Abstract
Background:
Improvement in complications of antitumor agents and surgery is important to enhance life quality and survival among patients with colon and colorectal cancer. It has been reported that some dietary components such as glutamine (Gln) have beneficial effects on these complications of cancer therapies. However, the results of studies are inconsistent in this area. We performed a review on randomized controlled trials (RCTs) evaluating the effects of Gln intake on complications related to therapeutic strategies of the colon and colorectal cancer.
Materials and Methods:
A systematic search was conducted in PubMed, Google Scholar, Cochrane Library, and SID databases to find the relevant literature, published before July 2015.
Results:
Nine RCTs of 217 screened articles were included in this systematic review. The results of the present review suggested that Gln intake among colon and colorectal cancer patients could reduce some complications induced by chemotherapy such as gut mucositis and diarrhea and improve nitrogen balance, immune system and wound healing after surgery, whereas benefits role of Gln on radiochemotherapy side effects were not provided.
Conclusion:
The role of Gln intake on some improvement of complications induced by cancer therapeutic methods and shorten the length of hospital stay may be promising and one that is worthy of further exploration.
Keywords: Cancer, colon, colorectal, glutamine, supplementation
INTRODUCTION
Among all cancer types, the colorectal cancer is common malignancy worldwide and more than 940,000 new cases and nearly 500,000 deaths occur each year in the world due to this cancer.[1,2] Although, a broad variety of treatment for these cancers are currently available, almost all clinically used antitumor treatments exhibit toxic side effects such as anorexia, constipation, depression, diarrhea, nausea/vomiting, and neuropathy among patients who receive these treatment regimen.[3,4] However, due to adverse reactions induced by these therapeutic strategies, life quality of cancer patients is affected, and many patients are at risk of treatment discontinuation.[4,5,6] Even, to reduce these complications, it may reduce the therapeutic dose of anticancer agents which lead to diminished efficacy of this medication.[7] Whereas, providing low toxicity treatment with maximum benefit is still an unsolved problem, evidence suggests that some dietary factors such as Vitamin E and probiotics may be helpful to relieve some patients’ symptoms such as oral mucositis and diarrhea related to cancer treatment.[8,9,10] Another important nutrient that associates with the complication of colorectal cancer treatment is Glutamine (Gln).[11] Gln is the most abundant non-essential amino acid in the plasma and amino acid pool that it is utilized as a fuel for rapid proliferation and growth of cells.[12,13] Furthermore, this nutrient is considered as an essential amino acid conditionally and its requirement increases in catabolic diseases including cancers.[14,15,16] Also, it was observed that Gln depletion among patients with colorectal cancer can lead to suppression of the T-cell response. Therefore, the immune system cannot destroy the cancer cells in this situation.[10,13] Furthermore, this nutrient may improve the immune system through the proliferation of lymphocyte and macrophages.[17] Whereas, antioxidant properties of this amino acid play a beneficial role in protection of structure and function of gastrointestinal tract, it can be a favorite option to reduce some complications of colorectal cancer treatment and improve gastrointestinal adverse events.[18,19,20]
Although, several studies demonstrated that there are beneficial effects of Gln on cancer complication,[20,21] some results were not consistent with them.[12,22] With respect to the contradictions in different studies and the importance of reducing the side effects of cancer treatments, this systematic review was conducted on randomized controlled trials (RCTs) to investigate the effects of Gln intake on several complications of chemotherapy, radiochemotherapy, and postoperation including, diarrhea, vomiting and T-cell dysfunction in patients with colon and colorectal cancer.[17,20,23]
MATERIALS AND METHODS
Search strategy
Systematic search was conducted in electronic databases including PubMed (www.pubmed.com), Google Scholar (scholar.google.com), Cochrane Library (www.cochrane.org), and SID (sid.ir) to identify studies assessing Gln effects on colon and colorectal cancer complications induced by therapeutic methods, published before July 2015. Search strategy was performed by two independent investigators according to the criteria of preferred reporting item for systematic review (PRISMA), without any language and time limitation. Search phrase was as follows: (“Glutamine” [mesh] OR “L-Glutamine” OR “D-Glutamine” OR “oral glutamine” OR “dietary glutamine” OR “supplement of glutamine”) AND (“Colonic Neoplasms” [mesh] OR “Colorectal Neoplasms” [mesh]). The title and abstract of all identified papers were assessed by two independent researchers and irrelevant articles were excluded. The full text of the remaining papers was examined to determine the illegible studies. In addition, we performed a manual search for using of references from review articles to identify other potentially eligible studies.
Eligibility criteria
We included all articles that focused on the effects of oral, supplement, injection or dietary Gln consumption on therapeutic side effects of the colon or colorectal cancer. Studies were excluded if they had not the clinical trial design and performed in animal or in vitro. Moreover, if researches were reported the association between serum Gln and cancer without Gln intake or the effect of Gln on overall gastrointestinal side effects of treatment without mentioning the specific findings for colon or colorectal cancer or administration of other supplements or drugs with Gln intake, we withdrawal them.
