Abstract
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an antioxidant-activated transcription factor that recently emerged as a critical regulator of cellular defense against oxidative and inflammatory lesions. Resveratrol (Res) is a natural phytoalexin that exhibits multiple therapeutic potentials, including antioxidative and anti-inflammatory effects in animals. Paraquat (PQ) is the second most widely used herbicide worldwide, but it selectively accumulates in human lungs to cause oxidative injury and fibrosis with high mortality. Here, we analyzed the molecular mechanism of the fibrogenic response to PQ and its inhibition by Res and Nrf2. PQ dose-dependently caused toxicity in normal human bronchial epithelial cells (BEAS-2B), resulting in mitochondrial damage, oxidative stress, and cell death. Res at 10 µM markedly inhibited PQ toxicity. PQ at 10 µM stimulated production of inflammatory and profibrogenic factors (tumor necrosis factor α, interleukin 6, and transforming growth factor β1) and induced the transformation of normal human lung fibroblasts (WI38-VA13) to myofibroblasts; both effects were inhibited by Res. Res strongly activated the Nrf2 signaling pathway and induced antioxidant response elementdependent cytoprotective genes. On the other hand, knockout or knockdown of Nrf2 markedly increased PQ-induced cytotoxicity, cytokine production, and myofibroblast transformation and abolished protection by Res. The findings demonstrate that Res attenuates PQ-induced reactive oxygen species production, inflammation, and fibrotic reactions by activating Nrf2 signaling. The study reveals a new pathway for molecular intervention against pulmonary oxidative injury and fibrosis.
Introduction
Pulmonary fibrosis is an irreversible stage of a large heterogeneous group of chronic lung diseases with a 5-year mortality rate larger than 50% (Husain and Kuman, 2005). Fibrosis in the lungs can result from genetic, infectious, autoimmune, idiopathic, and cancer pathology or arise from exposure to environmental and occupational agents, such as fibers and particles, metals, pesticides, and anticancer drugs (Bus and Gibson, 1984; Castranova and Vallyathan, 2000; Husain and Kuman, 2005; Rom, 2007). The underlying mechanism for lung fibrosis is unclear for the most part. Several cellular responses are commonly observed during lung fibrosis, including injury to airway and alveolar epithelia, macrophage activation, and transformation of fibroblasts into myofibroblasts. Myofibroblasts synthesize collagen 1 and α smooth muscle actin (αSMA) to promote fibrosis and scar formation (Fichtner-Feigl et al., 2006; Wynn, 2008; Bonner, 2010). The molecular events governing these fibrogenic reactions remain poorly understood. Although research trials are ongoing, there is no evidence that any medication can significantly help lung fibrosis at the present time.
Paraquat (PQ) is a highly effective, fast-acting, and nonselective herbicide widely used in the world. Human exposure to PQ by either respiratory or systemic route leads to the accumulation of PQ in the lungs, resulting in pulmonary edema, bronchial and alveolar destruction, and ultimately fibrosis with high mortality, which is in part caused by the lack of a specific antidote (Bus and Gibson, 1984). Chronic exposure to PQ is associated with liver damage, kidney failure, and Parkinsonian lesions in addition to fibrosis (Ossowska et al., 2006; Tanner et al., 2011). Upon entering cells, PQ undergoes cyclic single-electron reduction/oxidation through its quaternary ammonium nitrogen atoms and bipyridyl ring, producing reactive oxygen species (ROS) and PQ radicals. Redox cycling is believed to play an important role in initiating lung damage and fibrosis by PQ. How the oxidative signals from PQ interact with the pathways that underlie lung fibrogenic response is poorly understood.
Resveratrol (Res) is a phytoalexin polyphenol produced naturally by several plants when under attack by bacterial and fungal pathogens. Res exhibits multiple health-promoting properties including antioxidative, anticancer, anti-inflammatory, antiaging, blood sugar-lowing, and beneficial cardiovascular effects (Jang et al., 1997; Duffy and Vita, 2003; Leonard et al., 2003; Su et al., 2006; Valenzano et al., 2006; Elmali et al., 2007). Res alleviated bleomycin-induced lung injury in rats that typically progresses to fibrosis otherwise (Sener et al., 2007). Although the mechanism of protection by Res against bleomycin lung toxicity remains unclear, its beneficial effects on the vascular endothelium and lung epithelium involved activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (Kode et al., 2008; Ungvari et al., 2010).
Nrf2 is a cap “n” collar basic leucine zipper transcription factor ubiquitously expressed in mammalian cells. Nrf2 controls the antioxidant response element (ARE)-mediated expression of cellular detoxification enzymes and antioxidant proteins in response to a wide range of oxidant/antioxidant/electrophilic stimuli to protect the body (Leung et al., 2003; Talalay et al., 2003; Kobayashi et al., 2004; Kensler et al., 2007; Ma, 2008, 2010). Nrf2 and its binding protein Keap1 are redox sensors. Modification of critical cysteine residues in Keap1 and Nrf2 by oxidants and electrophiles leads to suppression of the ubiquitination and proteasomal degradation of Nrf2 (Kobayashi et al., 2004; He et al., 2006; He and Ma, 2009, 2010; Itoh et al., 2010; Taguchi et al., 2011). Activated Nrf2 translocates into the nucleus, dimerizes with small Maf proteins, and binds to ARE to up-regulate the transcription of cytoprotective genes. Nrf2-deficient mice have a tendency to develop autoimmune lesions and sponge-form leukoencephanopathy (Ma et al., 2006; Hubbs et al., 2007) and have increased susceptibility to toxicant-induced diseases, such as benzo[α]pyrene-induced cancer (Ramos-Gomez et al., 2001), metal toxicity (He et al., 2007, 2008), premature ovarian failure by 4-vinylcyclohexene diepoxide (Hu et al., 2006), and diabetic cardiomyopathy induced by high glucose (He et al., 2009). It is noteworthy that Nrf2 null mice are vulnerable to bleomycin and hypoxia-induced airway inflammation and lung fibrosis (Cho et al., 2004).
The purpose of the study was 3-fold: 1) to elucidate the molecular mechanism by which PQ damages human lung cells and induces fibrogenic response; 2) to examine the effect of Res on PQ pulmonary toxicity; and 3) to analyze the role and mechanism of action of Nrf2 in these processes. We found that PQ induced mitochondrial damage that, together with PQ redox cycling, caused heightened oxidative stress, leading to lung epithelial cell death, production of profibrogenic cytokines and growth factors, and myofibroblast transformation. Res effectively prevented PQ toxicity and blocked the fibrogenic reactions. Finally, Nrf2 was found to be critical for defense against PQ toxicity in normal cells and the protective effects of Res. The findings revealed a molecular pathway for intervention against PQ lung fibrosis and, potentially, other fibrotic diseases.
Materials and Methods
Reagents and Cell Culture
Reagents and cells were purchased from commercial vendors: PQ (N,N′-dimethyl-4,4′-bipyridynium dichloride; Thermo Fisher Scientific, Waltham, MA); Res (3,5,4′-trihydroxy- trans-stilbene; Enzo Life Sciences, Inc., Farmingdale, NY); tetramethylrhodamine ethyl ester (TMRE) and lipopolysaccharide (LPS) (Sigma, St. Louis, MO); MitoTracker deep red 633 (MTDR) and cell culture media (Invitrogen, Carlsbad, CA); and BEAS-2B, WI38- VA13, and RAW264.7 cells (American Type Culture Collection, Manassas, VA). Mouse embryonic fibroblast (MEF) cells were derived from Nrf2 wild-type (WT) and knockout (KO) mice and immortalized as described elsewhere (Bi et al., 2004; He and Ma, 2009). MEFs were cultured in Dulbecco’s modified Eagle’s medium with 10% FBS and 5% CO2. BEAS-2B cells were cultured in basal medium Eagle’s medium with additives from Lonza Walkersville, Inc. (Walkersville, MD). WI38-VA13 cells were cultured in α-modified Eagle’s medium with 10% FBS. Raw264.7 cells were grown in Dulbecco’s modified Eagle’s medium with 10% FBS.
