Abstract
Health professionals are interested in evaluating the risks that heavy metals pose to eco-receptors and humans. The objective of this study was to examine levels of mercury (Hg), lead (Pb), cadmium (Cd), and other contaminants in waterbirds nesting in the New York harbor in 2012 to determine (1) whether there were species and locational differences, and (2) whether consumption of eggs posed a health risk to predators or humans. For arsenic (As), Pb, Hg, and selenium (Se), species contributed more to variations in levels than location; for Cd and chromium (Cr), location was more significant. Mean metal levels differed among species for all metals, except Cd. Highest levels were As (great black-backed gulls, Larus marinus), Cr (great egret, Ardea alba), Pb (Canada goose, Branta canadensis), and Hg and Se (black-crowned night heron, Nycticorax nycticorax). There were significant locational differences only for herring gulls (Larus argentatus); significant differences were found for all metals. Levels of Hg and Pb may be sufficiently high in eggs of some species to produce adverse effects in predators that eat them. The proportion of samples above 0.3 ppm Hg (U.S. Environmental Protection Agency [EPA] freshwater criteria for freshwater fish), the contaminant of health concern, ranged from 0% (Canada goose, great egret), to 14 and 27% in gulls, to 50% (black-crowned night heron). Some herring gull, great black-backed gull, and black-crowned night heron eggs had 0.5 ppm or higher Hg. Thus, human consumption of eggs may pose a risk to fetuses and young children.
Health professionals, health risk assessors, government agencies, and the public are increasingly concerned about exposure to high contaminant levels. Humans and other eco-receptors are exposed through urban, suburban, and industrial development, local sources, and atmospheric deposition. Toxic chemicals are transported globally, including to urban and suburban environments (Evers et al., 2005; Hammerschmidt and Fitzgerald, 2006; Hammerschmidt et al., 2006). Atmospheric deposition is increasing because emissions are poorly regulated in industrialized nations, and there are few regulations in industrialized nations (Fitzgerald et al., 2005). Harbors, bays, and estuaries are vulnerable because of runoff from surrounding communities and rivers, from point-source pollution, and from natural geochemical processes (Fitzgerald and Lyons, 1973; Burger and Gochfeld, 2001). Mercury (Hg), lead (Pb), and cadmium (Cd) are the most significant contaminants in aquatic systems (Mailman, 1980; Fowler, 1990). Mercury, particularly methylmercury (MeHg), bioaccumulates up the food chain (Furness and Rainbow, 1990; Hahn et al., 1993; Bargagli et al., 1998, Burger, 2002; Gray, 2002). Cardwell et al. (2013), in an extensive review, concluded that Cd general did not biomagnify very well in aquatic food chains, and Pb never did. Understanding the movement of toxic chemicals through the food chain, particularly heavy metals, and levels reached in organisms is critical to determining risk to the organisms themselves, to predators that eat them, and to humans that might consume them.
This study examined levels of arsenic (As), Cd, chromium (Cr), Pb, Hg, and selenium (Se) in eggs of several species of waterbirds in the New York Harbor area to determine (1) whether there were species differences in metal levels, (2) whether there were locational differences, and (3) whether consumption of eggs posed a health risk to predators or humans that consumed them. The New York harbor ecosystem is complex, with several rivers, inlets, and bays. It was predicted that there would be interspecific differences because of variations in food types, but not locational differences in metal levels, as birds fly long distances. New York City is heavily industrialized with a population of more than 8 million people living adjacent to the New York/New Jersey (NY/NJ) harbor. The harbor itself is home to over 4,000 colonial waterbirds of 12 species, with nesting colonies on 17 of the harbor’s 19 undeveloped islands (Craig, 2013). Further, colonially nesting waterbirds have declined since the early 1990s in the NY/NJ harbor, giving rise to further concerns about possible population-level impacts to breeding birds from toxins (Burger and Gochfeld, 1997; Weseloh et al., 1997). The birds feed in the surrounding waters, and most species either arrive well before egg laying or are resident. Species examined represent different levels of the food chain, and included (from lowest to highest) Canada goose (Branta canadensis), herring gull (Larus argentatus), great egret (Ardea alba), great black-backed gull (Larus marinus), double-crested cormorant (Phalacrocorax auritus), and black-crowned night heron (Nycticorax nycticorax). For the region, levels of the food chain were determined from personal observations of foraging adults and food brought back to the nest, except for Canada goose, which is the only herbivore sampled. Canada geese are herbivorous, while black-crowned night herons eat large fish, crabs, and other animals.
Water birds that breed colonially are useful bioindicators of contaminants because they (1) are exposed to a wide range of chemicals, (2) occupy a wide range of trophic levels, (3) are easy to find, and (4) are numerous, and eggs can be collected without harming local populations (Custer, 2000; Nygard et al., 2001; Burger and Gochfeld, 2004; Kim and Koo, 2007; Custer et al., 2007). Gilbertson et al. (1987) also noted that contaminant levels in marine and coastal birds have lower coefficients of variation than do fish or marine mammals, making it easier to assess patterns. Eggs are often used as indicators of metal contamination because (1) females sequester metals in their eggs (Burger and Gochfeld, 1991, 1996; Lam et al., 2005), (2) concentrations of metals that are in eggs represent female exposure (Becker et al., 2002), usually from local exposure (Sanpera et al., 2000), (3) there is a high correlation between levels of contaminants in the diet of seabirds and levels in eggs, (4) eggs are easy to collect and store, and (5) removal of one egg from species with clutches of three or more does not adversely affect reproductive potential (since birds rarely raise as many young as their clutch).
Using birds as bioindicators provides data for managers, public policy makers, and the public, as well as health professionals. Such data are particularly useful in regional management plans (Elbin and Tsipoura, 2010). Toxicity varies both by area and toxin (Steinberg et al., 2004). Contaminated sediments remain a problem in the Hudson River and estuary (Parsons, 2001, 2003; U.S. Army Corps of Engineers, Port Authority of New York and New Jersey, 2009). Bird eggs are collected for human food extensively in some parts of the world and are sometimes collected by recent immigrants in the New York/New Jersey harbor estuary who may have collected them in their home countries (Burger et al., 2008, Burger, personal communication, 1980).
