INTRODUCTION
Attrition from HIV treatment programs in sub-Saharan Africa, estimated to be approximately 20% at one year after antiretroviral therapy (ART) initiation [1–3], is an enormous challenge that threatens gains achieved as a result of the public health scale-up of HIV care and treatment [4–6]. Attrition, often referred to as “loss to follow-up” (LTFU), does not provide information about an individual's true treatment or vital status.
Patients classified as “lost to follow-up” may 1) have transferred their care to another facility, unbeknownst to the original treatment site (often referred to as “silent transfer” or “remains connected to care”) [7,8]; 2) be disengaged from care and no longer on treatment; or 3) be dead. Studies that have physically traced patients classified as LTFU have discovered high proportions of patients in each of these three circumstances, with deaths accounting for 20-60% of those who were successfully traced [9– 11]. High rates of death, or connection to care among LTFU patients could potentially lessen the benefits of implementing programs that aim to reduce disengagement as patients who remain connected to care or who have died will not garner any benefit from such programs. Tetention/re-engagement strategies that discriminate between the possible circumstances of silent transfer, death, and true disengagement from care [10,12,13], or that can substantially reduce the rate of disengagement [14–18], however, may have the potential to improve patient outcomes. Therefore, interventions that seek to reduce disengagement or identify, trace, and re-link those patients who have become disengaged from care in sub-Saharan Africa may provide favorable value compared to other interventions designed to optimize outcomes for HIV-infected patients.
Mindful of the importance of distinguishing between patients who maintain a connection to care, those who have died, and true losses to care, we sought to evaluate the impact of alternative retention-in-care strategies in East Africa. In addition, we sought to ascertain the cost-effectiveness of the best retention-in-care strategies and to compare them to those of alternative resource uses for HIV-infected persons, in particular, to expansion of ART access.
METHODS
Overview
We expanded and re-calibrated a previously developed stochastic micro-simulation HIV model in order to account for retention-in-care dynamics [19]. We used this simulation to analyze the effectiveness and value of implementing retention-in-care strategies within a hypothetical East African HIV care and treatment program. Model parameters were informed by data from East African sources where possible, (Table 1). We compared scale up of these interventions to earlier ART provision as a measure of the potential health benefits forgone (e.g., opportunity cost) that could be gained by applying resources to other simultaneously resource-constrained decisions.
Table 1.
Key input parameters to computer simulation
| Parameter | Estimate (range considered) | Reference(s) |
|---|---|---|
| Characteristics of simulated cohort | ||
| Mean CD4 count (SD) | 287 (242) | AMPATH data |
| Mean age (SD) | 39 (9) | AMPATH data |
| Mean ART adherence | 70% | Adjusted for calibration |
| Loss to care probabilities | ||
| Probability of disengagement from clinic (per month) [LTFU] | 0.4-2.4%* (0.5x – 2.0x) | [3] |
| Relative risk of disengagement from clinic if pre-ART | 2 | [46–49] |
| Probability disengagement from care if disengaged from clinic | 27% (16-56%) | [8,13,25,40] |
| Probability of identification of disengagement | 100% | Assumption |
| Relative risk of treatment failure given disengaged from care | 3.32 | [50–54] |
| Intervention characteristics | ||
| Risk reduction intervention--Relative risk reduction on probability of disengagement | 40% (10-90%) | [14–18,26,27] |
| Cost of risk reduction intervention per person/month | $10 ($1-$50) | Assumption |
| Outreach intervention - Probability of tracing | 56% | [7,8,55] |
| Outreach intervention- Probability of re-linkage following successful tracing | 60% | [12,56] |
| Costs, 2014 US$ | ||
| Cost of pre-ART HIV care/annually | $429 | [57] |
| Cost of care while on 1st line ART/annually | $740 | [57] |
| Cost of care while on 2nd line ART/annually | $1453 | [57] |
| Cost of mortality/inpatient hospitalization (per event) | $220 | [58] |
ART: anti-retroviral therapy; LTFU: Loss to follow up [patient can be in one of three mutually exclusive health states—a) Alive and in care elsewhere; b) Alive and disengaged from care; c) Death which has been unrecognized]
Range reflects dependence of probability of disengagement on time spent in care (i.e. higher rate of disengagement in the first 6-12 months of clinical follow-up/treatment).
HIV disease progression simulation
This model has been previously validated by demonstrating its ability to predict clinical data in several distinct clinical cohorts [19–21]. Each simulated patient is assigned a CD4 count and VL from a relevant probability distribution reflecting an East African population in care, and the model tracks an individual's CD4 count, viral load, treatment status (on/off ART), and ART regimen (two regimens assumed to be available) over time, as well as the likelihood of experiencing an incident symptomatic HIV/AIDS related clinical event (e.g. opportunistic infection) or dying of an HIV-related or HIV-unrelated event. ART initiation is assumed to occur at a CD4 count of ≤200 cells/mm3. Although this does not reflect the current WHO recommendation [22], it does reflect a common situation in under-resourced programs [23,24]. Results were aggregated from large numbers of patient simulations in order to obtain stable estimates of the outcomes.