Cytotoxicity Assay
The CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI) was used to detect cell proliferation/cytotoxicity following the manufacturer’s instructions. Cells (1 × 105) were seeded in 96-well plates overnight. Absorbance at 490 nm (A490nm) was recorded, and means and S.D. were calculated from three samples for each treatment.
ROS Detection
Cells in an eight-well chamber slide were added with 5 µM dihydroethium (DHE; fluorophore for ROS; Invitrogen) 30 min before the end of treatment. Cells were fixed with 4% paraformaldehyde and mounted with a mounting solution containing DAPI that stains the nucleus. Images were taken by using a Zeiss LSM510 confocal microscope (Carl Zeiss Inc., Thornwood, NY) with a rhodamin- DAPI setting and fixed exposure time. Fluorescence intensity was quantified by using Optimus version 6.51 software (Media Cybernetics Inc., Bethesda, MD) and expressed in relative fluorescence units (RFU). Means and S.D. were calculated from five separate fields for each treatment.
Measurement of Mitochondrial Inner Membrane Potential
Cells cultured in an eight-well chamber slide were added with 50 nM TMRE and 1 µM MTDR 45 min before the end of treatment. Cells were fixed with 4% paraformaldehyde and mounted with a mounting solution containing DAPI for fluorescence microscopy. Quantification of fluorescence intensity was performed as described above for ROS detection. Alternatively, TMRE and MTDR fluorescence was measured by flow cytometry, and data were expressed in RFU.
Immunoblotting
Cells were lysed on ice with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors for 30 min. Cell lysate (40 µg) was immunoblotted with anti- Nrf2 or actin antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Protein bands were visualized by using enhanced chemiluminescence detection reagents from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK).
Detection of Cytokine
Cells were treated as shown in figure legends, and cell culture medium was collected. TNFα and IL-6 cytokines were detected by flow cytometry using mouse Flex TNFα and IL-6 beads (BD Biosciences, San Diego, CA) following the manufacturer’s instructions.
Immunofluorescent Staining
Cells in eight-well chamber slides were fixed with 4% paraformaldehyde, permeated with 0.5% Triton X-100, and stained with anti-αSMA antibody (Sigma) overnight at 4°C, followed by Alexa Fluor 594-conjugated second antibody (Invitrogen) for 1 h, avoiding light. Fluorescence images were taken and quantified as described above for ROS detection.
Apoptosis
BEAS-2B cells cultured in 96-well plates were added with 100 µl of the Caspase 3-Glo 3/7 reagent (Promega) and cultured at 37°C for 1h. Luminescence was measured in a plate-reading luminometer and expressed in relative light units (RLU).
Reporter Assay
RAW264.7 cells stably transfected with a TNFα promoter/luciferase reporter construct were as described previously (Ma et al., 2003). Cells were treated and lysed with a passive reporter lysis buffer (Promega). Cell lysate was centrifuged briefly to remove cell debris. Twenty microliters of supernatant was mixed with 100 µl of luciferase reagent (Promega), and luciferase activity was detected by using a TD 20/20 luminometer (Molecular Devices, Sunnyvale, CA). Luciferase activity was in RLU and normalized with protein concentrations.
Real-Time PCR
Cells were treated as shown in figure legends. Total RNA was isolated by using a QIAGEN RNA mini kit (QIAGEN, Valencia, CA). Five micrograms of total RNA was reverse-transcribed into cDNA with reverse transcriptase III (Invitrogen). TGFβ1 mRNA was amplified with gene-specific primers (primer sequences available on request) by using real-time PCR as described previously (He et al., 2009). Actin was used as an internal control.
siRNA Transfection
Cells were transfected with either the control siRNA (Santa Cruz Biotechnology, Inc) or Nrf2 siRNA specific for mouse Nrf2 (Invitrogen) as described previously (He et al., 2009). Knockdown of Nrf2 was confirmed by immunoblotting of Nrf2.
Northern Blotting
Total RNA of 3 µg each was fractionated in a 1.2% formaldehyde agarose gel, transferred to a supercharged nylon membrane, and blotted with the digoxigenin-labeled riboprobe prepared with digoxigenin-labeling reagents (Roche Applied Science, Indianapolis, IN). Plasmid constructs for riboprobes of Nqo1, Ho1, and actin were verified by sequencing. Northern signals were visualized by chemiluminescence using a digoxigenin RNA detection kit with CDP Star as a substrate (Roche Applied Science).
Results
Res Inhibits PQ-Induced Cell Death, ROS Production, and Mitochondrial Damage in Human Lung Cells
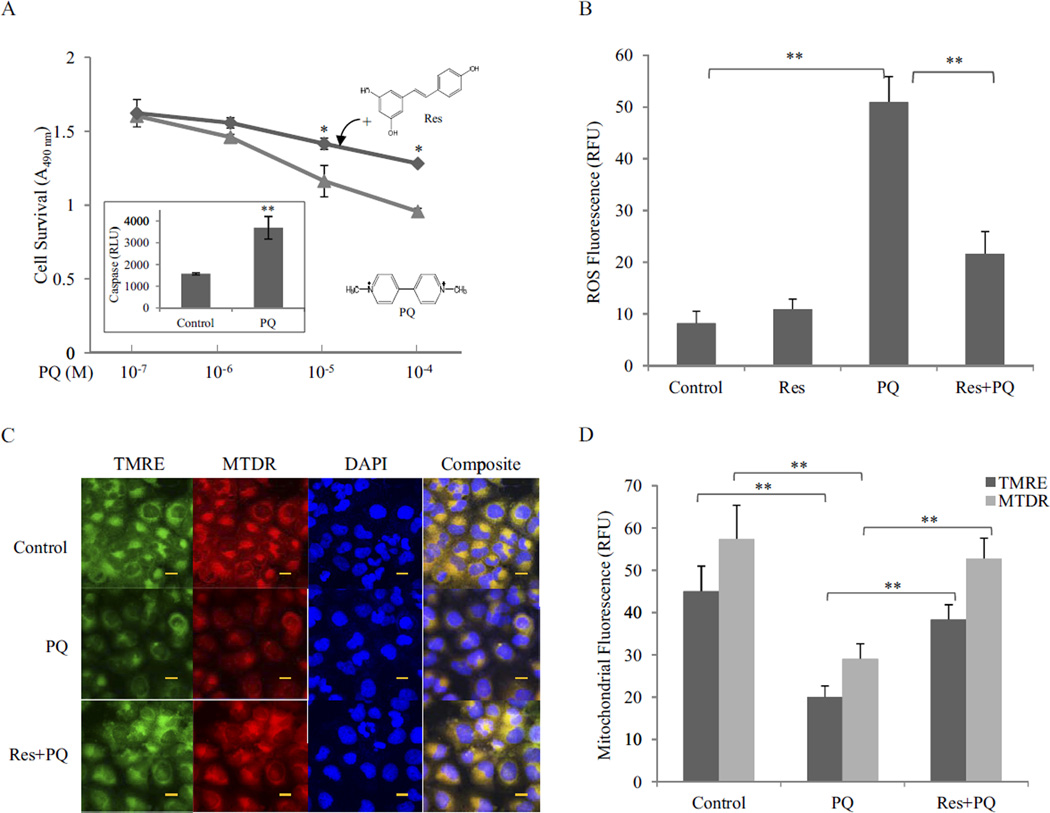
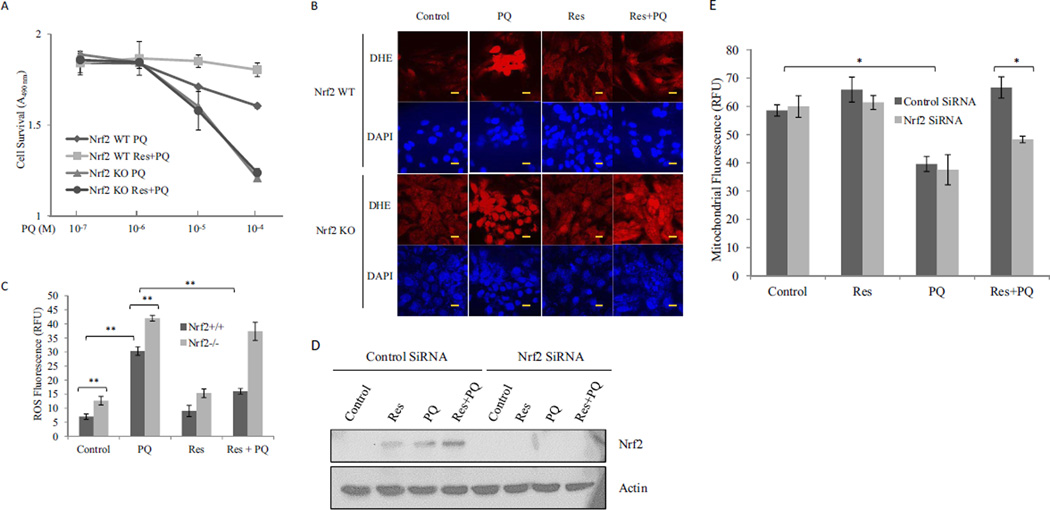
Exposure to PQ in the lungs typically causes extensive bronchial and alveolar destruction before fibrosis. BEAS-2B cells, a normal human bronchial epithelium cell line, were used to examine PQ cytotoxicity and the effect of Res. PQ at concentrations of 10−6 to 10−4 M induced cell toxicity dose-dependently (Fig. 1A). Toxicity was largely attributed to apoptosis as revealed by caspase 3/7 activation (Fig. 1A, inset). Res at 10 µM significantly reduced PQ toxicity at concentrations of 10−5 and 10−4 M.