Assessment of quality
All included studies in this review were scored by Jadad Quality Assessment Scores. The scale articles were assessed according to three items related to the validity of RCTs: “(1) Was the study randomized? (2) Was the study described as double blind? (3) Was there a description for logged dropouts?” It is composed of a total score of 0-5 points. If the total point is between 0-2 points and 3-5 points, the article is considered to be low quality or high quality, respectively.[24,25]
Data extraction
Information that have been extracted from each study included: First author name, year of publication, type of RCT, sample size and sex in each group, age range, type of intervention, duration of intervention, statistical adjustment, followed event, P-value, and quality score.
RESULTS
Detailed processes of the study selection were illustrated in Figure 1. Generally, 217 kinds of literature were identified via the initial electronic search; 8 of them were selected according to inclusion and exclusion criteria and 1 paper was added to hand search. These articles had a parallel design and were from the Asian and European countries. The average of intervention period was 5 days. The number of participants varied from 12 to 109 subjects and the mean age was 60 years. Table 1 provides a summary of the study characteristics included in this systematic review. These studies were classified into the three groups: Effects of Gln intervention in colon or colorectal cancer patients under chemotherapy (n = 4), radiochemotherapy (n = 1) and under surgery (n = 4).
Figure 1.
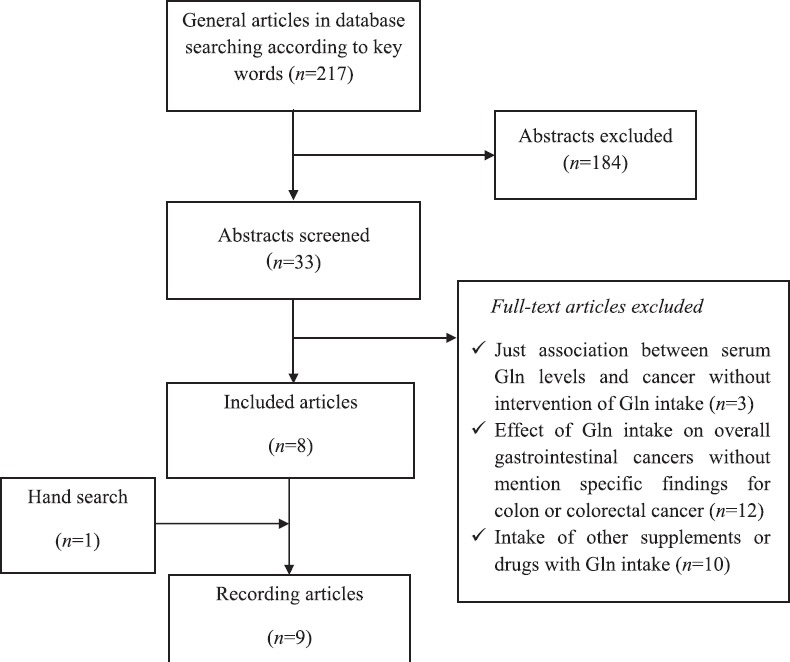
Flow chart of systematic literature search
Table 1.
Randomized controlled trials of Gln intake and complications of colon and colorectal cancer
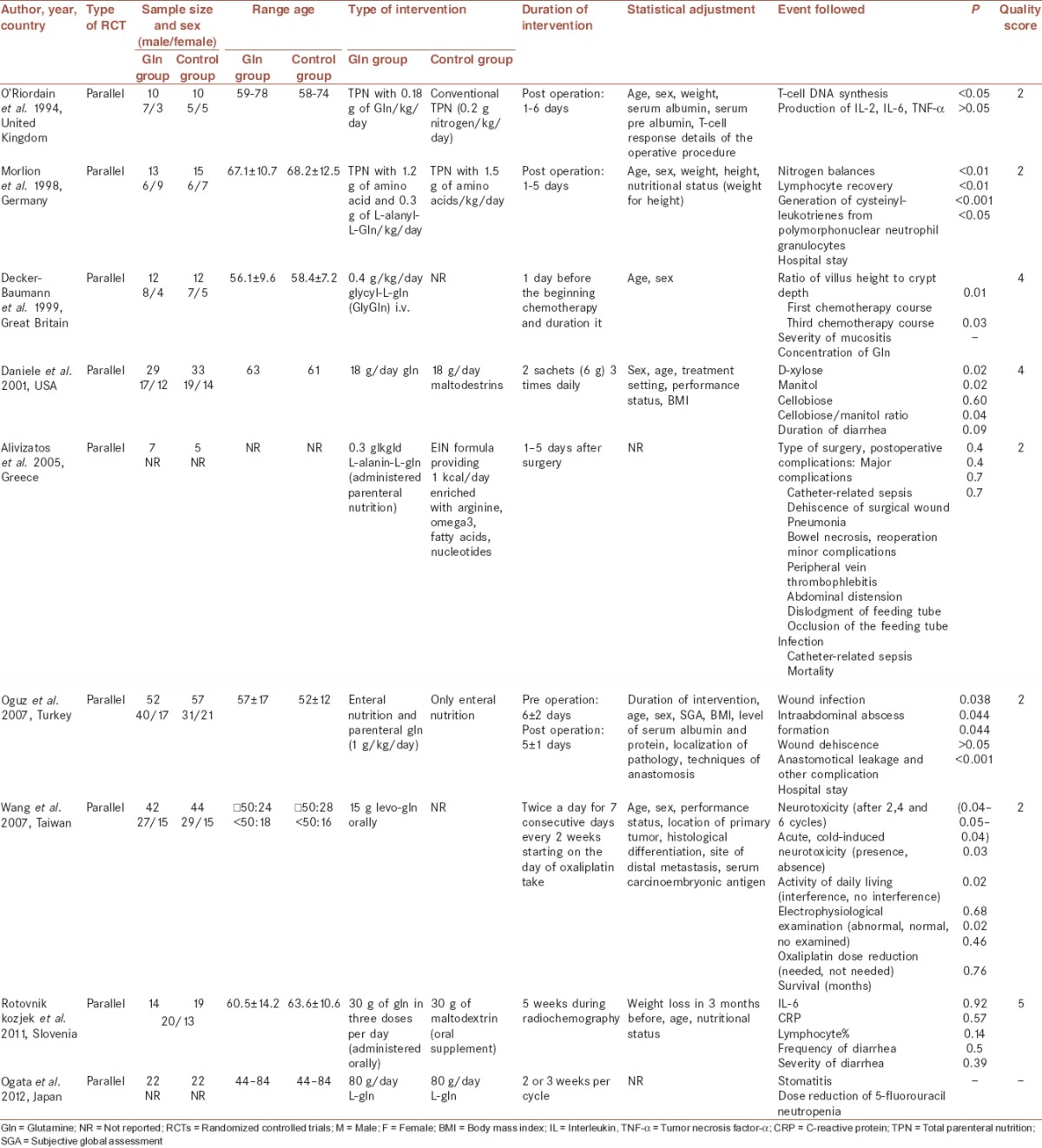
The glutamine intervention in colon or colorectal cancer patients under chemotherapy
One trail study assessed the effect of Gln on chemotropic side effects in colorectal metastatic patients among 4 female and 8 male with mean age 56.1 as the intervention group. These subjects consumed 0.4 g/kg glycine-Gln from 1 day before the beginning chemotherapy to the end of treatment period. All complications of metastatic colorectal cancer in duodenum and stomach before and after chemotherapy were classified, scored, and