Representation of LTFU, disengagement and re-engagement in simulation
Patients classified as LFTU have heterogeneous outcomes and may have a wide array of root causes of attrition. To account for this we added representation of the following clinical/treatment states to our computer simulation (Fig. 1):
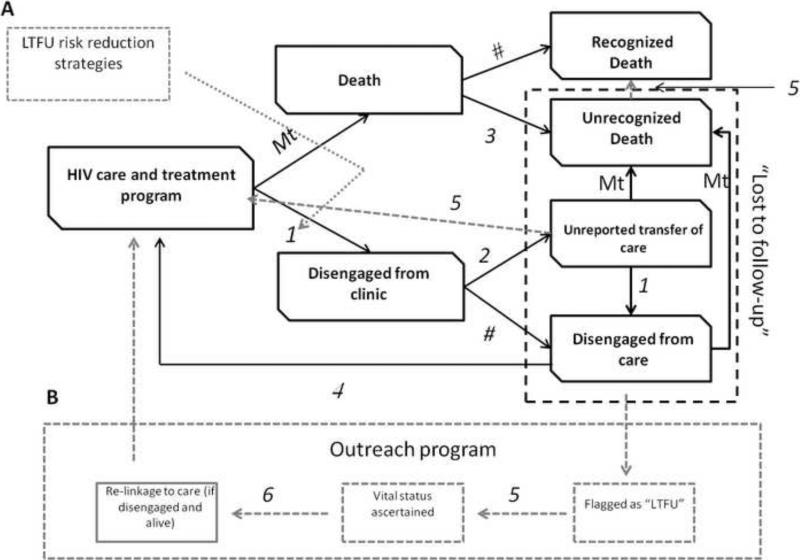
Figure 1.
Conceptual model of attrition from care and re-engagement in care and an intervention portfolio designed to mitigate drop-out from clinic and care within HIV treatment programs in sub-Saharan Africa. Cohort begins in HIV care and treatment state at start of simulation and can progress to a “disengaged from clinic” (lost to follow up) state either through death without appropriate surveillance (#3 in diagram) or through lost to clinic pathway (#1 in diagram). Patients considered “lost to follow-up” as classified by health facility reside in one of three mutually exclusive health states— Unrecognized death, unreported transfer of care, or disengaged from care. Patients who are transfers are assumed to continue on ART treatment. Patients disengaged from care are assumed to be no longer compliant with ART or OI prophylaxis. Disengaged patients will return to care (#4 in diagram) if they experience a new symptomatic AIDS event. Transition probabilities between states are represented by the following abbreviations—Mt: mortality, 1: probability of disengagement from clinic; 2: probability of unreported transfer given disengagement from clinic; 3: probability of death miss-classification; 4: return to care given disengaged from care; 5: probability of being successfully traced given outreach intervention component available; 6: probability of being successfully re-linked to care given successful tracing; #: complementary probability (e.g. 1-linked probability). Note: Not all state transitions are represented on figure for purposes of readability. For example, if patients are in “unrecognized transfer state” they are exposed to same risks of death or disengagement as if they were located in original care and treatment state) Grey dotted line boxes/arrows represent components (and their effects) of interventions designed to mitigate loss from HIV treatment programs. A- includes strategies which act to reduce the likelihood that patients disengage from clinic. B- includes outreach strategy which acts to trace, ascertain status of, and re-link those patients categorized as “disengaged from care” back to health system.
“Disengaged from clinic” [LTFU] – patient has not returned for follow-up care and treatment as requested and their vital status is uncertain. If a patient is “disengaged from clinic” they can be in one of three mutually exclusive health states.
“Death, unrecognized” – patient is deceased, however, surveillance system did not detect this outcome or death was not reported to health facility.
“Connected to care”– patient transferred care to another health facility or health provider and this was either not reported or incorrectly reported to initial treating facility; patient incorrectly categorized as lost. It is assumed that the patient is continuing to receive all relevant medications and care.
“Disengaged from care” (Care interruption) – patient is alive and no longer receiving care or treatment.
We simulate a cohort of HIV-infected persons initially in care. Patients can remain engaged in care or can become disengaged from clinic (LTFU) following one of two pathways. Firstly, an individual can suffer a mortality event which is not ascertained by the health facility where they receive care. As in earlier versions of this simulation mortality is closely associated with immune and virologic status (i.e. untreated patients with high VLs and lower CD4 counts have substantially higher mortality than patients on antiretroviral therapy who have suppressed VLs and immune recovery). Secondly, an individual can transfer their care to another health facility without this information being captured by the initial facility. Such “silent transfers” may be common in locales where robust health information systems such as electronic medical records or ART passports do not exist [8,25]. Finally, a patient may de-link or disengage from care and follow-up at the health facility but remain alive. Previous work has suggested that the LTFU rate is inversely associated with time spent in care, and this was adjusted for in the state transition probabilities governing disengagement from clinic [3]. Given the heterogeneity of LTFU rates reported in the literature, the outcomes associated with patients classified as such, and the incompletely understood relationship between these we chose to model the rates and probabilities of disengaging from clinic and the likelihood of de-linking from care independently.
Patients who remain engaged in care or who are LTFU but remain connected to care experience the same likelihood of future adverse outcomes (e.g. death, disengagement from care). Patients who disengage/de-link from care are assumed to be no longer compliant with ART and have similar probabilities of experiencing an adverse outcome as any other patient not on ART (controlling for immune and virologic status). We further assume that patients who are disengaged from care will re-link to care on their own if they experience a symptomatic AIDS-related event (more likely to occur once their CD4 count decreases below 200 cells/mm3). The methods underlying the re-calibration of this simulation are described in detail in the Supplementary Materials.
Representation of alternative retention-in-care strategies
We simulated alternative strategies exploring varying relative emphases: 1) a risk reduction intervention that seeks to reduce the chances of a patient from disengaging from clinic following enrolment; 2) an outreach intervention that seeks to find/ascertain/trace status of patients that are disengaged from clinic and re-link those that are disengaged from care. These component strategies were operationalized as follows.
