, Chen JJ, Chou SY, Chen CK, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34:355–8. doi: 10.1097/JCP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 18.Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23:767–76. doi: 10.1016/j.ajem.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Knott JC, Taylor DM, Castle DJ. Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006;47:61–7. doi: 10.1016/j.annemergmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Chan EW, Taylor DM, Knott JC, Phillips GA, Castle DJ, Kong DC. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: A multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61:72–81. doi: 10.1016/j.annemergmed.2012.07.118. [DOI] [PubMed] [Google Scholar]