Fig. 1.
Inhibition of PQ toxicity by Res. A, cytotoxicity. BEAS-2B cells were treated with PQ or PQ + Res (10 µM) for 24 h in triplicate. Cytotoxicity was detected by using CellTiter 96 AQueuos One Solution Reagent. Data represent means from three samples. Inset, apoptosis (caspase 3/7 activation) was measured in cells treated with PQ at 10 µM for 24 h. Caspase activity was expressed in RLU. B, ROS production. Cells were treated with Res (10 µM) for 16 h then with or without PQ (20 µM) for 24 h. ROS production was measured by using fluorophore DHE and expressed in RFU. Data represent means ± S.D. from three samples. C, mitochondrial damage. Cells were treated as in B. Mitochondrial damage was assayed by using fluorophores TMRE (green) and MTDR (red). DAPI (blue) was used to counterstain the nucleus. Composite images show the mitochondria in yellow (merge of green and red). Bar size, 20 nm. D, quantification of TMRE and MTDR fluorescence.*, p < 0.05; **, p < 0.01.
PQ undergoes redox cycling producing . and other radicals to initiate cell killing in plants and animals. Incubation of BEAS-2B cells with PQ (20 µM, 24 h) dramatically increased ROS production (5-fold) detected by DHE, a fluorophore specific for . (Fig. 1B). Res (Res + PQ; pretreated at 10 µM before PQ) significantly reduced the amount of PQ-induced ROS (~70%).
The mitochondria consume approximately 90% of the oxygen in cells for ATP synthesis and are a major source of ROS under both normal and pathological conditions. The observation that PQ substantially increased ROS production, whereas Res largely blocked PQ-induced ROS production suggests the involvement of mitochondria in PQ and Res effects. TMRE and MTDR are cationic lipophilic fluorophores that selectively accumulate in mitochondrial matrix in a mitochondrial inner membrane potential-dependent manner. Strong TMRE and MTDR florescence signals were found in the cytoplasm of control cells, indicating accumulation of the dyes in the mitochondrial matrix; however, the fluorescence was largely reduced and became diffusive upon treatment with PQ (20 µM; 24 h), indicating mitochondrial damage (Fig. 1, C and D). On the other hand, pretreatment with Res for 16 h followed by PQ restored the fluorescence intensity, indicating PQ-induced mitochondrial damage was largely prevented by Res.