1) Risk reduction
The risk reduction intervention attempts to reduce the likelihood that any patient will disengage from clinic after enrolment. Many interventions have been demonstrated to impact on the rate of “loss to follow-up,” and as such, wide uncertainty estimates characterize this intervention. Our initial estimate for its efficacy and cost are based upon a review of the literature including observational studies of interventions/strategies or cost-effectiveness analyses of interventions that impacted on disengagement rates [14–18,26,27]. We explored wide plausible intervals for estimates of intervention effectiveness (10%-90%) and cost ($1-$50 per patient per month) within our sensitivity analyses to represent the heterogeneous nature of these programs and the resources required for implementation.
2) Outreach
The outreach component consists of activities that are triggered when a patient becomes disengaged from clinic. Firstly, the patient is traced to determine their vital status. Secondly if tracing is successful and the patient is alive, attempts are made to re-link the individual to the treating health facility. Importantly, the outreach component has no impact on the rate at which loss from clinic or care is experienced [10,28–31].
Base case analyses
We compared the effects of alternative retention-in-care strategies on HIV-positive patients enrolled in care and treatment programs. We sought to identify the relative impact and cost effectiveness of the two interventions independently in comparison to a base case of either a) standard of care (null) scenario where no additional retention focused interventions were implemented or b) a scenario where ART is scaled up and available for all patients with a CD4≤ 350 cells/mm3. We used the model to predict several outcomes including life expectancy; quality adjusted life years (QALYs), as well as per-patient total costs for care and treatment. We estimated the health benefits as the difference between the life expectancy and or QALYs for simulations conducted under either base case assumption with those under the simulated implementation of a particular retention-in-care strategy. To estimate the cost-effectiveness we calculated incremental cost-effectiveness ratios (ICERs), or the difference in costs divided by the difference in effectiveness of the base case and intervention strategies. Costs are reported in 2014 US$ and both costs and QALYs are discounted at a rate of 3% annually. We evaluated the cost-effectiveness of the interventions considered from a payer perspective.
As a supplement to our opportunity-cost-based criteria, WHO guidelines were also used to define thresholds for cost-effectiveness. According to these guidelines an ICER of <1× the per capita GDP (~$1000 for Kenya was used in this analysis [32]) is considered to be “very cost-effective” while an ICER of <3× per capita GDP (~$3,000) is considered to be “cost-effective” [33]. Given the limitations with precision associated with any simulation, we assumed an ICER of >5× per capita GDP (~$5000) would almost certainly be considered unfavorable from a decision-making perspective, whereas an ICER between 3-5× GDP may be still be favorable accounting for these limitations.
Sensitivity analyses
We varied key parameters (see Table 1) in one – way sensitivity analysis across a plausible range of values to estimate which had the strongest effect on the cost-effectiveness including the rates associated with disengagement, the efficacy and costs of the intervention components, and the costs of ART and routine care. We then conducted multivariate sensitivity analyses using influential variables derived from the one-way sensitivity analysis in order to explore conditions under which intervention value may be enhanced or conditions under which intervention implementation would be a cost-effective addition to increasing ART access (i.e. expansion of earlier ART access to all HIV infected persons with CD4 count ≤ 350 cells/mm3).
RESULTS
Calibration of simulation
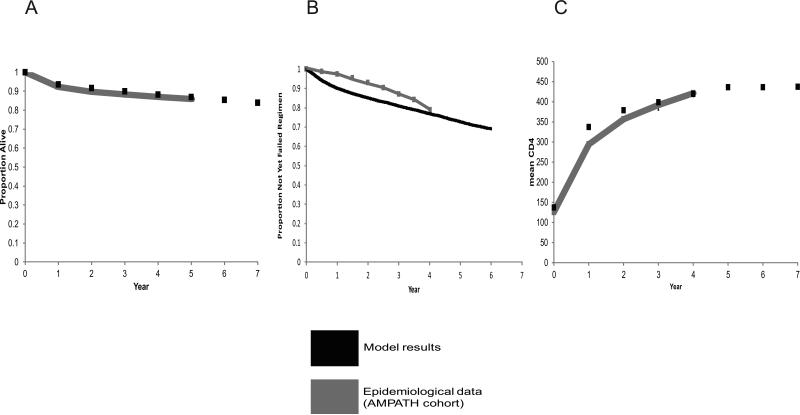
We pre-specified 3 calibration criteria in order to evaluate whether the model's predictions were compatible with observed results: mortality, time to antiretroviral treatment regimen failure, and mean change in CD4 (after ART initiation) (Fig. 2). We compared data from time trends over which data was available (2002-2010) for the criteria.
Figure 2A-C.
Re-calibration of survival (A), time to 1st ART regimen switch (B), and CD4 elevation. Simulation results are compared to clinical data from a large cohort in East Africa (Academic Model Providing Access to Healthcare [AMPATH]).
Effects on life expectancy of disengagement from care
The projected mean life expectancy for an HIV-infected adult who remains fully engaged in care is 16.0 years (9.5 QALYs). When the possibility of disengagement from clinic is considered, mean life expectancy decreases to 14.4 years (8.8 QALYs), a reduction of 1.6 years (0.7 QALYs).