assessed according to the World Health Organization (WHO) criteria characterized by no disorder, mucositis, and ulcer. The effect of Gln was estimated before and after a first and third period of chemotherapy by gastroscopy, duodenoscopy, and biopsy. The results showed that the ratio of villus height to crypt depth in the duration of chemotherapy increased in Gln group and decreased in control group significantly. Although decreased incidence of gastric and duodenal mucositis in Gln group was indicated, incidence and strength of chemotherapy side effects according to the WHO criteria did not improve in these patients.[19]
Wang et al. investigated whether oral Gln was effective in the prevention of peripheral neuropathy induced by chemotherapy. This study was performed among patients with colon or rectum cancer. Gln group received 15 g Gln once every 2 weeks, twice daily for 7 days. Although results showed that the Gln intake can reduce neuropathy symptom, it had no effect on median survival time and improvement of nerve condition.[26]
Another trial evaluated the effect of oral Gln on the toxicity of 5-fluorouracil (5-FU) as a chemotherapy drug among patients with colorectal cancer. Gln group 18 g/day Gln and placebo group 18 g/day maltodextrins received during 15 days that started 5 days before onset chemotherapy. The effect of 5-FU was assessed on intestinal absorption (IA) and intestinal permeability (IP) by measuring the D-xylose urinary excretion and cellobiose-mannitol test. Finally, the results showed that Gln supplementation could lead to the prevention of intestinal mucositis and diarrhea reduction and had a protective effect on IA and IP induced by 5-FU.[21]
In another study which assessed the effects of elemental Gln on prevention of stomatitis and neutropenia, a reduction in these complications were seen. In this study, 22 patients with colorectal cancer with 67 years average age received 80 g L-Gln per day for 2 or 3 weeks per cycle of chemotherapy.[27]
Generally, these studies suggested the decreased incidence of gastric and duodenal mucositis and diarrhea in Gln group. Also, Gln could reduce the neuropathy symptom, but there were not any changes in median survival time and improvement of nerve condition. Also, elemental Gln exerts the beneficial effects on stomatitis and neutropenia reduction.