Res Blocks PQ-Induced Fibroblast-to-Myofibroblast Transformation by Suppressing the Production of Inflammatory and Profibrogenic Factors
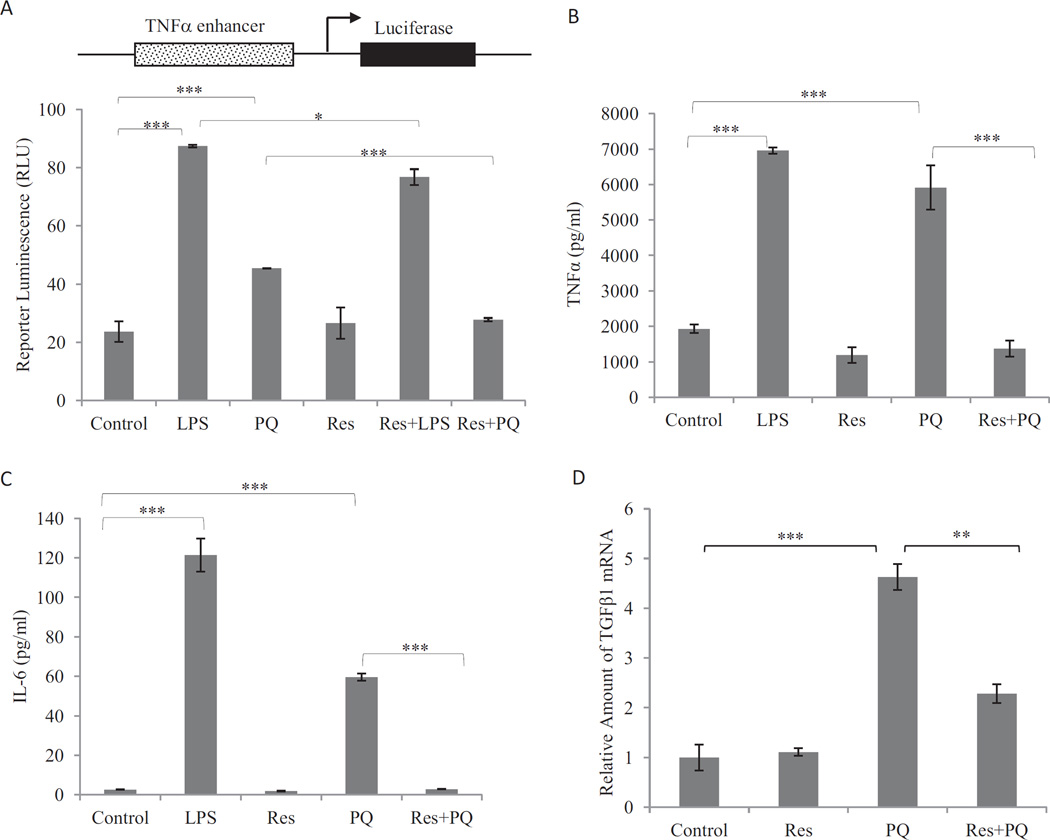
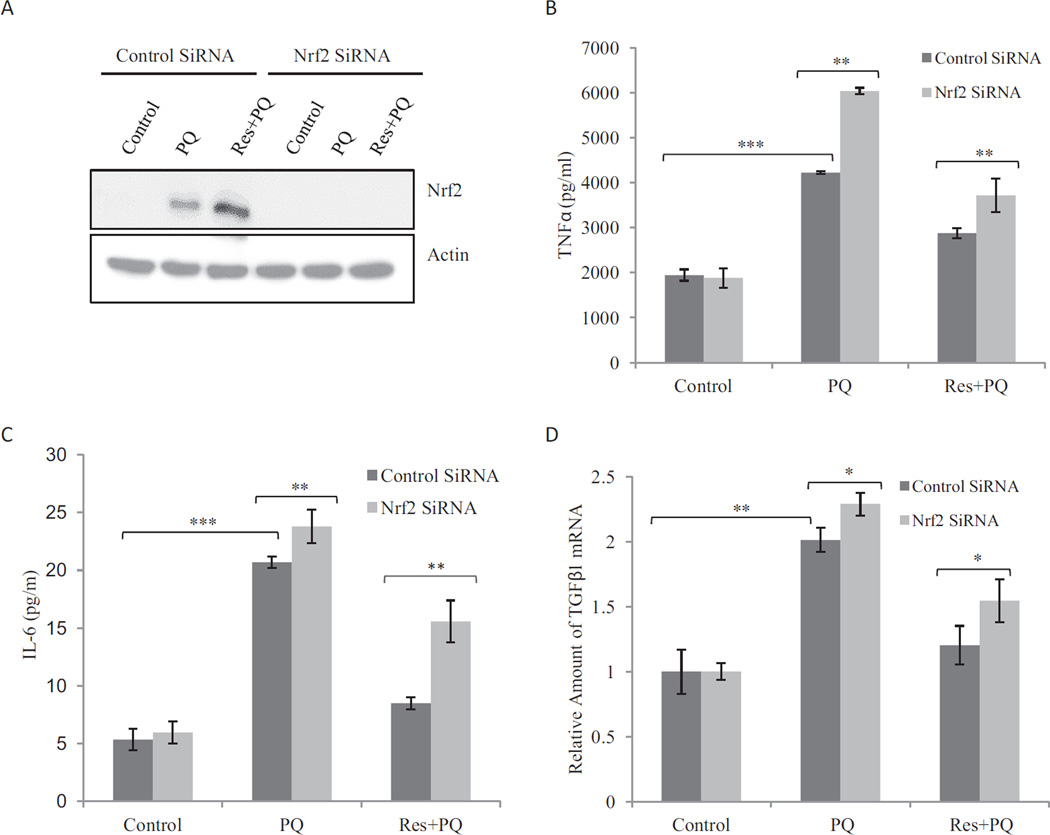
Fibrogenic agents induce the production and secretion of inflammatory and profibrogenic cytokines and growth factors from macrophages, epithelial cells, and fibroblasts. The soluble factors function as paracrines and autocrines to stimulate inflammation and fibrosis. To examine the effects of PQ and Res on the expression of profibrogenic factors, a luciferase reporter under the control of mouse TNFα promoter was examined for induction in RAW264.7 cells, a widely used macrophage cell line (Fig. 2A). PQ (10 µM; 16 h) induced reporter expression. Res itself (10 µM; 16 h) did not affect reporter expression, but cotreatment with Res blocked reporter induction by PQ. Res also weakly, but significantly, reduced reporter induction by LPS, a known inducer of TNFα. Expression of endogenous genes was examined. PQ (10 µM; 24 h) induced production of TNFα (Fig. 2B) and IL-6 (Fig. 2C) proteins in macrophages and TNFβ1 mRNA in fibroblasts (Fig. 2D). Pretreatment with Res at 10 µM for 16 h totally blocked induction of TNFα and IL-6 proteins and significantly diminished the induction of TNFβ1 mRNA by PQ.
Fig. 2.
Suppression of PQ-induced expression of TNFα, IL-6, and TNFβ1 by Res. A, TNFα promoter/luciferase reporter expression. Macrophages stably transfected with the TNFα promoter-luciferase reporter plasmid were treated with LPS (1 µg/ml; positive control), PQ (10 µM), Res (10 µM), LPS + Res, or PQ + Res for 16 h. Luciferase reporter expression was measured and expressed in RLU. B and C, secretion of TNFα (B) and IL-6 (C). Macrophages were pretreated with Res (10 µM) for 16 h followed by PQ (10 µM) for another 24 h (Res + PQ). LPS (1 µg/ml, 5 h) was a positive control for induction. The culture medium was assayed for TNFα (B) or IL-6 (C) proteins by flow cytometry using TNFα and IL-6 Flex beads. D, expression of TNFβ1 mRNA. WI38-VA13 cells were treated with PQ (10 µM) for 24 h or Res (10 µM) for 16 h followed by PQ for another 24 h (Res + PQ). Total RNA was assayed for TNFβ1 mRNA expression by using real-time PCR. Actin was used as a loading control. Data represent means ± S.D. from three samples.*, p < 0.05; **, p < 0.01; ***, p < 0.001.