Impact and cost effectiveness of retention-in-care focused interventions
The standard of care (null) scenario results in an average of 14.4 life years, 8.8 QALYs, with mean per-person discounted costs of $10,900. Implementing the risk-reduction strategy adds a mean of 1.2 life years or 0.6 QALYs at a mean incremental cost of $2200 to yield an ICER of $3700/QALY (compared to null scenario) (Table 2). Implementing an outreach intervention adds a mean of 0.1 life years, 0.1 QALYs at a mean incremental cost of $100 to yield an ICER of $1000/QALY. As implementation of this intervention had minimal health impacts and its inclusion in a combined retention package had no impact on cost-effectiveness results (data not shown) this component was not included in further multivariate sensitivity analysis. By comparison, ensuring complete expansion of ART treatment to all HIV infected persons with CD4 ≤ 350 cells/mm3 in line with previously adopted WHO guidelines (without any retention focused interventions) results in the addition of a mean of 3.8 life years, 2.9 QALYs at a mean incremental cost of $3900 to yield an ICER of $1300/QALY
Table 2.
Impact and cost effectiveness of an intervention aimed at reducing attrition (“loss to follow up”) within an East African HIV treatment program.
| Intervention(s) | Life Years | QALYs* | Costs* | ICER |
|---|---|---|---|---|
| No intervention | 14.4 | 8.8 | $10900 | --- |
| “LTFU” risk reduction | 15.6 | 9.4 | $13100 | ($3700)† |
| Full coverage of ART (CD4≤350 cells/mm3) | 18.2 | 10.7 | $14800 | $2000 |
| “LTFU” risk reduction +Full ART coverage | 19.6 | 11.3 | $17600 | $4700 |
LTFU: loss to follow up; ART: antiretroviral therapy; QALY: quality adjusted life year; ICER: incremental cost effectiveness ratio
discounted at a rate of 3%
intervention is “weakly dominated” by next most expensive intervention option (i.e. risk reduction intervention delivers health benefits for more $ per QALY [less efficiently] than full coverage of ART to all persons with CD4≤350 cells/mm3).
Cost-effectiveness of implementation of a retention in care focused intervention
We explored the impact of key parameters in the simulation on the health benefit, cost and value of retention in care intervention by varying their values across plausible ranges. When we varied parameters independently (i.e. one-way sensitivity analysis) the strongest influencers on the value of a retention focused intervention were, in order of importance: the cost of the retention in care intervention, the risk of a HIV patient becoming disengaged from clinic (LTFU), and the likelihood that a patient classified as LTFU was no longer connected to care (Supplementary Materials Figure S3). Importantly, there were no scenarios that we evaluated where the ICER for the retention in care intervention were favorable (i.e. less than) the ICER for earlier ART treatment (at a CD4 count of ≤350 cells/mm3). . If we assume, however, that all individuals who are LTFU are truly disengaged from care (rather than a proportion remaining connected to care) and that such patients do not return to care the cost effectiveness of the retention focused intervention approaches that of ART expansion (Supplementary Materials Figure S4).
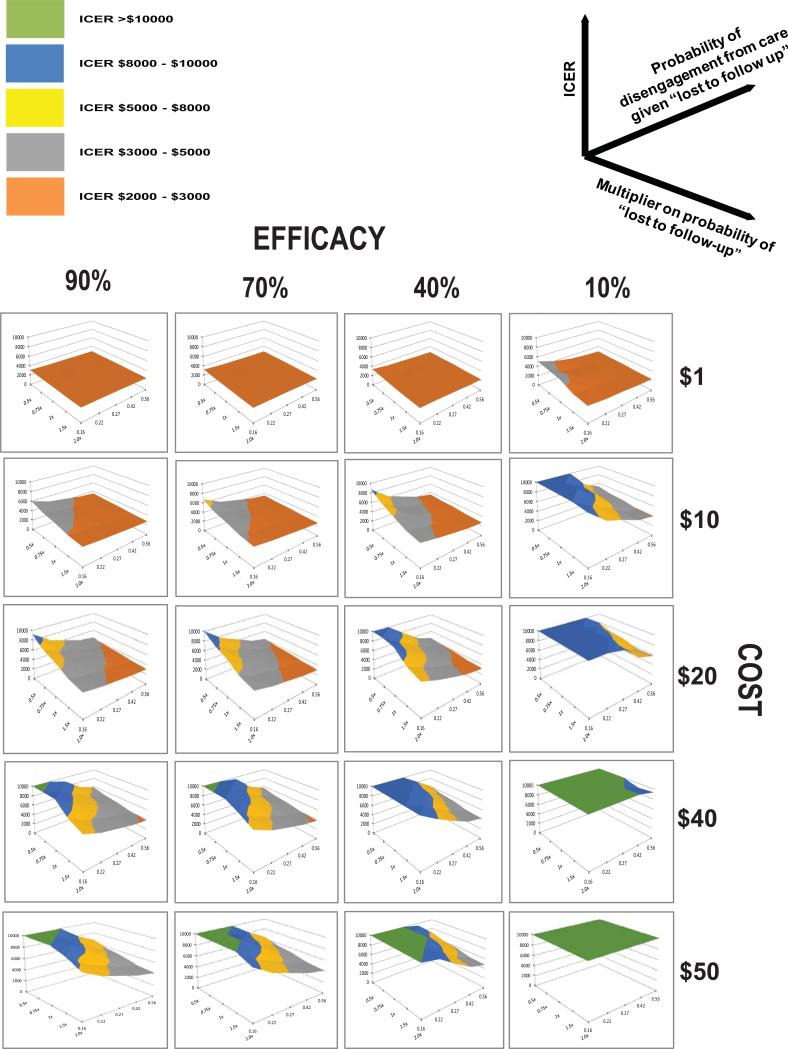
We evaluated in a multivariate sensitivity analysis scenarios that may result in cost – effective implementation of the retention in care intervention. When the monthly costs per patient were assumed to be $1 (or less) the cost-effectiveness ratio of the retention in care intervention was favorable (~$3000 or less) even if the intervention's efficacy was assumed to be only mild (10% reduction) as long as the likelihood of disengagement from clinic was greater than or equal to initial assumptions (Fig. 3). Even at an efficacy of 90%, and a high probability of disengagement from both clinic and care the cost effectiveness ratio of the retention focused intervention, however, was less favorable than earlier ART initiation (i.e. cost effectiveness ratio >$1300). When the monthly costs per patient were assumed to be $40 or more there were no scenarios where the cost-effectiveness ratio was less than $3000. When the monthly costs per patient were between $1-$40 the likelihood of the cost-effectiveness ratio remaining favorable was associated with an increasing probability of disengagement from clinic, an increasing probability of disengagement from care and an increasing efficacy of the intervention.