The glutamine intervention in colon or colorectal cancer patients under radiochemotherapy
One study was performed among men and women with a mean age of 73 years, with rectal cancer undergoing preoperative radiochemotherapy to examine the influence of Gln on quantity and quality of diarrhea and some inflammatory factors. Intervention and placebo groups received 30 g/day of Gln and maltodextrin orally, respectively. Some factors such as body weight, age, and nutritional status had been adjusted between two groups. This intervention was begun simultaneously with the start of radiochemotherapy and lasted for five weeks. Although there was no significant difference in frequency and severity of diarrhea and plasma levels of inflammatory indexes between two groups, compared with other studies, the dosages and duration of this intervention has been same or even more.[12]
The glutamine intervention in colon or colorectal cancer patients under surgery
In this regard, the operated patients with colorectal cancer received parenteral Gln (1 g/kg/day) plus enteral nutrition contained a standard isonitrogenous and isocaloric formula. There was no significant difference in the several characteristics of patients such as age (above 50 years) and index of malnutrition. Compared with patients in enteral nutrition group, the rate of wound infection, intraabdominal abscess formation, wound dehiscence and duration of hospital stay were significantly lower than Gln group after 5 days pre and postoperative intervention.[20]
Since the immune system among cancer patients with colorectal surgery is weakened, they are at risk for postoperative complications such as infection, hemorrhagic cytomegalovirus (CMV) colitis.[20,28] Hence, one study researched about the influence of Gln on peripheral blood T-cell response in these patients. Randomly, patients were divided into two groups that received conventional total parenteral nutrition (TPN) in the control group and plus 0.18 g of Gln/kg/day in the Gln group from days 1 to 6 after surgery. As a result, T-cell DNA synthesis increased in Gln group and led to improve immune function. Although, Gln is an essential substrate for monocyte metabolism, this intervention has no effect on interleukin (IL)-2, IL-6, and tumor necrosis factor (TNF) levels that were produced by the activated monocyte.[13]
Also, Morlion study assessed the effect of Gln on metabolic, immunologic and clinical variables among patients undergoing an elective resection of the colon or rectal cancer. Although intervention and control group received isonitrogenous and isoenergetic parenteral nutrition, 0.3 g/kg/day of l-alanyl-l-Gln was added to Gln group for 5 days after surgery. The mean of age, sex, weight and height were similar between two groups. Whereas, cysteinyl-leukotrienes (Cys-LTs), strong lipid mediators that are an essential prerequisite for host defense, generate from polymorphonuclear neutrophil granulocytes, it decreases with attenuated endogenous host defense. This survey reported that Gln administration could increase Cys-LTs concentration, improve immune response, and lymphocyte recovery. Also, improved nitrogen balance and shortened hospital stay was observed in this group.[17]
According to another report, Gln supplemented parenteral nutrition in colon cancer patients could not decrease gastrointestinal complication after surgery. In this study, 29 patients with malnutrition were selected and divide to enteral immune nutrition (EIN) (n = 14) enriched with omega-3 fatty acids, arginine, and nucleotides, and TPN-Gln groups (n = 15). Among these patients, there were five cases of EIN and seven subjects of TPN-group with colon cancer who had undergone colectomy. The findings represented that the side effects after surgery decreased non-significantly in TPN-Gln and EIN group (33.3% vs. 50%, P = 0.2). Also, when all postoperative complications divided into different types, there was no significant difference between the two groups regarding minor and major complications such as pneumonia, bowel necrosis, intractable diarrhea, peripheral vein thrombophlebitis, abdominal distension and cramps, and noninfection.[29]
Totally, although it can be noted that Gln may have beneficial effects on immune system, the improvement of several complications after surgery has been not observed in these studies.
DISCUSSION
Cancer and toxic effects resulting from antitumor therapy are the leading cause of mortality and morbidity among these patients. Therefore, the reduction of complications can lead to significant improvement in disease and increase life years of patients.[30] Our aim of this review was to assess the effects of Gln intake on chemotherapy, radiochemotherapy and surgery complications among colorectal or colon cancer patients. Although, we obtained controversial results in a few studies,[12,29] overall findings suggested that Gln supplementation could improve some these side effects. It seems that Gln administration can reduce mucositis, diarrhea and neuropathy among patients under chemotherapy.[19,21,23,27] Additionally, it could be associated with improvement in nitrogen balance, immune system and wound healing after surgery.[13,17,20,31] In this field, the mechanisms that caused the favorite effect of Gln can be classified as follows.