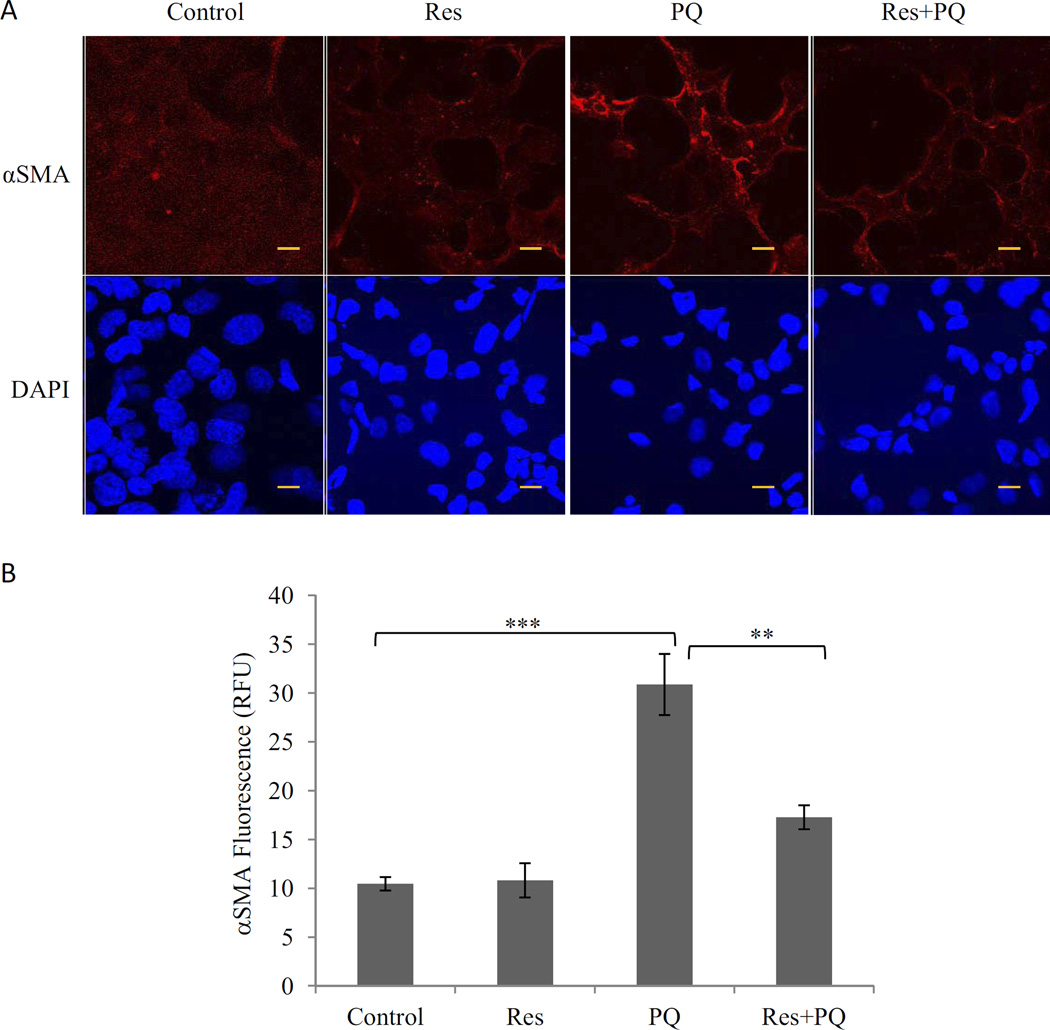
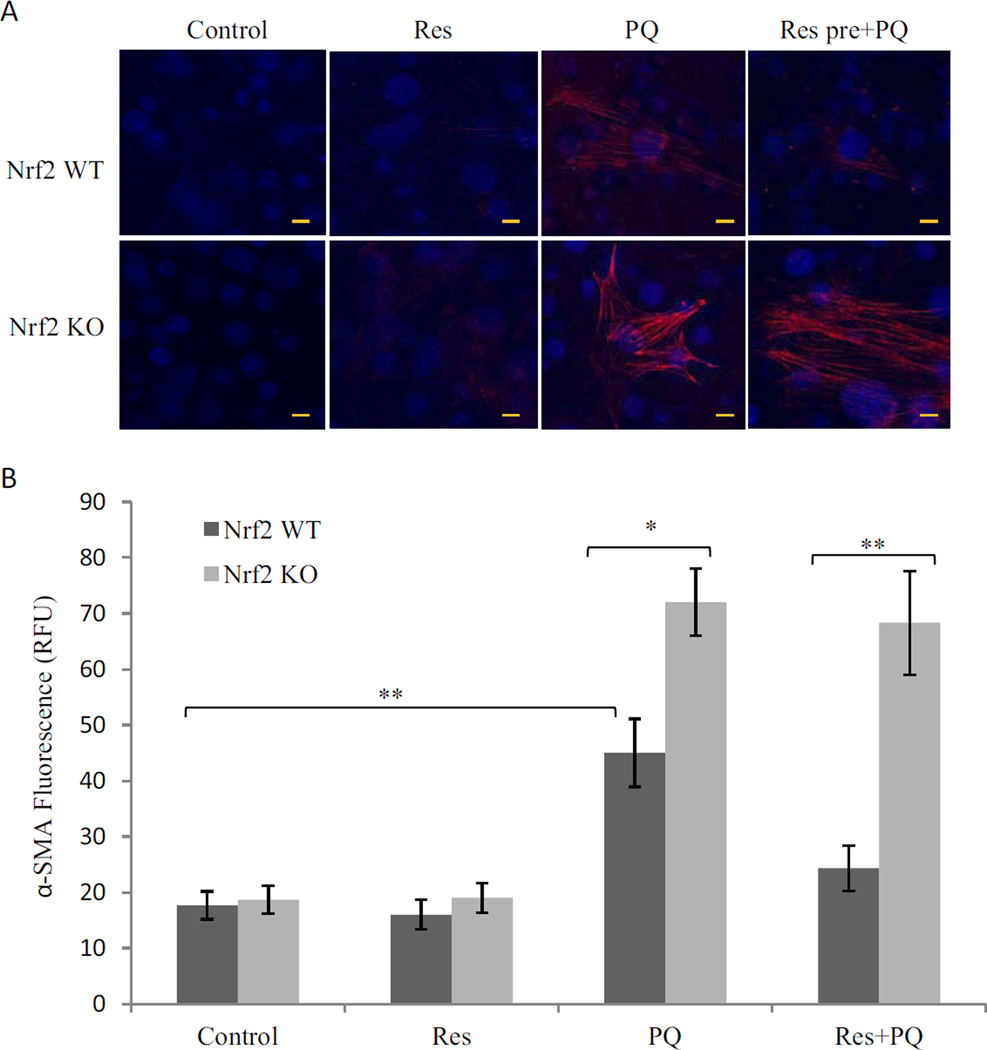
Transformation of fibroblast to myofibroblast is a critical cellular event in fibrosis because the latter synthesizes collagen I and αSMA, two major proteins involved in fibrosis and scar formation. WI38-VA13 cells, a normal human lung fibroblast cell line, were used to assess the effects of PQ and Res on myofibroblast transformation. PQ (10 µM; 24 h) induced a significant increase (>3-fold) in the amount of αSMA, indicating transformation of fibroblasts to myofibroblasts (Fig. 3). Res itself (10 µM) did not affect αSMA expression, but pretreatment with Res markedly reduced PQ-induced increase in αSMA. Thus, PQ stimulated myofibroblast transformation, whereas Res blocked the transformation.
Fig. 3.
Inhibition of PQ-induced myofibroblast transformation by Res. WI38-VA13 cells were treated with PQ (10 µM), Res (10 µM), or PQ + Res (pretreated for 16 h) for 24 h. Immunostaining was performed with an anti-αSMA antibody followed by Alex 549-conjugated second antibody (red). A, images were taking by confocal microscopy. The nucleus was counterstained with DAPI (blue). Bars, 20 nm. B, quantification of the images taken from five representative separate fields. **, p < 0.01; ***, p < 0.001.
Res Activates Nrf2 to Induce Cytoprotective Genes
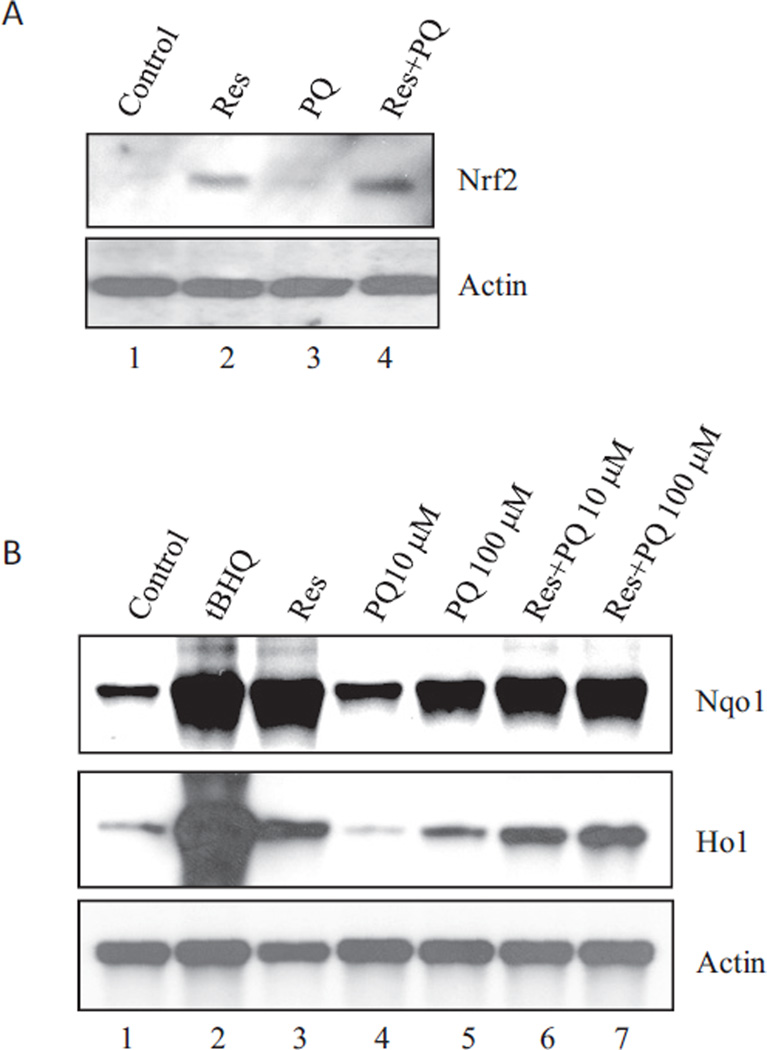
Nrf2 controls the cellular defense against oxidative and electrophilic chemicals and lesions. The profound oxidative effect by PQ and its inhibition by Res in lung cells raised the possibility that Nrf2 is involved in the actions of PQ and Res. As shown in Fig. 4A, Res (10 µM; 24 h) strongly and PQ (10 µM; 24 h) weakly increased the protein level of Nrf2 in BEAS-2B cells, indicating activation of Nrf2. Cotreatment with Res and PQ further increased Nrf2 protein, suggesting a synergy between Res and PQ for Nrf2 activation. Northern blotting was performed to analyze the induction of Nqo1 and Ho1, two representative target genes of Nrf2 (Fig. 4B). tBHQ (30 µM), a known inducer of Nqo1 and Ho1, strongly induced the genes. Res (10 µM) induced Nqo1 similarly to tBHQ and Ho1 to a less extent. PQ induced both genes at 100 but not 10 µM. Therefore, Res and, to a lesser extent, PQ activated Nrf2 signal transduction to induce cytoprotective genes.