METHODS
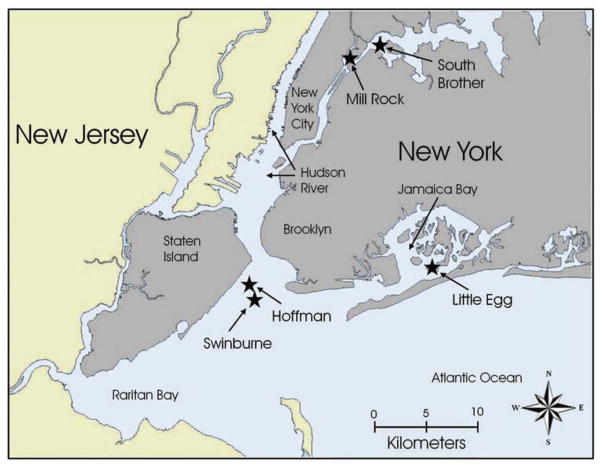
Eggs were collected from several colonies in the New York/New Jersey harbor, including South Brother, Mill Rock, Hoffman, Swinburne, and Little Egg (Figure 1). Although nesting colonies of waterbirds are located on several islands, not all species nest on each island, as available nest sites vary. Most heronries were located in vegetation with some poison ivy, low shrubs, or trees, gulls nested on the ground on sand or pebble beaches, cormorants nested on abandoned pilings, and Canada geese nested on the ground (solitarily), usually hidden by grasses or shrubs.
FIGURE 1.
Eggs were collected in late April through mid-May under appropriate federal and state permits. Only one freshly laid egg was collected per clutch; the smallest egg in each clutch was collected (last egg to be laid), and eggs were collected from widely separated locations within each colony. Eggs were labeled with a unique identifier, placed in a cushioned cooler, immediately taken back to the lab, and stored in a refrigerator for immediate analysis. Some eggs were frozen for archival purposes. All procedures were approved by the Rutgers University Animal Protocol Review Board.
In the lab, egg contents were emptied into acid-washed weigh boats, weighed, and then dried and reweighed. All samples were analyzed in the Elemental Laboratory of the Environmental and Occupational Health Sciences Institute of Rutgers University, in Piscataway, NJ. Whole egg contents were homogenized and digested individually in 70% nitric acid within microwave vessels for 10 min at 150 pounds per square inch (1.6 kg/sq cm), and subsequently diluted with deionized water.
Mercury was analyzed by cold vapor atomic absorption spectrophotometry, and other metals were analyzed by graphite furnace (flameless) atomic absorption. Mercury was analyzed as total Hg; about 90% is assumed to be methylmercury (Wolfe and Norman, 1998; Scheuhammer et al., 2001). All concentrations are expressed in nanograms per gram (ng/g, parts per billion) on a dry weight basis. Mean moisture content was as follows: cormorant (84%), great egret (81%), black-crowned night heron (80%), great black-backed and herring gulls (76%), and Canada goose (68%).
Instrument detection limits were 0.02 ng/g for As and Cd, 0.08 ng/g for Cr, 0.15 ng/g for Pb, 0.09 ng/g for Mn, 0.02 ng/g for Hg, and 0.7 ng/g for Se, but matrix detection limits were an order of magnitude higher for each metal. All specimens were run in batches that included a standard calibration curve and spiked specimens. The accepted recoveries on spiked specimens ranged from 90 to 115%. The coefficient of variation (CV) on replicate samples was usually less than 10%, and there were no discrepancies. Data (log transformed) were analyzed by analysis of variance (ANOVA) to determine differences among metals (Statistical Analysis System [SAS], 2005), and the Duncan’s multiple-range option with ANOVA (SAS, 2005) was used as a post hoc test of the significance of the differences among metals. A p < .05 was accepted as significant.
RESULTS
Between 19 and 83% of the variation in metal levels was explained by colony location or species (Table 1). Examining species × location added little to explaining variation because most species nested in few locations. Species explained most of the variation for As, Hg, Pb, and Se, while location explained more of the variation than did species for Cd and Cr. The most variation in metal levels was explained for Hg, and the least for Cd (Table 1).
TABLE 1.
Models explaining variations in contaminant levels in bird eggs collected in 2012 from New York / New Jersey harbor estuary. Metals data was log transformed for normality. NS = not significant
| Arsenic | Cadmium | Chromium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|
| Model | ||||||
| F | 4.6 | 1.6 | 4.1 | 3.8 | 32.3 | 15.6 |
| df | 12 | 12 | 12 | 12 | 12 | 12 |
| P | <0.0001 | NS | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| r2 | 0.40 | 0.19 | 0.39 | 0.35 | 0.83 | 0.69 |
| Factors entering | ||||||
| F (p) | ||||||
| Location | 3.9 (0.006) | 3.6 (0.01) | 10.9 (<0.0001) | 2.2 (0.08) | 10.9 (<0.0001) | 3.0 (0.02) |
| Species | 6.2 (<0.0001) | NS | 2.3 (0.05) | 6.7 (<0.0001) | 68.2 (<0.0001 | 25.4 (<0.0001) |
| Location X Species | 3.2 (0.03) | NS | 2.8 (0.04) | NS | 2.7 (0.05) | 2.8 (0.05) |
There were interspecific differences in all metal levels (except Cd), supporting prediction 1. No one species had the highest levels of metals. Instead, great black-backed gulls had the highest levels of As, great egrets had the highest levels of Cr, Canada geese had the highest levels of Pb, black-crowned night herons had the highest levels of Hg and Se, and there were no significant species differences for Cd (Table 2). Interspecific variation was as follows: (1) As varied from 5.5 ng/g (black-crowned night heron) to 159 ng/g (great black-backed gull), (2) Cd varied from 3.5 ng/g (great egret) to 7.2 ng/g (black-crowned night heron), (3) Cr varied from 40.2 ng/g (black-crowned night heron) to 176 ng/g (great egret), (4) Pb varied from 5.1 ng/g in great egret to 470 ng/g (Canada goose), (5) Hg varied from 15 ng/g (Canada goose) to 1408 ng/g in black-crowned night heron, and (6) Se varied from 1822 ng/g (herring gull) to 3720 ng/g (black-crowned night heron).
TABLE 2.