The mechanism of glutamine efficacy on improvement of gut mucositis
Mucositis is more important complication induced by chemotherapy that occurs in 40% of patients with standard chemotherapy and 100% of cases with high dose chemotherapy agents.[32] As a result, the increased proinflammatory factors caused by chemotherapy can lead to progress of inflammation in the mucosal lining of the digestive tract. Also, enhancement of vascular permeability, microbial colonization and infection are associated with mucositis.[33] It is claimed that Gln has a protective effect on intestinal mucosa, and it can prevent bacterial and endotoxin transfer from the intestinal lumen into the blood circulation.[34,35] It has been suggested that TNF-γ reduce the glutaminase enzyme activity. Subsequently, Gln availability can increase after the reduction of Gln break up.[36] The amount of this enzyme is low in intestinal and rectal cells. So, these cells uptake Gln and improve mucositis in these parts of the gut.[21] Moreover, administration of Gln above physiological concentrations versus low concentration or short time treatment can enhance proliferation of lymphocyte, monocyte and macrophages to relief the mucositis.[22] Furthermore, the antioxidant characteristic of Gln and its influence on arginine pathway and nitric oxide production have an important role on mucositis improvement. Gln can maintain the integrity and barrier function of intestinal cells via the prevention of nitric oxide production induced by arginine.[22,32] Additionally, it increased toxicity in tumor cells by down-regulation of glutathione as an antioxidative stress agent and enhance the effect of antitumor drugs.[11,21,26] Since, specific polymorphism in genes involved to toxicity effects of chemotherapeutic agents,[37] Gln through effect on genes expression, gens polymorphism, metabolism of chemotherapeutic drugs, and proinflammatory factors can induce toxicity in tumor cells.[32,38]
The effects of glutamine on neuropathy induced by chemotherapy
Peripheral neuropathy induced by chemotherapy is a serious adverse effect associated with neurotoxic agents used for cancer treatment.[39] These drugs disturb the axon's metabolic supply, resulting in sensory and motor neuron damage. According to a report, sensory symptoms include numbness, burning, pain, diminished vibratory and cutaneous sensation, and hyporeflexia can improve by Gln supplementation.[18] While, chemotropic drugs result to decrease level of nerve growth factor in plasma and make neurotoxic complication in these patients, dietary intervention by Gln can increase the expression of nerve growth factors and decrease neurotoxic effects of chemotherapy.[26,40]
The effects of glutamine on improvement negative nitrogen balance
Negative nitrogen balance status in the body can lead to increased rate of catabolism, protein analysis which resulted in malnutrition and depletion muscles mass.[41,42] Among patients undergoing cancer therapy, many factors such as insufficient dietary intake can lead to nutritional depletion.[17,20] In fact, in this situation an altered protein metabolism, concomitant negative nitrogen balance and changes in the pattern of plasma free amino acids lead to the depletion of plasma-free amino acids, especially Gln stores. It has two nitrogen groups in side chains that act as the most important transporter of circulating nitrogen.[14,20] Therefore, its supplementation affects nitrogen balance and increase retain of nitrogen, because of the quantity of nitrogen provided by Gln.[16,17]
The effects of glutamine on immune system
Augmentation of the immune system in cancer patients to destroy the tumor cells and reduction of susceptibility to infection after surgery is a considerable subject.[13,43] The role of Gln in proliferation capacity of T-lymphocytes and normal immune functions may be related to the production of energy in these cells. Also, it acts as a precursor of nucleotides required for cell proliferation and protein synthesis and turnover.[44,45,46] Since it is the substrate for T-cell DNA synthesis, the counts of circulating, total lymphocytes can increase by Gln that it leads to improving immune response.[47] Also, it has been observed that Gln deficiency can reduce production of some cytokine including IL-2 as the regulatory factors for defense system which resulted in activating the natural killer cells.[36,48]
Since, Gln can up-regulate antiapoptotic proteins and down-regulate proapoptotic proteins in T cells, it has the major role in the lymphocytes metabolism.[32,49] Generally, Gln boosts immune system response which may help to destroy tumor cells.
The effects of glutamine on wound healing
Surgery is one of therapeutic strategy in patients with colon and colorectal cancer that wounds creation is one of its outcomes. Wounds are a suitable place to penetrate microorganisms and for this reason, acceleration wound healing due to the prevention of infection is important.[50] Gln administration can help to improve poor wound healing after surgery through an effect on amelioration the laceration strength of wound and help to the synthesis of mature collagen.[47] Also, it has been assumed that fibroblasts, a type of cell that synthesizes the extra cellular matrix and collagen which plays a critical role in wound healing, use Gln as the main energy resource for proliferation.[34,51] In addition, proline, one of the Gln metabolism products, is essential for the collagen production. Furthermore, Gln can metabolize to arginine that it can promote wound healing in pharmacological doses. Therefore, this nutrient plays an important role in wound recovery.[20,44]
Nonetheless, there are doubts regarding the usefulness of such supplementation, because of the Gln role as an important fuel for tumor growth.[12,45] It has been illustrated that Gln consumption increases in tumor tissues and consequently decreases its concentration.[14] Hence, it is possible that Gln feeding can help to high gastrointestinal tract tumor cell turnover that it may lead to more growth of these cells.[52,53]
Several sources heterogeneity between studies including intervention duration, Gln dosage, the number of participants, and type of complications can lead to various findings.