Fig. 4.
Activation of Nrf2. A, Nrf2 protein stabilization. BEAS-2B cells were treated with Res (10 µM), PQ (10 µM), or PQ + Res for 24 h. Total cell lysates were immunoblotted with an anti-Nrf2 antibody. Actin was used as loading control. B, induction of Nqo1 and Ho1 mRNA. BEAS-2B cells were treated with tBHQ (30 µM), Res (10 µM), PQ (10 and 100 µM), Res + PQ (10 µM), or Res + PQ (100 µM) for 24 h. Total RNA was fractionated on 1.2% formaldehyde agarose gel. RNA bands were blotted with digoxigenin-labeled probes for Nqo1, Ho1, and actin and visualized with the CDP Star reagents and chemiluminescence.
Nrf2 Controls Defense against the Cytotoxicity, Oxidative Stress, and Mitochondrial Damage of PQ and Mediates Protection by Res
The role of Nrf2 in PQ toxicity and protection by Res was examined by comparing Nrf2 WT and KO MEFs. PQ at 10−5 and 10−4M caused significant toxicity in WT cells, and Res at 10 µM significantly reduced the toxicity (Fig. 5A) in agreement with the data for BEAS-2B cells (Fig. 1A). In KO cells, PQ induced significantly a higher level of toxicity than wild type and Res failed to rescue. The results indicate that Nrf2 is required for defense against PQ toxicity in normal cells and for protection by Res.
Fig. 5.
Nrf2-dependent inhibition of PQ toxicity. A, cell toxicity. Nrf2 WT and Nrf2 KO MEF cells were treated with PQ or PQ + Res (10 µM) for 24 h in triplicate. Cytotoxicity was measured and analyzed as described for Fig. 1. B, ROS production. Nrf2 WT and Nrf2 KO MEF cells were treated with PQ (10 µM), Res (10 µM), or PQ + Res for 24 h. ROS fluorescent probe DHE was added 30 min before the end of treatment to detect ROS. DAPI was used to counterstain the nucleus. Bars, 20 nm. C, quantification of fluorescence intensity of B. D and E, BEAS-2B cells were transfected with control siRNA or Nrf2 siRNA. The cells were then treated as described for B. D, knockdown of Nrf2 was examined by immunoblotting of Nrf2 with actin as the loading control. E, mitochondrial damage was assessed by using TMRE and flow cytometry. Data represent means ± S.D. from three samples. *, p < 0.05; **, p < 0.01.
In WT cells, PQ (10 µM) induced a dramatic increase of ROS production. Res (10 µM) alone did not significantly change the ROS level, but Res cotreatment blocked PQ-induced ROS production (Fig. 5, B and C). In KO cells, the basal ROS production was increased (compare control KO with control WT); PQ induced a high level of ROS; Res alone did not significantly change ROS level; and Res failed to block PQ-induced ROS production. The data revealed that Nrf2 is critically involved in the control of ROS production in cells exposed to PQ and is required for the suppression of PQ-induced oxidative stress by Res.
The effect of Nrf2 on PQ toxicity to BEAS-2B mitochondria was examined by using siRNA knockdown of Nrf2 (Fig. 5, D and E). Knockdown of Nrf2 significantly reduced the protein level of Nrf2 (Fig. 5D). Knockdown of Nrf2 or treatment with Res (10 µM) alone did not alter the mitochondrial inner membrane integrity, but PQ (10 µM) significantly reduced TMRE accumulation in mitochondrial matrix in both control and Nrf2 knockdown cells (Fig. 5E). Cotreatment with Res rescued the cells from PQ-induced mitochondrial damage in WT but not Nrf2 knockdown cells, indicating Nrf2 is required for Res protection against PQ toxicity to mitochondria.
Nrf2 Inhibits Induction of Profibrogenic Factors and Transformation of Myofibroblast by PQ
The effect of Nrf2 on the regulation of inflammatory and profibrogenic cytokine and growth factor expression by PQ and Res was examined in macrophages transfected with control or Nrf2 siRNA (Fig. 6). Nrf2 protein was significantly diminished in macrophages upon knockdown of Nrf2 (Fig. 6A). PQ (10 µM; 24 h) induced TNFα secretion from macrophages, and induction was significantly higher in Nrf2 knockdown cells, suggesting Nrf2 suppresses TNFα expression in macrophages (Fig. 6B). Res (pretreated for 16 h at 10 µM) blocked TNFα induction by PQ in control but not Nrf2 knockdown cells. Similar effects of Nrf2 knockdown were observed for the induction of IL-6 protein (Fig. 6C) and TNFβ1 mRNA (Fig. 6D) by PQ and inhibition by Res. Thus, Nrf2 inhibited PQ induction of the cytokines and growth factors in WT cells, and Nrf2 is required for Res to block induction by PQ.
Fig. 6.
Effect of Nrf2 on cytokine expression. RAW264.7 macrophages were transfected with control siRNA or Nrf2 siRNA and then treated with PQ (10 µM) or Res + PQ (pretreated with Res at 10 µM for 16 h followed by PQ for 24 h). A, knockdown of Nrf2 is shown by immunoblotting. B and C, TNFα (B) and IL-6 (C) proteins in the culture medium were detected by flow cytometry. D, induction of TNFβ1 mRNA was examined by real-time PCR. *, p < 0.05;**, p < 0.01; ***, p < 0.001.
PQ (10 µM; 24 h) induced transformation of Nrf2 WT fibroblasts into myofibrobasts as evidenced by marked increase in the synthesis of αSMA (Fig. 7). Res (10 µM) alone did not affect αSMA expression, but pretreatment with Res at 10 µM for 16 h markedly inhibited induction of αSMA by PQ. In Nrf2 KO fibroblasts, PQ induced significantly higher amounts of αSMA than in WT cells, and this increase was not affected by Res pretreatment. Therefore, Nrf2 inhibited PQ-induced myofibroblast transformation, and Nrf2 was required for suppression of PQ-induced fibrotic reaction by Res.
Fig. 7.
Nrf2-dependent suppression of myofibroblast transformation. A, Nrf2 WT and Nrf2 KO MEF cells were treated with PQ (10 µM), Res (10 µM), or Res + PQ (pretreated with Res at 10 µM for 16 h followed by PQ for 24 h). Fibroblast-to-myofibroblast transformation was assayed by immunostaining αSMA protein. Bars, 20 nm. B, quantification of fluorescence from five separate fields.*, p < 0.05; **, p < 0.01.
Discussion
The hallmark of PQ pulmonary toxicity is extensive tissue damage followed by fibrosis after either respiratory or systemic exposure (Bus and Gibson, 1984), which resembles the toxicity of a number of pulmonary toxicants, such as bleomycin and nitrofurantoin. In the case of PQ, the quaternary ammonium bipyridyl structure makes it prone to cyclic one-electron reduction/oxidation that causes production of ROS and depletion of reducing equivalents NADPH and GSH, both contributing to oxidative stress in cells. Thus, PQ serves as a valuable model compound for studying pulmonary toxicity and fibrosis, in particular, oxidative stress in lung fibrosis.