Figure 3.
Multivariate sensitivity analysis of critical factors influencing cost-effectiveness of a retention–in- care intervention. For each surface plot - X axis represents probability of no connection to care (i.e. disengaged from care) given patient is disengaged from clinic. Y axis represents multiplier on initial estimate of disengagement from clinic rates. Z axis represents incremental cost effectiveness ratio (ICER; $/QALY gained)) comparing intervention to null scenario (no retention intervention). Rows of plots represent simulations where values for effectiveness of risk reduction intervention are varied and include 10%, 40%, 70% and 90% risk reduction of disengagement from clinic. Columns of plots represent simulations where values for cost of intervention are varied and include $1, $10, $20, $40, $50 per person per month.
Value of retention in care package as next prioritized intervention
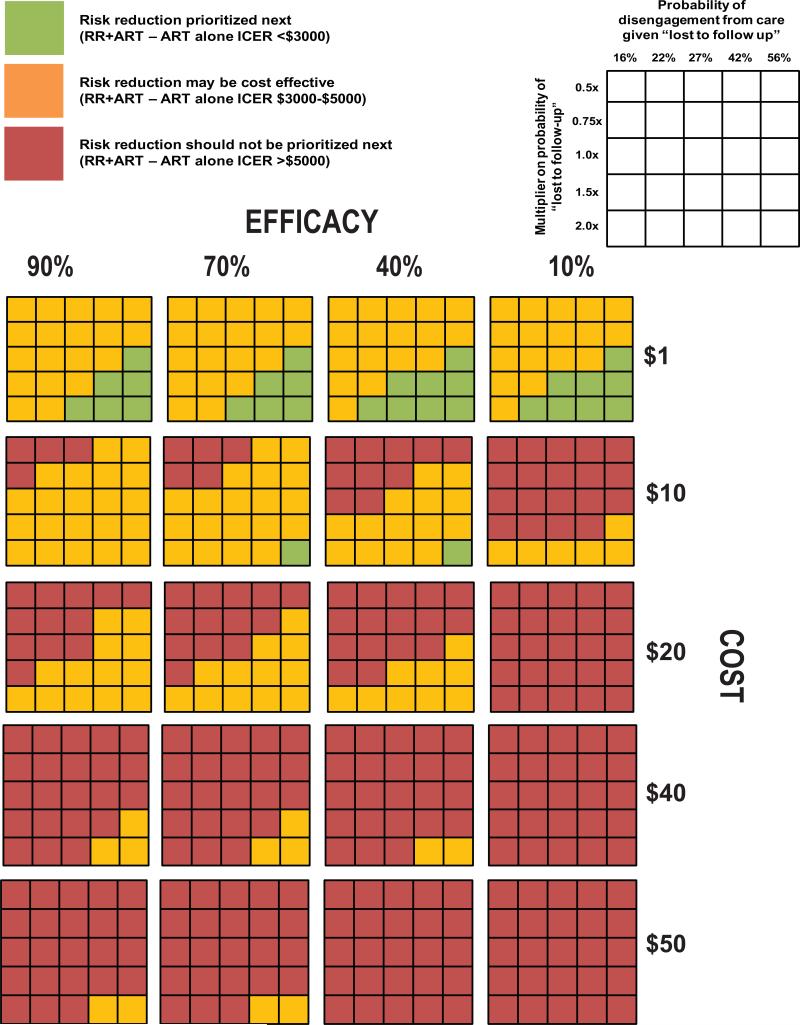
Given that under the model assumptions there was no scenario where a retention in care focused intervention was more favorable from a population health or economic perspective than earlier ART provision we explored under what conditions this intervention should be prioritized as the next best intervention (i.e. the addition of retention intervention to earlier ART retains an incremental cost effectiveness ratio that is considered favorable/cost-effective). When the retention in care intervention costs were assumed to be $1 (or less) the ICER of the retention in care intervention was favorable even if the intervention's efficacy was assumed to be only mild (10% reduction), as long as the likelihood of disengagement from clinic was at least 50 % greater than initial assumptions and the probability of disengagement from care was greater than 42% (Fig. 4). When the retention in care intervention monthly costs per patient were assumed to greater than or equal to $40 the ICER was almost always at least 5x the per capita GDP of Kenya (~$5000) and not likely to be favorable. When the monthly costs per patient were between $1-$40 the likelihood of retention in care intervention being a favorable next implementation option was associated with an increasing probability of disengagement from clinic, an increasing probability of disengagement from care and increasing efficacy of the intervention.
Figure 4.
