Abstract
Background
Compared with multiple daily injections (MDI), sensor-augmented pump (SAP) insulin therapy may reduce glycemic variability and oxidative stress in type 1 diabetes in a glycosylated hemoglobin (A1C)-independent manner.
Subjects and Methods
The STAR 3 study compared SAP with MDI therapy for 1 year. Week-long continuous glucose monitoring studies were conducted at baseline and 1 year for assessment of glycemic variability in both groups. Soluble CD40 ligand (CD40L), a biomarker of inflammation and thrombocyte function, was measured at baseline and 1 year. Subjects were classified according to treatment group and 1-year A1C levels (<6.5%, 6.5–6.9%, 7–7.9%, ≥8%). Glycemic parameters were compared between SAP and MDI subjects in each A1C cohort.
Results
At 1 year, sensor glucose values at A1C levels ≥6.5% were similar in the SAP and MDI groups. However, sensor glucose SD and coefficient of variation (CV) values were lower at A1C levels <8% among SAP than among MDI subjects; the overall between-group difference was significant for both SD (P<0.01) and CV (P=0.01). The overall mean amplitude of glycemic excursion was similar in MDI and SAP groups (P=0.23). CD40L levels fell over the course of the study in both groups, but the between-group difference was not significant (P=0.18). CD40L concentrations were unrelated to A1C, change in A1C from baseline, or glycemic variability.
Conclusions
At comparable A1C levels of <8%, SAP reduced glycemic variability as measured by SD and CV compared with MDI. SAP may provide beneficial reductions in the number and severity of glycemic excursions.
Introduction
Excessive glycemic variability, along with sustained hyperglycemia and episodic hypoglycemia, characterizes the dysglycemia in diabetes,1 and minimization of glycemic variability has been suggested as a goal in the management of diabetes.2 Quantification of glycemic variabilty depends on repeated glucose measurements, which can be obtained with continuous glucose monitoring (CGM) systems that are now widely available. The SD and coefficient of variation (CV) (the ratio of the SD to the mean) of CGM values are widely used to summarize glycemic variability, but other metrics, including the mean amplitude of glycemic excursion (MAGE), are sometimes used to emphasize particular aspects of subjects' time–glucose profiles. Because the magnitude of the glycemic excursions contributing to MAGE must exceed some predefined threshold (typically 1 SD), MAGE is insensitive to low-amplitude variability and also insensitive to the asymmetric risks associated with hypo- and hyperglycemia.3
Glycemic variability may contribute to the risk of complications of diabetes through induction of inflammation and oxidative stress. An important inflammatory pathway involves CD40 (a costimulatory protein found on the surface of antigen-presenting cells) and its ligand, CD40L (also known as CD154 and expressed by activated T cells). The soluble ligand has been proposed as a biomarker of inflammation, thrombocyte activation, and oxidative stress, and its level is elevated in several disease states, most notably cardiovascular disease.
Sensor-augmented pump (SAP) therapy is associated with rapid and sustained improvement in glycosylated hemoglobin (A1C) values. In the recent STAR 3 trial,4 subjects on multiple daily injections (MDI) therapy were randomized to receive either optimized MDI therapy or SAP therapy. At the end of the 1-year randomized study phase, the baseline A1C level (8.3% in the two study groups) had decreased to 8.1% in the MDI group compared with 7.5% in the SAP group (P<0.001). The optional 6-month continuation phase then allowed subjects in the SAP group to stay on SAP and allowed subjects in the MDI group to cross over to SAP therapy. Continuation phase results5 confirmed that the lower mean A1C value seen with SAP therapy was sustained for 18 months. Mean A1C values among MDI subjects who crossed over to SAP therapy for the continuation phase also fell significantly compared with the 1-year value. Data from the study phase of STAR 3 were analyzed to determine whether and to what extent SAP therapy is associated with reductions in glycemic variability and/or levels of CD40L in an A1C-independent manner.