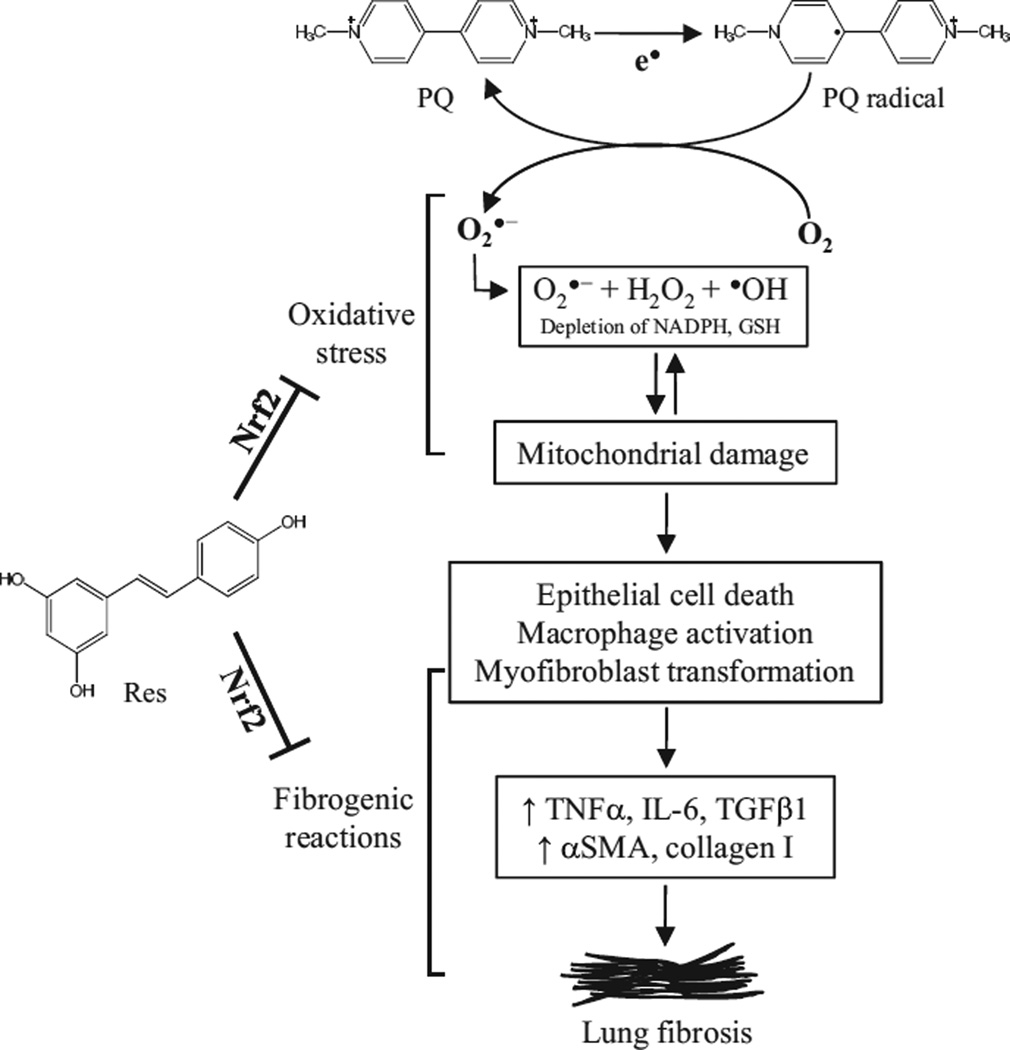
In this study, we analyzed the mechanism of the fibrogenic response to PQ and its inhibition by Res and Nrf2 in lung cells. Taken together, the findings illustrate a molecular model in which PQ induces oxidative stress and fibrotic reactions in lung cells, whereas Res suppresses PQ-induced oxidative stress and fibrogenic responses by activating the Nrf2 pathway (Fig. 8). PQ induces oxidative stress through both redox cycling and mitochondrial damage. Oxidative stress stimulates fibrogenic responses by promoting production and secretion of inflammatory and profibrogenic factors from macrophages and other lung cells, transformation of fibroblasts to myofibroblasts, and synthesis of αSMA and collagen 1. Res activates Nrf2 and induces cytoprotective genes to inhibit PQ-induced ROS production, mitochondrial damage, cytokine and growth factor expression, and myofibroblast transformation.
Fig. 8.
A working model for PQ-induced lung fibrosis and protection by Res through Nrf2. Within lung cells, PQ undergoes one-electron redox cycling, leading to the production of PQ radicals and ROS (, H2O2, and -OH), and depletion of reducing equivalents (NADPH, GSH), both damaging the mitochondria and further increasing ROS production to result in a vicious cycle of oxidative stress. Oxidative stress and epithelial damage stimulate macrophages as well as epithelial and fibroblast cells to produce inflammatory and profibrogenic cytokines and growth factors to induce myofibroblast transformation and, ultimately, fibrosis. Res inhibits both the oxidative damage and fibrogenic reactions by PQ. Nrf2 is required for both defense against PQ oxidative and fibrogenic toxicity in normal cells and protection by Res against PQ through upregulating cytoprotective gene expression.
The mitochondria are a major organelle for oxygen consumption and ROS production in cells. We found that mitochondria are a major target of PQ and damage to mitochondria contributes to oxidative stress induced by PQ. In this regard, PQ-induced mitochondrial damage and PQ redox cycling form a vicious cycle to cause heightened oxidative stress in lung cells. The observations that loss of Nrf2 in cells exacerbated PQ-induced ROS production and mitochondrial toxicity, whereas antioxidant Res blocked PQ oxidative stress and toxicity, support the notion that oxidative stress and mitochondrial damage are major mechanisms by which PQ causes lung damage and fibrosis.
Res is one of the promising natural products with multiple health-promoting effects (Vang et al., 2011). In this study, Res was shown, for the first time, to inhibit PQ-induced oxidative damage, expression of profibrogenic cytokines and growth factors, and transformation of fibroblasts into myofibroblasts effectively. Several lines of evidence support that Nrf2 mediates the protective effect of Res against PQ lung toxicity and fibrosis. First, Res elevated the Nrf2 protein level and induced Nrf2/ARE-controlled cytoprotective genes Nqo1 and Ho1 in lung cells. Second, PQ induced oxidative stress and fibrogenic reactions that were more severe in Nrf2 KO than WT cells. Third, Res could reverse or attenuate PQ-induced ROS production and fibrogenic responses in WT but not KO cells. Finally, a body of literature exists indicating a critical role of Nrf2 in Res functions. For instance, activation of Nrf2 is an important mechanism by which Res exerts its protective effects in the vascular endothelium (Ungvari et al., 2010). Res protects against cigarette smokemediated oxidative damage by activating Nrf2 in human lung epithelial cells (Kode et al., 2008), and both Nrf2 and Res protect against bleomycin-induced lung damage and fibrosis (Cho et al., 2004; Sener et al., 2007).
Cytokines and growth factors are involved in both the early inflammatory and late fibrotic phases of lung fibrosis through paracrine and autocrine mechanisms. TNFα is one of the early cytokines consistently found in animal models of pulmonary fibrosis and plays a cardinal role in the pathogenesis of this disease. TNFα activates nuclear factor-κB and activator protein 1 that regulate the proliferation of fibroblasts and expression of cellular matrix deposition genes, such as matrix metalloproteinase-9. IL-6 promotes fibrogenesis either alone or in concert with TNFα. IL-6 mRNA level is elevated in idiopathic pulmonary fibrosis (Smith et al., 1998). Overexpression of IL-6 in the lungs does not seem to be sufficient to induce marked fibrosis, but an abnormal IL-6 level has been consistently detected in animal and human lung fibrosis samples (Baecher-Allan and Barth, 1993; Moodley et al., 2003). We found that PQ significantly increased the expression of TNFα and IL-6 in macrophages, but pretreatment with Res prevented induction of TNFα and IL-6 by PQ.