Multivariate analysis of the estimated value of adding a retention–in- care intervention to earlier ART. For each 5×5 grid - rows represent varying assumptions on the probability of no connection to care (i.e. disengaged from care) given patient is disengaged from clinic; columns represent varying assumptions on the multiplier on initial estimate of disengagement from clinic rates. Rows of 5x5 grids represent simulations where values for effectiveness of risk reduction intervention are varied and include 10%, 40%, 70% and 90% relative risk reduction of disengagement from clinic. Columns of 5x5 grids represent simulations where values for cost of intervention are varied and include $1, $10, $20, $40, $50 per person per month. Each cell is color coded to represent whether the addition of the retention in care intervention to full coverage of an earlier ART policy (at a CD4 count of ≤ 350 cells/mm3) would have be favorable from a economic perspective. Cells shaded green represents an ICER of less than $3000 (<3x per capita GDP), cells shaded orange represent an ICER $3000-$5000 (3-5× per capita GDP), and cells shaded red represent an ICER >$5000 (>5× per capita GDP).
DISCUSSION
Using a computer simulation of HIV disease progression and retention-in-care dynamics in East Africa, we found that disengagement from care had a clinically relevant effect on life expectancy—an average loss of 1.6 years (0.7 QALYs). Additionally, an intervention designed to improve retention-in-care increased QALYs compared to no intervention. An outreach intervention (i.e. physical tracing and relinking) alone was associated with both minimal costs and minimal benefits under our initial assumptions. A risk reduction strategy had a more pronounced impact on clinical outcomes (greater increase in QALYs) when compared to outreach. This strategy, however, was a more expensive intervention.
We found that the factors with the strongest influence on the value of a retention-in-care intervention included risk of disengagement from clinic (LTFU), risk of loss of connection to care if disengaged, the effectiveness, and the cost of the intervention. Under conditions where cost is 1/10th of initial estimates and the risk reduction intervention at least as efficacious as initially assumed implementation of retention in care intervention would be a cost effective use of resources. Furthermore, if costs exceed $40 per month per person such an intervention is unlikely to achieve cost-effectiveness regardless of other parameter values.
Another key finding of our analysis is that where resources are limited investing in retention-in-care focused interventions may be less preferable when “benchmarked” to other potential HIV care and treatment options, including earlier ART initiation (at CD4 count ≤350 cells/mm3 instead of ≤200 cells/mm3). Our results demonstrate that the value (i.e. the health benefit for resource expended) of earlier ART treatment is greater than implementation of the retention in care strategies we considered (and no improvement in ART access). This is likely a function of our conservative assumptions regarding the proportion of persons classified as LTFU that are truly disengaged from care (i.e. only about 25%), the likelihood of these individuals returning to care (all will return if become symptomatic), and the assumption that the cost of the intervention is borne by all individuals whether or not they are or become lost. In scenarios where these assumptions were substantially relaxed the retention focused intervention is on par in terms of value with earlier ART treatment and in some cases (i.e. very low cost or very high efficacy) it may even provide greater health benefits on average (Supplementary Materials Figure S4). Similarly, relaxing these assumptions improves the overall value of a retention in care intervention whereby it is cost effective (ICER $3000) across a much broader array of intervention cost and efficacy values than under the initial assumptions of our analysis. Under our initial assumptions, however, scenarios may exist where implementation of retention in care package would be a valuable next strategy to implement following full implementation of earlier ART therapy for HIV infected persons seeking care.
In our multivariate sensitivity analysis if the cost of the intervention is less than $10 per person per month it may have favorable value under some conditions (i.e. cost-effective by compared WHO standards). Furthermore, if the cost could be brought below $1 it would be likely be considered cost effective and it would be a preferred next option following scale-up of earlier ART therapy if implemented in an environment where LTFU rates are high and where many of those classified as lost were actually disengaged from care. For instance, in settings where robust electronic medical records have been implemented, or where “ART passports” are commonly used, “silent transfers” are less common [34] and patients classified as LTFU are more likely to be either deceased or alive and disengaged from care. Strategies aimed at improving retention in this setting would provide greater health benefits and do so more efficiently than in a setting where such information and tracking systems do not exist because, all else being equal, fewer persons would experience the health benefits of the intervention (maintenance of viral suppression as a result of robust ART compliance) as a result of the increased likelihood of misclassification. Though our simulation was not designed to evaluate how such information systems and or other physical tracking systems might impact these decisions or how they might assist with prioritization of such interventions these are important questions for future study.
Our results are somewhat different from another published cost-effectiveness study that demonstrated that strategies for LTFU prevention could result in a substantial savings of life years and be considered “very cost-effective” under many scenarios [35]. If the effectiveness of that study's intervention (equivalent to the risk reduction strategy in our study) was at least 41% it would be considered “cost-effective” under the cost assumptions most similar to ours, whereas implementation of this intervention in our model results in an ICER > 3×GDPpc. The other study, however, did not account for the fact that significant proportions of persons categorized as “lost-to-follow-up” may remain connected to care and therefore dilute the health benefit observed for the resource invested in a retention program.
There is a growing body of evidence that provides insight into the underlying causes and factors contributing to the poor retention-in-care experiences of many HIV care and treatment programs in the developing world [31,36–41]. Patient out of pocket expenses for opportunistic infection prophylaxis or antiretroviral treatment and transportation costs/distance from a health center have been shown to be associated with disengagement in Africa and poorer clinical outcomes. In addition, interventions to alleviate these costs to patients have been demonstrated to improve adherence and retention-in-care [15,42,43]. Further research would be useful in assisting decision makers in tailoring and prioritizing strategies aimed at alleviating and ameliorating these barriers.