One of the limitations in this review is related to little attention regarding the role of Gln in cancer progression among all studies. Also, we should consider other limitations including short duration of interventions and low quality of most studies.[13,17,19,29] Also, the studies had no exact and clear mechanisms to the explanation of Gln effectiveness. Furthermore, in all included studies, diet was not measured before and after the intervention. Thus, the potential biases related to confounding factors might occur. Nonetheless, the present systematic review has several strengths. Firstly, we systematically reviewed the articles with different therapeutic methods for cancer. Indeed, the effect of Gln on most complications induced by treatment in these patients was assessed. Secondly, in these reviewed studies, the effects of Gln on the patients with various stages of colon and colorectal cancer were investigated that can give a more comprehensive view regarding the Gln efficacy.
CONCLUSION
The main finding of this systematic review suggested that Gln intake among colon and colorectal cancer patients can reduce mucositis, diarrhea and neuropathy induced by chemotherapy and improve nitrogen balance, immune system, and wound healing after surgery. Further studies with long-term follow-up, a high number of participants, and consideration to adverse effects of Gln on tumor growth should be conducted.
Financial support and sponsorship
This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
NRJ, SM, searched databases and selected articles. NRJ, SM, RGH, GHA and MM wrote the manuscript. All authors have read and approved the content of the manuscript.
REFERENCES
- 1.Zhang SW, Chen WQ, Kong LZ, Li LD, Lu FZ, Li GL, et al. An analysis of cancer incidence and mortality from 30 cancer registries in China, 1998<2002. Zhongguo Zhong. 2006;15:430–48. [Google Scholar]
- 2.Jin P, Wu ZT, Li SR, Li SJ, Wang JH, Wang ZH, et al. Colorectal cancer screening with fecal occult blood test: A 22-year cohort study. Oncol Lett. 2013;6:576–82. doi: 10.3892/ol.2013.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKibbin T, Frei CR, Greene RE, Kwan P, Simon J, Koeller JM. Disparities in the use of chemotherapy and monoclonal antibody therapy for elderly advanced colorectal cancer patients in the community oncology setting. Oncologist. 2008;13:876–85. doi: 10.1634/theoncologist.2008-0061. [DOI] [PubMed] [Google Scholar]
- 4.Hung A, Mullins CD. Relative effectiveness and safety of chemotherapy in elderly and nonelderly patients with stage III colon cancer: A systematic review. Oncologist. 2013;18:54–63. doi: 10.1634/theoncologist.2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract 2012. 2012 doi: 10.1155/2012/282570. 282570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capra S, Ferguson M, Ried K. Cancer: Impact of nutrition intervention outcome – nutrition issues for patients. Nutrition. 2001;17:769–72. doi: 10.1016/s0899-9007(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 7.Piasek A, Bartoszek A, Namiesnik J. Phytochemicals that counteract the cardiotoxic side effects of cancer chemotherapy. Postepy Hig Med Dosw (Online) 2009;63:142–58. [PubMed] [Google Scholar]
- 8.Ben-Arye E, Polliack A, Schiff E, Tadmor T, Samuels N. Advising patients on the use of non-herbal nutritional supplements during cancer therapy: A need for doctor-patient communication. J Pain Symptom Manage. 2013;46:887–96. doi: 10.1016/j.jpainsymman.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Argyriou AA, Chroni E, Koutras A, Iconomou G, Papapetropoulos S, Polychronopoulos P, et al. A randomized controlled trial evaluating the efficacy and safety of Vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: Final results. Support Care Cancer. 2006;14:1134–40. doi: 10.1007/s00520-006-0072-3. [DOI] [PubMed] [Google Scholar]
- 10.Pace A, Savarese A, Picardo M, Maresca V, Pacetti U, Del Monte G, et al. Neuroprotective effect of Vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21:927–31. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 11.Medina MA. Glutamine and cancer. J Nutr. 2001;131(9Suppl):2539S–42S. doi: 10.1093/jn/131.9.2539S. [DOI] [PubMed] [Google Scholar]
- 12.Rotovnik Kozjek N, Kompan L, Soeters P, Oblak I, Mlakar Mastnak D, Možina B, et al. Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: A randomised double blinded, placebo controlled pilot study. Clin Nutr. 2011;30:567–70. doi: 10.1016/j.clnu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 13.O’Riordain MG, Fearon KC, Ross JA, Rogers P, Falconer JS, Bartolo DC, et al. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann Surg. 1994;220:212–21. doi: 10.1097/00000658-199408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldin E, Aptekar L, Siguencia J, Tsvang E, Fich A, Zimmerman J. Reduced glutamine content in colonic polyps. Scand J Gastroenterol. 1996;31:345–8. doi: 10.3109/00365529609006408. [DOI] [PubMed] [Google Scholar]