TNFβ1 is a critical regulator of cell growth and differentiation as well as matrix protein production. Elevated production of TNFβ1 has been associated with human and rodent chronic inflammatory and fibrotic diseases. For instance, bleomycin-induced pulmonary fibrosis is accompanied by enhanced expression of TNFβ1, collagen 1, and αSMA (Zhang et al., 1996). TNFβ induces the proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through extracellular signal-regulated kinase/Akt inhibition and Pten restoration, whereas Res inhibits this process (Fagone et al., 2011). PQ increased TNFβ1 production, but pretreatment with Res for 16 h could prevent PQ-induced TNFβ1 expression.
The primary effecter cells in fibrosis are myofibroblasts, which are highly synthetic for collagen 1, have a contractile phenotype, and are characterized by de novo synthesis of αSMA stress fiber. Myofibroblasts may derive from activation/proliferation of resident lung fibroblasts, through epithelial-mesenchymal differentiation, or via recruitment of circulating fibroblastic stem cells. Resting fibroblasts express only β- and γ-actin isoforms (Herman, 1993; Khaitlina, 2001), whereas activated fibroblasts synthesize αSMA. Res could inhibit αSMA expression in hepatic stellate cells (Kawada et al., 1998). Olson et al. (2005) demonstrated an inhibitory role of Res in cardiac fibroblast proliferation and myofibroblast formation by reducing αSMA expression and stress fiber organization in cardiac fibroblasts. Res also alleviates bleomycin- induced pulmonary fibrosis (Sener et al., 2007). We found that PQ treatment for 24 h elevated αSMA expression in lung fibroblast WI38VA13 cells, but pretreatment with Res for 16 h inhibited PQ-induced αSMA expression, providing the first evidence that Res could effectively prevent PQinduced fibrogenic response in lung cells. These data provide a rationale for exploring the therapeutic and chemoprotective potentials of Res for the treatment and prevention of pathologic conditions involving pulmonary fibrosis.
ABBREVIATIONS
- αSMA
αsmooth muscle actin
- ARE
antioxidant response element
- DAPI
4,6-diamidino-2-phenylindole
- DHE
dihydroethium
- FBS
fetal bovine serum
- Ho1
heme oxygenase 1
- IL
interleukin
- KO
knockout
- LPS
lipopolysaccharide
- MEF
mouse embryonic fibroblast
- MTDR
MitoTracker deep red 633
- Nqo1
NAD(P)H:quinone oxidoreductase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PCR
polymerase chain reaction
- PQ
paraquat
- Res
resveratrol
- RFU
relative fluorescence unit
- RLU
relative light unit
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- tBHQ
tert-butylhydroquinone
- TGF
transforming growth factor
- TMRE
tetramethylrhodamine ethyl ester
- TNF
tumor necrosis factor
- WT
wild type
Footnotes
Authorship Contributions
Participated in research design: He, Wang, Szklarz, Bi, and Ma.
Conducted experiments: He, Wang, and Ma.
Contributed new reagents or analytic tools: Szklarz and Bi.
Performed data analysis: He, Wang, and Ma.
Wrote or contributed to the writing of the manuscript: He, Wang, and Ma.
References
- Baecher-Allan CM, Barth RK. PCR analysis of cytokine induction profiles associated with mouse strain variation in susceptibility to pulmonary fibrosis. Reg Immunol. 1993;5:207–217. [PubMed] [Google Scholar]
- Bi Y, Palmiter RD, Wood KM, Ma Q. Induction of metallothionein I by phenolic antioxidants requires metal-activated transcription factor 1 (MTF-1) and zinc. Biochem J. 2004;380:695–703. doi: 10.1042/BJ20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC. Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair. 2010;3:15. doi: 10.1186/1755-1536-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect. 2000;108(Suppl 4):675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Vita JA. Effects of phenolics on vascular endothelial function. Curr Opin Lipidol. 2003;14:21–27. doi: 10.1097/00041433-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. Effects of resveratrol in inflammatory arthritis. Inflammation. 2007;30:1–6. doi: 10.1007/s10753-006-9012-0. [DOI] [PubMed] [Google Scholar]
- Fagone E, Conte E, Gili E, Fruciano M, Pistorio MP, Lo Furno D, Giuffrida R, Crimi N, Vancheri C. Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Exp Lung Res. 2011;37:162–174. doi: 10.3109/01902148.2010.524722. [DOI] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 × Keap1 × Cul3 complex and recruiting Nrf2 × Maf to the antioxidant response element enhancer. J Biol Chem. 2006;281:23620–23631. doi: 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- He X, Lin GX, Chen MG, Zhang JX, Ma Q. Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol Sci. 2007;98:298–309. doi: 10.1093/toxsci/kfm081. [DOI] [PubMed] [Google Scholar]
- He X, Ma Q. NRF2 cysteine residues are critical for oxidant/electrophilesensing, Kelch-like ECH-associated protein-1-dependent ubiquitination-proteasomal degradation, and transcription activation. Mol Pharmacol. 2009;76:1265–1278. doi: 10.1124/mol.109.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Ma Q. Critical cysteine residues of Kelch-like ECH-associated protein 1 in arsenic sensing and suppression of nuclear factor erythroid 2-related factor 2. J Pharmacol Exp Ther. 2010;332:66–75. doi: 10.1124/jpet.109.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman IM. Actin isoforms. Curr Opin Cell Biol. 1993;5:48–55. doi: 10.1016/s0955-0674(05)80007-9. [DOI] [PubMed] [Google Scholar]
- Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol Cell Biol. 2006;26:940–954. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Benkovic SA, Miller DB, O’Callaghan JP, Battelli L, Schwegler-Berry D, Ma Q. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170:2068–2076. doi: 10.2353/ajpath.2007.060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain AN, Kuman V. The lung. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saunders; 2005. pp. 711–772. [Google Scholar]
- Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265–1274. doi: 10.1002/hep.510270512. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Khaitlina SY. Functional specificity of actin isoforms. Int Rev Cytol. 2001;202:35–98. doi: 10.1016/s0074-7696(01)02003-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- Ma Q. Xenobiotic-activated receptors: from transcription to drug metabolism to disease. Chem Res Toxicol. 2008;21:1651–1671. doi: 10.1021/tx800156s. [DOI] [PubMed] [Google Scholar]
- Ma Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther. 2010;125:376–393. doi: 10.1016/j.pharmthera.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kinneer K, Ye J, Chen BJ. Inhibition of nuclear factor κB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Mol Pharmacol. 2003;64:211–219. doi: 10.1124/mol.64.2.211. [DOI] [PubMed] [Google Scholar]
- Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, Mutsaers SE, Thompson PJ, Knight DA. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol. 2003;163:345–354. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005;288:H1131–H1138. doi: 10.1152/ajpheart.00763.2004. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Smiałowska M, Kuter K, Wierońska J, Zieba B, Wardas J, Nowak P, Dabrowska J, Bortel A, Biedka I, et al. Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: implications for Parkinson’s disease. Neuroscience. 2006;141:2155–2165. doi: 10.1016/j.neuroscience.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom WN. Asbestosis, pleural fibrosis, and lung cancer. In: Rom WN, Markowitz SB, editors. Environmental and Occupational Medicine. Lippincott Williams & Wilkins, Philadelphia: 2007. pp. 298–316. [Google Scholar]
- Sener G, Topaloğlu N, Sehirli AO, Ercan F, Gedik N. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm Pharmacol Ther. 2007;20:642–649. doi: 10.1016/j.pupt.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1α expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64:528–536. [PubMed] [Google Scholar]
- Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast α-smooth muscle actin expression and contractile phenotype in bleomycin- induced pulmonary fibrosis. Am J Pathol. 1996;148:527–537. [PMC free article] [PubMed] [Google Scholar]