Limitations and strengths
Our study included several limitations. We did not include the downstream effects of preventing patients from disengaging from care or re-linking disengaged patients to treatment such as reduced HIV transmission. Including secondary infections may have made our results even more favorable to an RIC portfolio of interventions, since maintaining a low viral load greatly reduces the risk of transmission [44,45]. We modeled only two sequential available ART regimens, however, it is difficult to ascertain the magnitude and direction of bias this assumption has on our results. Whenever possible, we derived model inputs from East Africa data, but when local data estimates were unavailable, we used data from elsewhere in sub-Saharan Africa. Finally, we only compared the clinical effectiveness and cost-effectiveness of two broad HIV interventions (expansion of ART access and mitigation of disengagement from care), whereas broader portfolios of interventions may be under consideration. A notable strength of our analysis, however, is that it resolves a shortcoming of previous work: a person categorized as loss-to-follow up may not actually be disengaged from the health care system (i.e. may have died or may be receiving treatment elsewhere) and therefore may not derive any health benefits for the resources invested in the efforts to retain them. Not considering this important circumstance would likely result in over-estimation of the cost-effectiveness of retention in care focused interventions.
Conclusions
In summary, our results suggest that programs should scale up or optimize their retention programs if they have already achieved broad, early ART coverage among their HIV infected population. Under conditions where LFTU is common, disengagement from care is likely and retention programs can be scaled-up relatively inexpensively this may be a valuable next option on the implementation “menu” for policy and decision makers in this region.
ACKNOWLEDGMENTS
This study was supported by NIAAA 1R01AA017385-01 (Computer simulation of HIV epidemic in sub-Saharan Africa) and NIAID 1R01 AI099970-01 (Implementation science to optimize HIV prevention in East Africa).
The authors would like to thank Elizabeth Stevens for her assistance with preparation of the manuscript. This work has used computing resources at the High Performance Computing Facility of the Center for Health Informatics and Bioinformatics at the New York University Langone Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rosen S, Gill CJFMP. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen SFMP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011:8. doi: 10.1371/journal.pmed.1001056. page-range. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review. Trop Med Int Health. 2010;15(Suppl. 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurgens R, Cohen J, Tarantola D, Heywood M, Carr R. Universal voluntary HIV testing and immediate antiretroviral therapy. Lancet. 2009;373:1079. doi: 10.1016/S0140-6736(09)60644-9. author reply 1080–1. [DOI] [PubMed] [Google Scholar]
- 5.Sow PS, Bissagnene E, Kityo C, Bennink R, et al. Implementation of an antiretroviral access program for HIV-1 infected individuals in resource-limited settings: clinical results from 4 African countries. JAIDS. 2007;44:262–7. doi: 10.1097/QAI.0b013e31802bf109. [DOI] [PubMed] [Google Scholar]
- 6.Ivers LC, Doucette KKD. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 7.Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–7. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6:e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigel R, Hochgesang M, Brinkhof MW, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon JH, Elliott JH, Hong SY, et al. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One. 2013;8:e56047. doi: 10.1371/journal.pone.0056047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the “Back-to-Care” project in Lilongwe, Malawi. Trop Med Int Health. 2010;15(Suppl. 1):82–9. doi: 10.1111/j.1365-3156.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 13.Krebs DW, Chi BH, Mulenga Y, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–7. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 14.Lamb MR, El-Sadr WM, Geng E, Nash D. Association of adherence support and outreach services with total attrition, loss to follow-up, and death among ART patients in sub-Saharan Africa. PLoS One. 2012;7:e38443. doi: 10.1371/journal.pone.0038443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emenyonu N, Habyarimana J, et al. Cash transfers to cover clinic transportation costs improve adherence and retention in care in a HIV treatment program in Rural Uganda.. 17th Conf. Retroviruses Opportunistic Infect.; 2010. [Google Scholar]
- 16.Kohler PK, Chung MH, McGrath CJ, et al. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. AIDS. 2011;25:1657–61. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braitstein P, Siika A, Hogan J, et al. A clinician-nurse model to reduce early mortality and increase clinic retention among high-risk HIV-infected patients initiating combination antiretroviral treatment. J Int AIDS Soc. 2012;15:7. doi: 10.1186/1758-2652-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke MF, Kaigamba F, Socci AR, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56:1319–26. doi: 10.1093/cid/cis1193. [DOI] [PubMed] [Google Scholar]
- 19.Braithwaite RS, Nucifora K a, Yiannoutsos CT, et al. Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives? J Int AIDS Soc. 2011;14:38. doi: 10.1186/1758-2652-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braithwaite RS, Justice AC, Chang C-CH, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–8. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite RS, Roberts MS, Chang CCH, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model. Ann Intern Med. 2008;148:178–85. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organisation Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2010 [Google Scholar]
- 23.Organization WH. Global update on HIV treatment 2013: results, impact and opportunities. World Health Organization; Geneva: 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130630_treatment_report_en.pdf. [Accessed Month day year] [Google Scholar]
- 24.Kranzer K, Lawn SD, Johnson LF, et al. Community viral load and CD4 count distribution among people living with HIV in a South African Township: implications for treatment as prevention. J Acquir Immune Defic Syndr. 2013;63:498–505. doi: 10.1097/QAI.0b013e318293ae48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20:365–79. doi: 10.1111/tmi.12434. [DOI] [PubMed] [Google Scholar]