Concentrations of metals in eggs collected in 2012 from the New York / New Jersey Harbor estuary. Given are means ± SE. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. Duncan letter groupings on log transformed data, different letters indicate significant differences. All values are in ng/g (ppb dry weight.) NS = Not significant
| Species | Canada Goose | Herring Gull | Great Egret | Great Black-backed Gull | Double-crested Cormorant | Black-crowned Night Heron | X2 Comparison |
|---|---|---|---|---|---|---|---|
| N | 10 | 49 | 6 | 11 | 10 | 10 | |
| Arsenic | 50.8 ± 27.1 | 57.4 ± 13.4 | 8.1 ± 5.0 | 159 ± 42.8 | 4.1 ± 3.3 | 5.5 ± 3.3 | 22.7 (0.0004) |
| B | B | B | A | B | B | ||
| Cadmium | 5.6 ± 1.7 | 5.3 ± 0.7 | 3.5 ± 1.0 | 4.5 ± 1.7 | 6.9 ± 1.7 | 7.2 ± 4.0 | 2.8 (NS) |
| A | A | A | A | A | A | ||
| Chromium | 61.5 ± 12.6 | 85.2 ± 12.8 | 176 ± 53.3 | 55.3 ± 16.0 | 79.6 ± 17.4 | 40.2 ± 9.53 | 10.0 (0.08) |
| B | B | A | B | A, B | B | ||
| Lead | 470 ± 178 | 138 ± 43.8 | 5.1 ± 3.3 | 62.7 ± 22.5 | 11.0 ± 7.4 | 212 ± 86.2 | 27.6 (<0.0001) |
| A | B | C | B | C | B | ||
| Mercury | 15 ± 2.3 | 706 ± 83 | 779 ± 183 | 1049 ± 172 | 1094 ± 167 | 1408 ± 305 | 33.0 (<0.0001) |
| B | A | A | A | A | A | ||
| Selenium | 1430 ± 144 | 1822 ± 63 | 3317 ± 463 | 2164 ± 112 | 2830 ± 105 | 3720 ± 221 | 57.9 (<0.0001) |
| D | C | A, B | C | B | A |
There were significant locational differences in all metals for herring gulls (Table 3). As was highest on Hoffman, Cd was highest on Little Egg (and Hoffman), Cr was highest on Little Egg, Pb was highest on Swinburne, and Hg and Se were highest on Mill Rock. There were no locational differences in metal levels for black-crowned night heron, Canada goose, double-crested cormorant, and great egret. Great black-backed gulls could only be collected from one island (Table 3). Thus, prediction 2 was supported for all species except herring gull, the species that occurred in the greatest number of colonies.
TABLE 3.
Concentrations of metals in eggs collected in 2012 from the New York/New Jersey Harbor estuary as a function of location. Given are means ± SE. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb dry weight.) NS=Not significant
| Species | N | Arsenic
|
Cadmium
|
Chromium
|
Lead
|
Mercury
|
Selenium
|
|---|---|---|---|---|---|---|---|
| mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | ||
| Canada Goose | |||||||
| South Brother | 7 | 21.8 ± 14.0 | 6.7 ± 2.4 | 52.6 ± 19.5 | 313 ± 72.6 | 16.7 ± 3.1 | 1529 ± 192 |
| Mill Rock | 3 | 118 ± 79.7 | 3.1 ± 1.3 | 76.3 ± 5.4 | 837 ± 587 | 11.0 ± 1.5 | 1200 ± 115 |
| X2 Comparison | NS | NS | NS | NS | NS | NS | |
| Herring Gull | |||||||
| South Brother | 9 | 81.1 ± 17.3 | 3.8 ± 1.3 | 67.4 ± 15.3 | 49.7 ± 39.0 | 794 ± 74.9 | 1742 ± 132 |
| Mill Rock | 10 | 20.7 ± 12.0 | 5.5 ± 1.9 | 113 ± 35.7 | 40.3 ± 23.9 | 1420 ± 236 | 2360 ± 124 |
| Hoffman | 10 | 143.3 ± 50.1 | 7.3 ± 1.6 | 95.6 ± 34.9 | 162 ± 57.5 | 474 ± 80.7 | 1720 ± 98 |
| Swinburne | 10 | 20.0 ± 13.3 | 2.1 ± 0.6 | 26.0 ± 4.3 | 245 ± 152.2 | 237 ± 38.6 | 1630 ± 84 |
| Litte Egg | 10 | 24.2 ± 16.7 | 7.8 ± 1.6 | 122 ± 24.6 | 186 ± 136.1 | 615 ± 159 | 1652 ± 128 |
| X2 Comparison | 15.4 (0.004) | 11.8 (0.02) | 17.2 (0.002) | 10.2 (0.04) | 26.5 (<0.0001) | 16.6 (0.002) | |
| Great Egret | |||||||
| South Brother | 1 | 0.2 | 0.0 | 32.0 | 0.2 | 433 | 2400 |
| Mill Rock | 5 | 9.7 ± 5.8 | 4.2 ± 0.8 | 223 ± 33.3 | 6.1 ± 3.9 | 848 ± 208 | 3500 ± 521 |
| Great Black-backed Gull | |||||||
| S Brother | 11 | 159 ± 42.8 | 4.5 ± 1.7 | 55.3 ± 16.0 | 62.7 ± 22.5 | 1049 ± 172 | 2164 ± 112 |
| Double-crested Cormorant | |||||||
| South Brother | 10 | 4.1 ± 3.3 | 6.9 ± 1.7 | 79.6 ± 17.4 | 11.0 ± 7.4 | 1094 ± 167 | 2830 ± 105 |
| Black-crowned Night Heron | |||||||
| South Brother | 5 | 1.8 ± 1.6 | 3.9 ± 1.8 | 53.8 ± 16.8 | 370 ± 141 | 1835 ± 468 | 3520 ± 297 |
| Mill Rock | 5 | 9.1 ± 6.2 | 10.4 ± 7.9 | 26.6 ± 5.9 | 54.1 ± 34.0 | 981 ± 331 | 3920 ± 334 |
| X2 Comparison | NS | NS | NS | 3.0 (0.08) | NS | NS | |
Because of the potential for adverse effects from Hg on the birds themselves, or their predators, the percent of samples that fell into different levels was computed for each species. The present study reports levels in nanograms per gram dry weight, but most regulatory levels for human consumption are given in ppm, wet weight, and thus wet weights were used to compute percent of samples above different levels (Table 4). The percent of egg samples above 0.3 ppm Hg (the current U.S. Environmental Protection Agency [EPA] freshwater fish criterion) ranged from 0 (Canada goose), to 50% for black crowned-night herons. The percent of samples above 0.5 ppm was 30% for night herons, 14% for herring gulls, and 27% for great black-backed gulls. Canada geese had no levels above 300 ng/g (Table 4). For gulls, eggs were observed to be collected by people for consumption.
TABLE 4.
Potential human health effects from people eating eggs collected from the New York/New Jersey harbor estuary. Given are percent above different regulatory action levels for mercury (ppb wet weight)
| Species | N | % above 0.30 ppm | % above 0.50 ppm | % above 1.0 ppm |
|---|---|---|---|---|
| Canada Goose | 9 | 0% | 0% | 0% |
| Herring Gull | 49 | 14% | 2% | 0% |
| Great Egret | 6 | 0% | 0% | 0% |
| Great Black-backed Gull | 11 | 27% | 9% | 0% |
| Double-crested Cormorant | 10 | 10% | 0% | 0% |
| Black-crowned Night Heron | 10 | 50% | 10% | 0% |
DISCUSSION
There were significant interspecific differences in all metal levels (except Cd), and there were locational differences only for herring gull. Most metal levels varied by an order of magnitude among species. Several species had Hg levels above 300 ng/g, the level of concern for human foods (the U.S. EPA freshwater criterion for fish). Each of these aspects is discussed in the following.