- 15.Smith RJ, Wilmore DW. Glutamine nutrition and requirements. JPEN J Parenter Enteral Nutr. 1990;14(4 Suppl):94S–99S. doi: 10.1177/014860719001400412. [DOI] [PubMed] [Google Scholar]
- 16.Fürst P, Albers S, Stehle P. Evidence for a nutritional need for glutamine in catabolic patients. Kidney Int Suppl. 1989;27:S287–92. [PubMed] [Google Scholar]
- 17.Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, et al. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: A randomized, double-blind, controlled study. Ann Surg. 1998;227:302–8. doi: 10.1097/00000658-199802000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2008;42:1481–5. doi: 10.1345/aph.1L179. [DOI] [PubMed] [Google Scholar]
- 19.Decker-Baumann C, Buhl K, Frohmüller S, von Herbay A, Dueck M, Schlag PM. Reduction of chemotherapy-induced side-effects by parenteral glutamine supplementation in patients with metastatic colorectal cancer. Eur J Cancer. 1999;35:202–7. doi: 10.1016/s0959-8049(98)00389-x. [DOI] [PubMed] [Google Scholar]
- 20.Oguz M, Kerem M, Bedirli A, Mentes BB, Sakrak O, Salman B, et al. L-alanin-L-glutamine supplementation improves the outcome after colorectal surgery for cancer. Colorectal Dis. 2007;9:515–20. doi: 10.1111/j.1463-1318.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 21.Daniele B, Perrone F, Gallo C, Pignata S, De Martino S, De Vivo R, et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: A double blind, placebo controlled, randomised trial. Gut. 2001;48:28–33. doi: 10.1136/gut.48.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidal-Casariego A, Calleja-Fernández A, de Urbina-González JJ, Cano-Rodríguez I, Cordido F, Ballesteros-Pomar MD. Efficacy of glutamine in the prevention of acute radiation enteritis: A randomized controlled trial. JPEN J Parenter Enteral Nutr. 2014;38:205–13. doi: 10.1177/0148607113478191. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Ping X, Yu B, Liu F, Ni X, Li J. Clinical trial: Prophylactic intravenous alanyl-glutamine reduces the severity of gastrointestinal toxicity induced by chemotherapy - A randomized crossover study. Aliment Pharmacol Ther. 2009;30:452–8. doi: 10.1111/j.1365-2036.2009.04068.x. [DOI] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Chung JH, Lee SW. Assessing the quality of randomized controlled urological trials conducted by korean medical institutions. Korean J Urol. 2013;54:289–96. doi: 10.4111/kju.2013.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang WS, Lin JK, Lin TC, Chen WS, Jiang JK, Wang HS, et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007;12:312–9. doi: 10.1634/theoncologist.12-3-312. [DOI] [PubMed] [Google Scholar]
- 27.Ogata Y, Takeuchi M, Ishibashi N, Kibe S, Takahashi K, Uchida S, et al. Efficacy of Elental on prevention for chemotherapy-induced oral mucositis in colorectal cancer patients. Gan To Kagaku Ryoho. 2012;39:583–7. [PubMed] [Google Scholar]
- 28.Saito M, Ishino A, Ito T, Sakuma T, Matsuzaki M, Katagata N, et al. Hemorrhagic cytomegalovirus colitis in a postoperative colon cancer patient. Case Rep Oncol. 2013;6:109–13. doi: 10.1159/000348711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alivizatos V, Athanasopoulos P, Makris N, Karageorgos N. Early postoperative glutamine-supplemented parenteral nutrition versus enteral immunonutrition in cancer patients undergoing major gastrointestinal surgery. J BUON. 2005;10:119–22. [PubMed] [Google Scholar]
- 30.Guo MG, Feng Y, Liu JZ, Zheng Q, Di JZ, Wang Y, et al. Factors associated with mortality risk for malignant colonic obstruction in elderly patients. BMC Gastroenterol. 2014;14:76. doi: 10.1186/1471-230X-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morlion BJ, Siedhoff HP, Joosten U, Köller M, König W, Fürst P, et al. Immunomodulation after parenteral glutamine administration in colorectal surgery. Langenbecks Arch Chir Suppl Kongressbd. 1996;113:342–4. [PubMed] [Google Scholar]