Research Design and Methods
The design and conduct of STAR 3 are described separately.6 In brief, 495 subjects were randomized to either SAP (n=247) or MDI (n=248) therapy for 1 year. Subjects in the SAP arm used the Paradigm REAL-Time system (Medtronic MiniMed, Inc., Northridge, CA). Blinded CGM data were collected from all subjects at baseline using iPro recording devices and Sof-sensor glucose sensors (Medtronic). At the end of the study, blinded CGM data were again collected from MDI subjects for 1 week. Soluble CD40L was measured at baseline and 12 months by a colorimetric enzyme-liked immunosorbent assay (R&D Systems, Minneapolis, MN). Subjects were stratified into four groups according to 12-month A1C levels (<6.5%, 6.5–6.9%, 7.0–7.9%, and ≥8%). Glycemic parameters including mean sensor glucose (MSG), SD, CV, and MAGE were calculated and compared between SAP and MDI subjects in each A1C cohort.
Results
The number of subjects in each of the four A1C cohorts is shown in Table 1. Most of the subjects with A1C <6.5% were in the SAP group, and most of the subjects with A1C ≥8% were in the MDI group. Only three subjects in the MDI group (all adults) had A1C values <6.5%.
Table 1.
Subjects in Each Glycosylated Hemoglobin Cohort from Sensor-Augmented Pump and Multiple Daily Injection Treatment Arms
| 12-month A1C | SAP (n) | MDI (n) |
|---|---|---|
| <6.5% | 16 (14A, 2P) | 3 (3A, 0P) |
| 6.5–6.9% | 33 (28A, 5P) | 17 (13A, 4P) |
| 7.0–7.9% | 124 (88A, 36P) | 88 (69A, 18P) |
| ≥8.0% | 45 (21A, 24P) | 106 (61A, 45P) |
A, adult (19–70 years old); A1C, glycosylated hemoglobin; MDI, multiple daily injections; P, pediatric (7–18 years old); SAP, sensor-augmented pump.
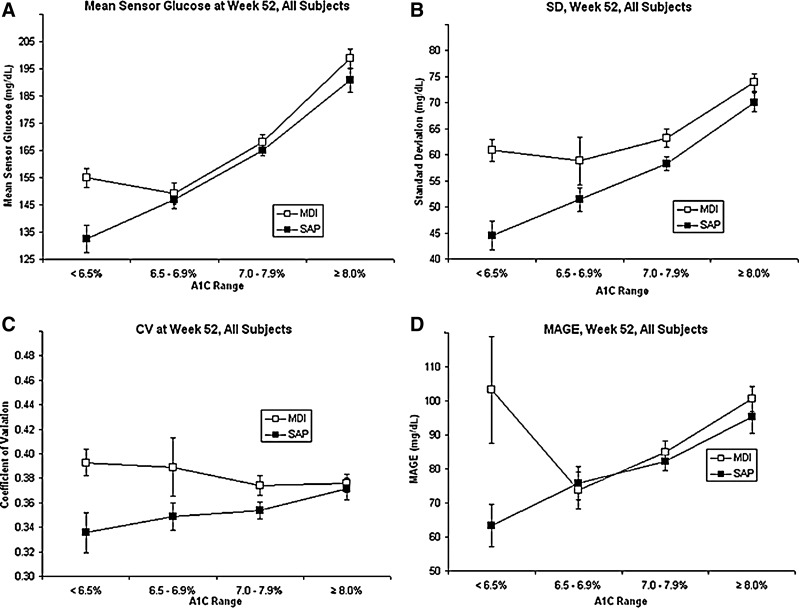
Figure 1 shows MSG values and three summary statistics of glycemic variability (CV, SD, and MAGE) in each of the four A1C cohorts. For subjects in the SAP treatment arm, MSG values increased across the four A1C cohorts, and at A1C levels ≥6.5%, MSG values were similar in the SAP and MDI groups (Fig. 1A). A treatment group difference was evident with respect to both SD (Fig. 1B) and CV (Fig. 1C) at A1C levels <8%, with lower variability seen in the SAP group. The overall between-groups difference was significant for both SD and CV (P<0.01 and P=0.01, respectively). As A1C increased to ≥8%, the differences between SD and CV in the SAP and MDI groups narrowed. The overall difference between MAGE values calculated for SAP and MDI groups was not significant (P=0.21) (Fig. 1D).
FIG. 1.
Sensor glucose and glycemic variability in the STAR 3 Study, by glycosylated hemoglobin (A1C) range: (A) mean sensor glucose; (B) SD of sensor glucose values; (C) coefficient of variation (CV) of sensor glucose values; and (D) mean amplitude of glycemic excursion (MAGE) of sensor glucose values. Data are mean±SE values. MDI, multiple daily injections; SAP, sensor-augmented pump.