Interspecific Differences and Trophic Level
Species that forage in coastal estuaries are more vulnerable to pollutants than terrestrial species because (1) there is potential for rapid movement of contaminants in water, (2) chemicals can be stored in bottom sediments, providing a pool for years to come, (3) contaminants move quickly through the food chain, and (4) Hg and some other metals bioaccumulate as a function of size and age (Stewart et al., 1997; Burger and Gochfeld, 2000a; Becker et al., 2002). Metals levels generally reflect food-chain relationships (Burger, 1993; Becker et al., 2002), foraging locations (Borga et al., 2006), and diet/prey types (Monteiro et al., 1996; Bryan et al., 2012). Species foraging at top trophic levels usually have higher metal levels than those at lower levels (Burger, 2002; Becker et al., 2002; Frederick et al., 2002). However, even within the same trophic level, birds feeding in different places can forage on different amounts of prey, and prey fish can have higher levels one place compared to another. Further, some prey accumulate higher metal levels than others (e.g., some small invertebrate-foraging fish can have higher levels of contaminants that larger fish-eating fish). Thus, trophic level alone does not always account for bioaccumulation of metals. Further, some metals bioaccumulate (e.g., Hg), while others do not (e.g., Se). Some metals (e.g., Se) are regulated in the body (Drown et al., 1986; Roels et al., 1992), while others (e.g., Cd, Pb, Hg) are not naturally found in the body and are toxic (Eisler, 1987, 2000; Burger and Gochfeld, 2000b).
In the present study there were interspecific differences in metal levels (except for Cd), but no one species had the highest levels of all metals, or the lowest levels of all metals. Canada geese are at the lowest trophic level (consuming only vegetation), and black-crowned night heron and double-crested cormorant are at the highest (consuming mainly large fish). Canada geese had the highest levels of Pb, but the lowest levels of Hg and Se, suggesting they obtain Pb from vegetation or from soil consumed while eating vegetation. High Pb levels may be legacy contamination in soil from the prior use of leaded gasoline in the region. Birds that probe or peck for invertebrates or roots can consume sediments at a rate of 7–30% of their diet (Beyer et al., 1994; Beyer and Fries, 2002).
While the geese were clearly low on the trophic scale, the other species all eat some fish. Cormorants eat only fish, while the other species eat other vertebrates as well as fish. Hg levels provide the best indication of trophic level, in order of Canada goose < herring gull < great egret, great black-backed gull < double-crested cormorant < black-crowned night heron, although the differences among the latter species were not significantly different. For metals other than Hg, differences may partly be explained by diet. All species eat a range of fish, from small forage fish to larger fish; gulls even eat quite large dead fish washed up on the shore (Burger and Elbin, personal communication, 2000–2010). Great egrets and night herons both eat invertebrates and amphibians. Thus the interspecific differences may be predominantly due to food/prey types, prey sizes, and locational differences.
Locational Differences
There were no significant locational differences in metals levels, except for herring gulls. The lack of a difference for these species may be due to their nesting on only two different colony sites that were close together (e.g., Mill Rock and S. Brother), while herring gulls nested on five that were farther apart. Mill Rock and S. Brother are both in the East River, far from the main harbor. When the herring gull data for Mill Rock and S. Brother are compared, there were no significant differences for any metal. However, there were significant differences in metal levels for all the metals for herring gulls. The levels from the lower harbor were generally lower compared to those in the colonies from the East River for Cr and Hg, but not Pb or the other contaminants. Data suggest that contaminants are not moving together, or that there are local point sources that need to be examined.
Implications for Population Dynamics
Declines in some waterbird species in the NY/NJ harbor estuary gave rise to questions about whether contaminants could account for the declines. New York City Audubon has been conducting surveys of nesting colonial waterbirds for 32 years (Craig, 2013). The number of nesting pairs of colonial waterbirds reached a high of 2233 pairs in 1993, declined by 32% in 2002 to a low of 1519 nesting pairs, and increased to 2047 by 2010. Although black-crowned night herons are the numerically dominant species in the harbor, they have suffered greater declines than some of the other species (Padula et al., 2010). At the same time, breeding colonies have moved from islands in the lower, inner harbor (on the Arthur Kill and Kill Van Kull waterways) to islands in the inner, upper harbor (on the East River), and more recently to islands in the lower outer harbor (off the eastern shore of Staten Island; Craig, 2010). The cause of colony declines and population movements has been a major concern for resource managers, and contaminants need to be considered. Immunological investigations in herring gulls and black-crowned night herons from the Hudson–Raritan estuary suggest that polychlorinated biphenyls (PCB) and dioxins contribute to immunosuppression (Grasman et al., 2013), and other contaminants may exert a similar effect. Metal levels in eggs are significant because they (1) indicate female exposure from local sources, (2) indicate any potential for reproductive and hatchling effects, and (3) serve as an early warning of other potential ecosystem effects if levels are elevated.
Date from the present study provide a basis to examine potential effects of heavy metals, metalloids, and Se in eggs on reproductive success and population stability. Mercury is the primary metal of concern in terms of health effects on organisms themselves and on the predators that might consume them (Burger and Gochfeld, 1997; Eisler, 2000). Egg concentrations of Hg seem to be the best predictor of Hg risk to avian reproduction (Wolfe et al., 1998; Nichols et al., 1999). Effects of Hg on egrets have been demonstrated (Bouton et al., 1999; Spalding et al., 2000a, 2000b). Mercury levels as low as 500 ng/g (wet weight) produce adverse effects for developing embryos, including mortality, lowered hatching rates, higher chick defects, and other neurobehavioral deficits (Eisler, 1987, 2000; Burger and Gochfeld, 1997; Fisk et al., 2005), although there are interspecific differences (Heinz et al., 2012). Severe effects usually occur at 1000 to 3000 ng/g (Eisler, 2000). Seabirds seem less vulnerable to Hg than other birds (Thompson and Furness, 1998). In the present study, mean Hg levels in all species were below 1408 ng/g dry weight (= 352 ng/g wet weight). In individuals, levels were as high as 3401 ng/g dry weight (= 680 ng/g wet weight), in black-crowned night herons from S. Brother, and 3207 ng/g dry weight (= 801 ng/g wet weight) in herring gulls from Mill Rock. S. Brother and Mill Rock are close together, and located in the East River, far from the main estuary. However, sensitive birds might exhibit effects at dietary Hg concentrations of 50 to 500 ng/g wet weight, and sensitive mammals display harmful effects at dietary levels of 1100 ng/g wet weight (Eisler, 1987; World Health Organization [WHO], 1990). Thus, there is some potential that Hg levels in eggs, particularly of night herons, cormorants, and great black-backed gulls, might produce adverse effects on other sensitive bird and mammal species that eat them.