- 32.Deniel N, Marion-Letellier R, Charlionet R, Tron F, Leprince J, Vaudry H, et al. Glutamine regulates the human epithelial intestinal HCT-8 cell proteome under apoptotic conditions. Mol Cell Proteomics. 2007;6:1671–9. doi: 10.1074/mcp.M600428-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis – complicating the treatment of cancer. Neoplasia. 2004;6:423–31. doi: 10.1593/neo.04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sipahi S, Gungor O, Gunduz M, Cilci M, Demirci MC, Tamer A. The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: A retrospective analysis of diabetic haemodialysis patients. BMC Nephrol. 2013;14:8. doi: 10.1186/1471-2369-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pattanshetti VM, Powar RS, Godhi AS, Metgud SC. Enteral glutamine supplementation reducing infectious morbidity in burns patients: A randomised controlled trial. Indian J Surg. 2009;71:193–7. doi: 10.1007/s12262-009-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohde T, MacLean DA, Klarlund Pedersen B. Glutamine, lymphocyte proliferation and cytokine production. Scand J Immunol. 1996;44:648–50. doi: 10.1046/j.1365-3083.1996.d01-352.x. [DOI] [PubMed] [Google Scholar]
- 37.Sukhotnik I, Pollak Y, Coran AG, Pilatov J, Bejar J, Mogilner JG, et al. Glutamine attenuates the inhibitory effect of methotrexate on TLR signaling during intestinal chemotherapy-induced mucositis in a rat. Nutr Metab (Lond) 2014;11:17. doi: 10.1186/1743-7075-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2(6 Suppl 3):3–8. [PubMed] [Google Scholar]
- 39.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer. 2008;44:1507–15. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Stubblefield MD, Vahdat LT, Balmaceda CM, Troxel AB, Hesdorffer CS, Gooch CL. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: A clinical and electrophysiologic study. Clin Oncol (R Coll Radiol) 2005;17:271–6. doi: 10.1016/j.clon.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen AN, Cederholm T. Health effects of protein intake in healthy elderly populations: A systematic literature review. Food Nutr Res. 2014:58. doi: 10.3402/fnr.v58.23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilar A, Giménez J, Gómez-Campos E, Cardona L, Borrell A. d15N value does not reflect fasting in mysticetes. PLoS One. 2014;9:e92288. doi: 10.1371/journal.pone.0092288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stockmann C, Schadendorf D, Klose R, Helfrich I. The impact of the immune system on tumor: Angiogenesis and vascular remodeling. Front Oncol. 2014;4:69. doi: 10.3389/fonc.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKay D, Miller AL. Nutritional support for wound healing. Altern Med Rev. 2003;8:359–77. [PubMed] [Google Scholar]
- 45.Wilmore DW, Shabert JK. Role of glutamine in immunologic responses. Nutrition. 1998;14:618–26. doi: 10.1016/s0899-9007(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhang G, Ducatelle R, Pasmans F, D’Herde K, Huang L, Smet A, et al. Effects of helicobacter suis gamma-glutamyl transpeptidase on lymphocytes: Modulation by glutamine and glutathione supplementation and outer membrane vesicles as a putative delivery route of the enzyme. PLoS One. 2013;8:e77966. doi: 10.1371/journal.pone.0077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Costa MA, Campos AC, Coelho JC, de Barros AM, Matsumoto HM. Oral glutamine and the healing of colonic anastomoses in rats. JPEN J Parenter Enteral Nutr. 2003;27:182–5. doi: 10.1177/0148607103027003182. [DOI] [PubMed] [Google Scholar]
- 48.Olofsson PE, Forslund E, Vanherberghen B, Chechet K, Mickelin O, Ahlin AR, et al. Distinct migration and contact dynamics of resting and il-2-activated human natural killer cells. Front Immunol. 2014;5:80. doi: 10.3389/fimmu.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueno PM, Oriá RB, Maier EA, Guedes M, de Azevedo OG, Wu D, et al. Alanyl-glutamine promotes intestinal epithelial cell homeostasis in vitro and in a murine model of weanling undernutrition. 2011;301:G612–22. doi: 10.1152/ajpgi.00531.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anders A, Nordhausen B, Zeuschner Z, Fabrizius K. Prevention of infections in surgery of the colon. Zentralbl Chir. 1984;109:1097–106. [PubMed] [Google Scholar]
- 51.Kesici U, Kesici S, Ulusoy H, Yucesan F, Turkmen AU, Besir A, et al. Effects of glutamine on wound healing. Int Wound J. 2015;12:280–4. doi: 10.1111/iwj.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Dwyer ST. Glutamine supplementation in colorectal cancer. Colorectal Dis. 2007;9:852. doi: 10.1111/j.1463-1318.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 53.Sykorova A, Horacek J, Zak P, Kmonicek M, Bukac J, Maly J. A randomized, double blind comparative study of prophylactic parenteral nutritional support with or without glutamine in autologous stem cell transplantation for hematological malignancies–three years’ follow-up. Neoplasma. 2005;52:476–82. [PubMed] [Google Scholar]