- 26.Fatti G, Meintjes G, Shea J, et al. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61:e50–8. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 27.Torpey KE, Kabaso ME, Mutale LN, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3:e2204. doi: 10.1371/journal.pone.0002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alamo ST, Wagner GJ, Sunday P, et al. Electronic medical records and same day patient tracing improves clinic efficiency and adherence to appointments in a community based HIV/AIDS care program, in Uganda. AIDS Behav. 2012;16:368–74. doi: 10.1007/s10461-011-9996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estill J, Tweya H, Egger M, et al. Tracing of patients lost to follow-up and HIV transmission: mathematical modeling study based on 2 large ART programs in Malawi. J Acquir Immune Defic Syndr. 2014;65:e179–86. doi: 10.1097/QAI.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhuna B, Taremba K, et al. Outcomes of patient active tracing by lay community workers in a decentralized HIV care program of rural Malawi.. 5th IAS Conf. HIV Pathog. Treat. Prev.; 2009; 1 more name. [Google Scholar]
- 31.Tweya H, Feldacker C, Estill J, et al. Are they really lost? “true” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PLoS One. 2013;8:e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bank TW. Gross Domestic Product per capita. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. [Accessed Month day, year]
- 33.Edejer TT, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CLJBR, editors. Making choices in health: WHO guide to cost-effectiveness analysis. WHO; Geneva: 2003. [Google Scholar]
- 34.Frontieres MS. Providing antiretroviral therapy for mobile populations: lessons learned from a cross border ARV programme in Musina, South Africa. 2012 Available at: http://www.msfaccess.org/sites/default/files/MSF_assets/HIV_AIDS/Docs/AIDS_report_ARTformo bilepops_ENG_2012.pdf. [Accessed Month day, year]
- 35.Losina E, Toure H, Uhler LM, et al. Cost-effectiveness of preventing loss to follow-up in HIV treatment programs: a Cote d’Ivoire appraisal. PLoS Med. 2009;6:e1000173. doi: 10.1371/journal.pmed.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng EH, Glidden DV, Emenyonu N, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15(Suppl. 1):63–69. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng EH, Musinguzi N, Emenyonu N, Bwana MB, BDR Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. JAIDS. 2010;53:405–11. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7:234–44. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Health. 2010;15(Suppl. 1):48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weigel R, Hochgesang M, Brinkhof MW, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisson GP, Gross R, Lo Re V, et al. Out-of-pocket costs of HAART limit HIV treatment responses in Botswana’s private sector. AIDS. 2006;20:1333–6. doi: 10.1097/01.aids.0000232245.36039.2b. [DOI] [PubMed] [Google Scholar]
- 42.Harries AD, Zachariah R, Lawn SD, Rosen S. Strategies to improve patient retention on antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15(Suppl. 1):70–5. doi: 10.1111/j.1365-3156.2010.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zachariah R, Massaquoi M, Kocholla L, et al. Payment for antiretroviral drugs is associated with a higher rate of patients lost to follow up than those offered free-of-charge therapy in Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2008;102:288–93. doi: 10.1016/j.trstmh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Quinn TC, Sewankambo N, Serwadda MB, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. NEJM 200AD. 342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 45.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(Suppl 1):43–7. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15-24 years of age) enrolled in HIV care. AIDS. 2014;28:559–68. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Govindasamy D, Meghij J, Kebede Negussi E, et al. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings--a systematic review. J Int AIDS Soc. 2014;17:19032. doi: 10.7448/IAS.17.1.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geng EH, Bwana MB, Muyindike W, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63:e64–71. doi: 10.1097/QAI.0b013e31828af5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills EJ, Funk A, Kanters S, et al. Long-term health care interruptions among HIV-positive patients in Uganda. J Acquir Immune Defic Syndr. 2013;63:e23–7. doi: 10.1097/QAI.0b013e31828a3fb8. [DOI] [PubMed] [Google Scholar]
- 51.Luebbert J, Tweya H, Phiri S, et al. Virological failure and drug resistance in patients on antiretroviral therapy after treatment interruption in Lilongwe, Malawi. Clin Infect Dis. 2012;55:441–8. doi: 10.1093/cid/cis438. [DOI] [PubMed] [Google Scholar]
- 52.Mann M, Diero L, Kemboi E, et al. Antiretroviral treatment interruptions induced by the Kenyan postelection crisis are associated with virological failure. J Acquir Immune Defic Syndr. 2013;64:220–4. doi: 10.1097/QAI.0b013e31829ec485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pai NP, Tulsky JP, Lawrence J, et al. Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults. Cochrane Database Syst Rev. 2005:CD005482. doi: 10.1002/14651858.CD005482. [DOI] [PubMed] [Google Scholar]
- 54.Ncaca LN, Kranzer K, Orrell C. Treatment interruption and variation in tablet taking behaviour result in viral failure: a case-control study from Cape Town, South Africa. PLoS One. 2011;6:e23088. doi: 10.1371/journal.pone.0023088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rachlis B, Ochieng D, Geng E, et al. Evaluating outcomes of patients lost to follow-up in a large comprehensive care treatment program in western Kenya. J Acquir Immune Defic Syndr Published Online First. 2015 Jan;:16. doi: 10.1097/QAI.0000000000000492. doi:10.1097/QAI.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochieng D, Braitstein P, et al. O V. Patient tracking and retention in a resource-constrained setting: the AMPATH experience in western Kenya.. 4th IAS Conf. HIV Pathog. Treat Prev; 2007; 1 more author. volume: Abstract n. [Google Scholar]
- 57.2013 Report on Costs of Treatment in the President's Emergency Plan for AIDS Relief (PEPFAR) Available at: http://www.pepfar.gov/documents/organization/212059.pdf. [Aceesed Month day, year]
- 58.Guinness L, Arthur G, Bhatt SM, et al. Costs of hospital care for HIV-positive and HIV-negative patients at Kenyatta National Hospital, Nairobi, Kenya. AIDS. 2002;16:901–8. doi: 10.1097/00002030-200204120-00010. [DOI] [PubMed] [Google Scholar]