Lead and Cd are the next most important metals in terms of toxicity and occurrence in marine ecosystems (Mailman, 1980). Cadmium levels in eggs that are toxic to developing embryos are unclear, but exposure was associated with reduced growth rates and poor fledging success in heron chicks (Spahn and Sherry, 1999), and young are more susceptible to Cd than adults (Wren et al., 1995). Bird predators are adversely affected by Cd levels of 1000 ng/g (wet weight) or higher (Eisler, 1985). Cadmium levels in all species examined in this study were low, and pose no apparent risk to the birds themselves or to their predators.
Lead decreases survival and growth, creates metabolic abnormalities, and is a neurotoxin that produces cognitive and behavioral deficits in vertebrates (Eisler, 1988; Agency for Toxic Substances and Disease Registry [ATSDR], 1997; Burger and Gochfeld, 2000b), although the levels producing these effects differ depending upon tissue. Dietary levels as low as 100–500 ng/g (wet weight) may produce learning deficits in some vertebrates (Eisler, 1988). In the present study, Pb levels were generally low, except for Canada goose eggs. Mean Pb egg levels for Canada goose were 836 ng/g (= 268 ng/g wet weight, Mill Rock) and 312 ng/g (= 119 ng/g, wet weight, S. Brother). In contrast, Pb levels from Canada geese from the nearby Meadowlands were only 54 ng/g (Tsipoura et al., 2011). Canada geese feed on vegetation, and likely remain local, so the higher metal levels at Mill Rock bear consideration in terms of predators that might eat them. High Pb levels in geese may originate from soil ingested while eating roots of vegetation, and Pb in soil may be from legacy contamination due to metal in paint or gasoline.
High levels of Se from agricultural runoff were associated with hepatic lesions, liver changes, congenital malformations, lowered reproductive success, and adult mortality in birds at Kesterson Reservoir in California (Ohlendorf et al., 1986, 1989). Selenium concentrations of 3000 ng/g (wet weight) in eggs resulted in adverse effects in lab studies (Heinz, 1996), while Se levels of 1000 ng/g (wet weight) are toxic to other wildlife that eat eggs (Lemly, 1993). In the present study, mean Se levels ranged from 1430 (= 400 ng/g, wet weight) to 3720 ng/g (= 744 ng/g, wet weight, black-crowned night herons), which are below the levels that produce adverse effects in lab studies. The potential for Se to moderate effects of Hg toxicity, either by sodium selenite protecting against MeHg poisoning or by the binding of MeHg to Se compounds, is intriguing, but not well studied for birds themselves or for egg predators (Sell and Horani, 1976; Ralston and Raymond, 2010). However, studies with breeding mallards (Anas platyrynchos) exposed to MeHg and Se in combination resulted in more deformities than either compound administered by itself, and were antagonistic (Heinz et al., 2011, 2012). The ameliorating effect of Se and Hg on toxicity in wild bird eggs (and egg predators) requires considerably more research before any conclusions can be drawn. However, both Se and Hg levels were highest in the same species (e.g., black-crowned night heron).
There are few lab or field experiments on effects of Cr on birds, although Burger and Gochfeld (1995, 2000b) found that Pb and Cr produced similar neurobehavioral effects in herring gulls. Overall, the results of this study suggest that levels of Hg and Pb may be sufficiently high in the eggs of some species, and in some individual eggs, to produce adverse effects to developing embryos and predators that eat them. It should be noted, however, that these data are from only one year, although they involve 6 species and 96 eggs. In this context, however, many investigators found that Hg levels have not declined over the last 30 years in heron, gull, and tern eggs, while Cd levels fell (Chunsheng et al., 2003; Burger and Gochfeld, 2004; Frederick et al., 2004; Weseloh et al., 2011; Burger, 2013; Burgess et al., 2013). However, patterns are often complex and vary with location, habitat, and pollution sources (Weseloh et al., 2006).
Potential Risk to Human Consumers
Although it is illegal, some individuals were observed collecting gull eggs for consumption in the NY/NJ harbor estuary (Burger, personal communication, 1980), and presumably would collect eggs of other species if available. Methylmercury is one of the main contaminants of concern for subsistence foods because it can be sufficiently high in some fish to produce adverse human health effects (Institute of Medicine [IOM], 1991, 2006, Gochfeld, 2003; Hightower and Moore 2003), particularly to developing fetuses (National Research Council [NRC], 2000; Joint Expert Committee on Food Additives [JECFA], 2011).
The U.S. Food and Drug Administration (FDA) action level for MeHg in fish (which should be similar for other foods) is 1 ppm (1 μg/g wet weight; FDA, 2005), a regulatory action level rather than a risk level. None of the eggs were above this level. In 1982 the European Commission set an Environmental Quality Standard for Hg; the mean concentration in Hg of a representative sample of fish shall not exceed 0.3 ppm (wet weight). The U.S. EPA (2001) promulgated 0.3 ppm as an ambient freshwater quality standard in 2001. In the present study, the percentage above 0.3 ppm varied (Table 4): cormorant eggs (10%), herring gull (14%), great black-backed gull (27%), and night heron (50%). The latter three had some individual eggs with levels above 0.5 ppm. Regardless of the regulatory level for Hg, it is clear that consumption of eggs of the species examined in this study (except Canada goose and great egret) from nesting waterbird colonies in the NY/NJ harbor may pose a risk to fetuses and young children. Caution needs to be applied by subsistence consumers if they are eating several meals of bird eggs per day or week.
Reaching the key vulnerable population who might be consuming waterbird eggs from the NY/NJ harbor estuary region is difficult because it appears to be primarily a recent immigrant population that might not read English signs or adhere to such warnings. Signs in a range of languages with pictorial messages might be effective. It is often low-income, vulnerable, minority populations who are exposed to high levels of contaminants through consumption of fish, shellfish, and other subsistence foods (Burger and Gochfeld, 2011). Even so, it is critical for health professionals, managers, and the public to be aware of the potential health problem from eating wild bird eggs.
Acknowledgments
FUNDING
We particularly thank many people who aided in egg collection, chemical and statistical analysis, and logistics, including C. Jeitner, T. Pittfield, E. Craig, E. Tobon, J. Rowden, D. Manry, B. Lysenko, F. Arengo, and D. Riepe. This research was funded by the Eppley Foundation, National Institute of Environmental Health Sciences (NIEHS: P30ES005022), and Rutgers University.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/uteh
References
- Agency for Toxic Substances and Disease Registry. Toxicological profile for lead. Atlanta, GA: Agency for Toxic Substances and Disease Registry, U.S. Public Health Service; 1997. [Google Scholar]
- Bargagli R, Monaci F, Sanchez-Hernandez JC, Cateni D. Biomagnification of mercury in an Antarctic marine coastal food web. Mar Ecol Prog Ser. 1998;169:65–76. [Google Scholar]
- Becker PH, Gonzalez-Solis J, Behrends B, Croxall J. Feather mercury levels in seabirds at South Georgia: Influence of trophic position, sex, and age. Mar Ecol Prog Ser. 2002;243:261–269. [Google Scholar]
- Beyer WN, Connor EE, Gerould S. Estimates of soil ingestion by wildlife. J Wildl Manage. 1994;58:375–382. [Google Scholar]
- Beyer WN, Fries GF. Toxicological significance of soil ingestion by wild and domestic animals. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of Ecotoxicology. 2. Lewis Publishers; Boca Raton, FL: 2002. pp. 151–166. [Google Scholar]
- Borga K, Campbell L, Gabrielsen BW, Norstrom RJ, Muir D, Fisk A. Regional and species specific bioaccumulation of major and trace elements in Arctic seabirds. Environ Toxicol. 2006;25:2927–2936. doi: 10.1897/05-574r1.1. [DOI] [PubMed] [Google Scholar]
- Bouton SN, Frederick PC, Spalding MG, McGill H. Effects of chronic, low concentrations of dietary methylmercury on the behavior of juvenile great egrets. Environ Toxicol Chem. 1999;18:1934–1939. [Google Scholar]
- Bryan AL, Brant HA, Jagoe CH, Romanek CS, Brisbin IL. Mercury contamination in nestling wading birds relative to diet in the southeastern United States: A stable isotope analysis. Arch Environ Contam Toxicol. 2012;63:144–152. doi: 10.1007/s00244-011-9745-0. [DOI] [PubMed] [Google Scholar]
- Burger J. Metals in avian feathers: Bioindicators of environmental pollution. Rev Environ Toxicol. 1993;5:203–311. [Google Scholar]
- Burger J. Food chain differences affect heavy metals in bird eggs in Barnegat Bay, New Jersey. Environ Res. 2002;90:33–39. doi: 10.1006/enrs.2002.4381. [DOI] [PubMed] [Google Scholar]
- Burger J. Temporal trends (1989–2011) in levels of mercury and other heavy metals in feathers of Great Egrets (Ardea alba) nesting in Barnegat Bay, NJ. Environ Res. 2013;122:11–17. doi: 10.1016/j.envres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Cadmium and lead in common terns (Aves: Sterna hirundo): Relationship between levels in parents and eggs. Environ Monit Assess. 1991;16:253–258. doi: 10.1007/BF00397612. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Heavy metal and selenium concentrations in eggs of herring gulls (Larus argentatus): Temporal differences from 1989 to 1994. Arch Environ Contam Toxicol. 1995;29:192–197. doi: 10.1007/BF00212970. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Heavy metal and selenium levels in Franklin’s gull (Larus pipixcan) parents and their eggs. Arch Environ Contam Toxicol. 1996;30:487–491. doi: 10.1007/BF00213400. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Risk, mercury levels, and birds: Relating adverse laboratory effects to field biomonitoring. Environ Res. 1997;75:160–172. doi: 10.1006/enrs.1997.3778. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Metals in albatross feathers from Midway Atoll: Influence of species, age, and nest location. Environ Res. 2000a;82:207–221. doi: 10.1006/enrs.1999.4015. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Effects of lead on birds (Laridae): A review of laboratory and field studies. J Toxicol Environ Health B. 2000b;3:59–78. doi: 10.1080/109374000281096. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Effects of chemicals and pollution on seabirds. In: Schreiber EA, Burger J, editors. Biology of marine birds. Boca Raton, FL: CRC Press; 2001. pp. 485–525. [Google Scholar]
- Burger J, Gochfeld M. Metals levels in eggs of common tern (Sterna hirundo) in New Jersey: Temporal trends from 1971–2002. Environ Res. 2004;94:336–343. doi: 10.1016/S0013-9351(03)00081-1. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Conceptual environmental justice model for evaluating chemical pathways of exposure in low-income, minority, Native American, and other unique exposure populations. Am J Pubic Health. 2011;101:S64–S73. doi: 10.2105/AJPH.2010.300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Snigaroff D, Snigaroff R, Stamm T, Volz C. Assessment of metals in down feathers of female common eiders and their eggs from the Aleutians: Arsenic, cadmium, chromium, lead, manganese, mercury, and selenium. Environ Monitor Assess. 2008;143:247–256. doi: 10.1007/s10661-007-9973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess NM, Bond AL, Hebert CE, Nugebauer E, Champoux L. Mercury trends in herring gull (Larus argentatus) eggs from Atlantic Canada, 1972–2008: Temporal change or dietary shift. Environ Pollut. 2013;172:216–222. doi: 10.1016/j.envpol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Cardwell RD, DeForest DK, Brix KV, Adams WJ. Do Cd, Cu, Pb, and Zn biomagnify in aquatic ecosystems? Rev Environ Contam, Toxicol. 2013;226:101–122. doi: 10.1007/978-1-4614-6898-1_4. [DOI] [PubMed] [Google Scholar]
- Chunsheng L, Cornett J, Ungar K. Long-term decrease of cadmium concentrations in the Canadian Arctic air. Geophys Res Lett. 2003;30:1256–1259. [Google Scholar]
- Craig E. New York City Audubon’s Harbor Herons Project: 2010 Nesting survey—25th Annual report. New York, NY: New York City Audubon; 2010. [Google Scholar]
- Craig E. New York City Audubon’s Harbor Herons Project: 2013 Nesting survey report. New York, NY: New York City Audubon; 2013. [Google Scholar]
- Custer TW. Environmental contaminants. In: Kushlan JA, Hafner H, editors. Heron conservation. New York, NY: Academic Press; 2000. pp. 251–267. [Google Scholar]
- Custer TW, Custer CM, Eichorst BA, Warburn D. Selenium and metal concentrations in waterbirds eggs and chicks at Agassiz National Wildlife Refuse, Minnesota. Arch Environ Contam Toxicol. 2007;53:103–109. doi: 10.1007/s00244-006-0139-7. [DOI] [PubMed] [Google Scholar]
- Drown DB, Oberg SG, Sharma RP. Pulmonary clearance of soluble and insoluble forms of manganese. J Toxicol Environ Health. 1986;17:201–212. doi: 10.1080/15287398609530816. [DOI] [PubMed] [Google Scholar]
- Eisler R. Biological report 85 (1.2) Washington, DC: U. S. Fish & Wildlife Service; 1985. Cadmium hazards to fish, wildlife and invertebrates: A synoptic review. [Google Scholar]
- Eisler R. Biological report 85 (1.10) Washington, DC: U. S. Fish & Wildlife Service; 1987. Mercury hazards to fish, wildlife and invertebrates: A synoptic review. [Google Scholar]
- Eisler R. Biological report 85 (1.4) Washington, DC: U. S. Fish & Wildlife Service; 1988. Lead hazards to fish, wildlife and invertebrates: A synoptic review. [Google Scholar]
- Eisler R. Handbook of chemical risk assessment: Health hazards to humans, plants and animals. Boca Raton, FL: CRC Press; 2000. Selenium; pp. 1649–1705. [Google Scholar]
- Elbin SB, Tsipoura NK, editors. Harbor herons conservation plan—NY/NJ harbor region. NY-NJ Harbor Estuary Program; New York, New York: 2010. [Google Scholar]
- Environmental protection agency (EPA) Freshwater criterion for fish. 2001 < http://www.epa.gov/fedrgstr/EPA-WATER/2001/January/Day-08/w217.htm>.
- Evers DC, Burgess NM, Champous L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology. 2005;14:191–221. doi: 10.1007/s10646-004-6269-7. [DOI] [PubMed] [Google Scholar]
- Fisk AT, deWit CA, Wayland M, Kuzyk ZZ, Burgess N, Letcher R, Braune B, Norstrom R, Blum SP, Sandau C, Lie E, Larsen HJS, Skaare JU, Muir DCG. An assessment of the toxicological significance of anthropogenic contaminants in Canadian arctic wildlife. Sci Total Environ. 2005;351–352:57–93. doi: 10.1016/j.scitotenv.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Fitzgerald WF, Lyons WB. Organic mercury compounds in coastal waters. Nature. 1973;242:452–453. doi: 10.1038/242452a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald WF, Engstrom DR, Lamborg CH, Tseng CM, Balcom PH, Hammerschmidt CR. Modern and historic atmospheric mercury fluxes in northern Alaska: Global sources and Arctic depletion. Environ Sci Technol. 2005;39:557–568. doi: 10.1021/es049128x. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Answers. 2005 http;//www.fda.gov/bbs/topics/ANSWERS/2005.
- Fowler SW. Critical review of selected heavy metal and chlorinated hydrocarbon concentrations in the marine environment. Mar Environ Res. 1990;29:1–64. [Google Scholar]
- Frederick PC, Spalding MG, Dusek R. Wading birds as bioindicators of mercury contamination in Florida, USA: Annual and geographic variation. Toxicol Chem. 2002;21:163–167. [PubMed] [Google Scholar]
- Frederick PC, Hylton B, Health JA, Spalding MG. A historical record of mercury contamination in southern Florida (USA) as inferred from avian feather tissue. Environ Toxicol Chem. 2004;23:1474–1478. doi: 10.1897/03-403. [DOI] [PubMed] [Google Scholar]
- Furness RW, Rainbow PS. Heavy metals in the marine environment. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- Gilbertson M, Eliot JE, Peakall DB. Seabirds as indicators of marine pollution. In: Diamond AW, Filion F, editors. The value of birds. Cambridge, UK: International Council for Bird Preservation; 1987. pp. 231–248. ICBP Tech. Publ. 6. [Google Scholar]
- Grasman KA, Echols KR, May TM, Peterman PH, Gale RW, Orazio CE. Immunological and reproductive health assessment in herring gulls and black-crowned night herons in the Hudson–Raritan estuary. Environ Toxicol Chem. 2013;32:548–501. doi: 10.1002/etc.2089. [DOI] [PubMed] [Google Scholar]
- Gray J. Biomagnification in marine systems: The perspective of an ecologist. Mar Pollut Bull. 2002;45:46–52. doi: 10.1016/s0025-326x(01)00323-x. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Safety. 2003;56:174–179. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Hahn H, Hahn K, Stoeppler M. Bird feathers as bioindicators in areas of the German Environmental Specimen Bank—Bioaccumulation of mercury in food chains and exogenous deposition of atmospheric pollution with lead and cadmium. Sci Total Environ. 1993;139:259–270. doi: 10.1016/0048-9697(93)90025-2. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch Environ Contam Toxicol. 2006;51:416–424. doi: 10.1007/s00244-005-0265-7. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Tseng CM. Biogeochemical cycling of methylmercury in lakes and tundra watersheds of Arctic Alaska. Environ Sci Technol. 2006;40:1204–1211. doi: 10.1021/es051322b. [DOI] [PubMed] [Google Scholar]
- Heinz GH. Selenium in birds. In: Beyer WM, Heinz WM, editors. Environmental contaminants in wildlife: Interpreting tissue concentrations. Boca Raton, FL: Lewis; 1996. pp. 447–458. [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR. A comparison of the teratogenicity of methylmercury and selenomethionine injected into bird eggs. Arch Environ Contam Toxicol. 2011;62:519–528. doi: 10.1007/s00244-011-9717-4. [DOI] [PubMed] [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA. Species differences in the sensitivity of avian embryos to methylmercury. Arch Environ Contam Toxicol. 2012;56:129–138. doi: 10.1007/s00244-008-9160-3. [DOI] [PubMed] [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Seafood safety. Washington, DC: National Academy Press; 1991. [Google Scholar]
- Institute of Medicine. Seafood choices: Balancing benefits and risks. Washington, DC: National Academy Press; 2006. [Google Scholar]
- Joint Expert Committee on Food Additives. Evaluation of certain contaminants in food. Seventy-second report of the joint FAO/WHO Expert Committee. 2011 http://whqlibdoc.who.int/trs/WHO_TRS_959_eng.pdf.
- Kim J, Koo TH. The use of feathers to monitor heavy metal contamination in herons, Korea. Arch Environ Contam Toxicol. 2007;53:435–441. doi: 10.1007/s00244-006-0196-y. [DOI] [PubMed] [Google Scholar]
- Lam JCW, Tanabe S, Lam MHW, Lamm PKS. Risk to breeding success of waterbirds by contaminants in Hong Kong: Evidence from trace elements in eggs. Environ Pollut. 2005;138:481–490. doi: 10.1016/j.envpol.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Lemly DA. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ Monit Assess. 1993;28:83–100. doi: 10.1007/BF00547213. [DOI] [PubMed] [Google Scholar]
- Mailman RB. Heavy metals. In: Perry JJ, editor. Introduction to environmental toxicology. New York, NY: Elsevier; 1980. pp. 34–43. [Google Scholar]
- Monteiro LR, Costa V, Furness RW, Santos RS. Mercury concentrations in prey fish indicate enhanced bioaccumulation in mesopelagic environments. Mar Ecol Prog Ser. 1996;141:21–25. [Google Scholar]
- National Research Council. Toxicological effects of methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Nichols J, Bradbury S, Swartout J. Derivation of wildlife values for mercury. J Toxicol Environ Health B. 1999;2:325–355. doi: 10.1080/109374099281160. [DOI] [PubMed] [Google Scholar]
- Nygard T, Lie E, Roy N, Steinnes E. Metal dynamics in an Antarctic food chain. Mar Pollut Bull. 2001;42:598–602. doi: 10.1016/s0025-326x(00)00206-x. [DOI] [PubMed] [Google Scholar]
- Ohlendorf HM, Hothem RL, Bunck CM, Aldrich TW, Moore JR. Relationship between selenium concentrations and avian reproduction. Trans 51st NA Wildlife Res Conf. 1986;51:330–342. [Google Scholar]
- Ohlendorf H, Hothem RL, Walsh D. Nest success, cause-specific nest failures and hatchability of aquatic birds at selenium contaminated Kesterson Reservoir and a reference site. Condor. 1989;91:787–796. [Google Scholar]
- Padula V, Burger J, Newman SH, Elsin S, Jeitner C. Metals in feathers of black-crowned night heron (Nycticorax nyctiucorax) chicks from the New York harbor estuary. Arch Environ Contam Toxicol. 2010;59:157–165. doi: 10.1007/s00244-009-9427-3. [DOI] [PubMed] [Google Scholar]
- Parsons KC. Regional patterns of wading bird productivity in northeastern U.S. estuaries. Waterbirds. 2001;24:323–330. [Google Scholar]
- Parsons KC. Chemical residues in cormorants from New York Harbor and control locations. Final Report to NYC DEC. 2003 http://passaic.sharepointspace.com/Public%20Documents/Chemical%20Residues%20in%20Cormorants%20from%20New%20York%20Harbor%20and%20Control%20Location.pdf.
- Ralston VC, Raymond LJ. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. J Ind Med. 1992;49:25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanpera C, Morere M, Ruiz X, Jover L. Variability of mercury and selenium levels in clutches of Auduoin’s gull (Larus audouinii) breeding at the Chafarinas Islands, southwest Mediterranean. Arch Environ Contam Toxicol. 2000;30:119–123. doi: 10.1007/s002440010087. [DOI] [PubMed] [Google Scholar]
- Scheuhammer AM, Perrault JA, Bond DE. Mercury, methylmercury, and selenium concentrations in eggs of common loons (Gavia immer) from Canada. Environ Monitor Assess. 2001;72:79–94. doi: 10.1023/a:1011911805216. [DOI] [PubMed] [Google Scholar]
- Sell JL, Horani FG. Influence of selenium on toxicity and metabolism of methylmercury in chicks and qual. Nutr Rep Int. 1976;14:439–447. [Google Scholar]
- Spahn SA, Sherry TW. Cadmium and lead in exposure associated with reduced growth rates, poorer fledging success of little blue heron chicks (Ehretta caerulea) in South Louisiana wetlands. Arch Environ Contam Toxicol. 1999;37:377–384. doi: 10.1007/s002449900528. [DOI] [PubMed] [Google Scholar]
- Spalding MG, Frederick PC, McGill HC, Bouton SN, McDowell LR. Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J Wildl Dis. 2000a;36:411–411. doi: 10.7589/0090-3558-36.3.411. [DOI] [PubMed] [Google Scholar]
- Spalding MG, Frederick PC, McGill HC, Bouton SN, Richey LJ, Schumacher IM, Blackmore CGM, Harrison J. Histologic, neurologic, and immunologic effects of methylmercury in captive great egrets. J Wildl Dis. 2000b;36:423–435. doi: 10.7589/0090-3558-36.3.423. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis System. SAS users’ guide. Cary, NC: SAS Institute, Inc; 2005. [Google Scholar]
- Steinberg N, Suszkowski DJ, Clark L, Way J. A report to the NY/NJ Harbor Estuary Program. New York, NY: Hudson River Foundation; 2004. Health of the harbor. The first comprehensive look at the State of the NY/NJ Harbor estuary. [Google Scholar]
- Stewart FM, Phillips RA, Catry P, Furness RW. Influence of species, age and diet on mercury concentrations in Shetland seabirds. Mar Ecol Prog Ser. 1997;151:237–244. [Google Scholar]
- Thompson DR, Furness RW. Seabirds as biomonitors of mercury inputs to epipelagic and mesopelagic marine food chains. Sci Total Environ. 1998;213:299–305. [Google Scholar]
- Tsipoura N, Burger J, Newhouse M, Jeitner C, Gochfeld M, Mizrahi D. Lead, mercury, cadmium, chromium, and arsenic levels in eggs, feathers, and tissues of Canada geese of the New Jersey Meadowlands. Environ Res. 2011;111:775–784. doi: 10.1016/j.envres.2011.05.013. [DOI] [PubMed] [Google Scholar]
- U.S. Army Corps of Engineers and the Port Authority of New York and New Jersey. Draft Hudson–Raritan Estuary comprehensive restoration plan. 2009 http://www.nan.usace.army.mil/Portals/37/docs/harbor/Harbor%20Program%20Images/CRP%20vol1.pdf.
- Weseloh D, Pekarik C, De Solla SR. Spatial patterns and rankings of contaminant concentration in herring gull eggs from 15 sites in the Great lakes and connecting channels, 1998–2002. Enviro Monitor Assess. 2006;113:265–284. doi: 10.1007/s10661-005-9084-6. [DOI] [PubMed] [Google Scholar]
- Weseloh DVC, Moore DJ, Hebert CE, de Solla SR, Braune BM, McGoldrick D. Current concentrations and spatial and temporal trends in mercury in Great Lakes herring gull eggs, 1974–2009. Ecotoxicology. 2011;20:1644–1658. doi: 10.1007/s10646-011-0755-5. [DOI] [PubMed] [Google Scholar]
- Weseloh DVC, Rodrigue J, Blokpoel H, Ewins PJ. Contaminant concentrations in eggs of black terns (Chilidonias niger) from southern Ontario and southern Quebec, 1989–1996. Colonial Waterbirds. 1997;20:604–616. [Google Scholar]
- Wolfe MF, Norman D. Effects of waterborne mercury on terrestrial wildlife at Clear Lake. Evaluation and testing of a predictive model. Environ Toxicol Chem. 1998;17:214–227. [Google Scholar]
- Wolfe MF, Schwarzbach S, Sulaiman RA. Effects of mercury on wildlife: A comprehensive review. Environ Toxicol Chem. 1998;17:146–160. [Google Scholar]
- World Health Organization. International programme on chemical safety-methylmercury. Environ Health Crit. 1990;101:42–58. [Google Scholar]
- Wren CD, Harris S, Harttrup NA. Ecotoxicology of mercury and cadmium. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of exotoxicology. Boca Raton, FL: Lewis; 1995. pp. 392–423. [Google Scholar]