Mean CD40L levels fell over the course of the study by 32.9 pg/mL in the SAP group and rose by 50.4 pg/mL in the MDI group; the between-group difference was not significant (P=0.18). CD40L levels were not correlated with body mass index, A1C, hypoglycemic exposure (measured by area under the curve for sensor glucose values <70 mg/dL), or SD.
Discussion
CGM data can be used to estimate the “glycemic triumvirate”1 of ambient glucose levels, the number and severity of glycemic excursions, and glycemic variability. There is particular interest surrounding glycemic variability, and several recent studies have confirmed its importance. In a recent study of 54 patients with type 2 diabetes initiating insulin pump therapy, glucose was monitored with patient-blinded CGM systems; reduced glycemic variability was associated with improved health utility (as measured by the EuroQol-5 Dimensions scale) and increased treatment preference (as measured by the Insulin Delivery System Rating Questionnaire).7 Elevated glycemic variabilty (MAGE ≥3.4 mmol/L) displayed significant value in predicting coronary artery disease in patients with type 2 diabetes, whereas A1C did not.8 In a third study of persons with type 2 diabetes using CGM devices, it was found that the risk of hypoglycemia was completely or virtually eliminated in the face of low glycemic variability (SD <1.7 mmol/L), irrespective of the mean glucose level or treatment modality.9 Minimization of glycemic excursion should be a criterion by which diabetes treatment strategies are evaluated, and wider use of real-time CGM in clinical practice may play a key role in minimization of glycemic variability and superoxide overproduction.10
Glycemic excursions are also common in type 1 diabetes, and young children may be especially vulnerable to extremely high and low glucose values because of their increased insulin sensitivity and variability in their activity levels and food intake. Average daily risk range scores have been proposed as a summary statistic of glycemic variability in children and appear to be more informative if calculated from CGM data rather than capillary blood glucose readings.11
Although A1C values correlate strongly with mean glucose values (detected by CGM or by frequent blood sampling), there is no comparably stable biomarker of glycemic variability. There appears to be a weak inverse relationship between osteocalcin and MAGE in type 2 diabetes,12 and the glucagon response to insulin-induced hypoglycemia in patients with type 1 diabetes may be impaired in times of high glucose variability (as measured by CV and continuous net overall glycemic action).13
The role of CD40–CD40L interactions in oxidative stress, inflammation, and vascular disease has been examined.14 Elevated CD40L levels have been noted in people with metabolic syndrome,15 and CD40L may play a role in autoimmune disease by induction of pro-inflammatory cytokines.16 CD40L is elevated in type 1 diabetic nephropathy17 and increases in type 1 diabetes after hypoglycemia,18 suggesting a mechanism for hypoglycemia-induced vascular injury. Acute hyperglycemia results in elevated urinary excretion of CD40L.19 Based on these and earlier reports, CD40L was considered as a potential biomarker for glycemic variability, and the STAR 3 study included it as an exploratory end point. However, CD40L does not appear to be related to A1C, to the change in A1C from baseline, or to glycemic variability. Because STAR 3 volunteers were excluded for cardiovascular disease or uncontrolled hypertension, baseline CD40L values may be representative of a relatively healthy population of subjects with type 1 diabetes. The exclusion of subjects with type 2 diabetes and the wide range of baseline CD40L values represent limitations of the study, and the design may have been inadequate to detect short-term fluctuations in CD40L levels as they relate to glycemic variability. Further studies of CD40L values in insulin-taking patients with comorbid conditions are warranted.
At comparable A1C levels of <8%, subjects on SAP had lower glycemic variability as measured by SD and CV than did subjects on MDI, which may help form the basis of improved glycemic control provided by the pump system. The similar MAGE values among the SAP and MDI treatment groups at A1C values of ≥6.5% may reflect the relative insensitivity of this metric to small fluctuations in glucose concentration. It should be noted that the three subjects in the MDI group who achieved A1C values of <6.5% had paradoxically higher MSG values and much higher MAGE values than the 17 MDI subjects in the 6.5–6.9% A1C cohort, which may reflect a tendency toward frequent, short, high-amplitude fluctuations in the former group. Based on these results, SAP may be recommended to improve A1C and reduce glycemic variability.
Acknowledgments
The authors thank Francine R. Kaufman, M.D., and Scott W. Lee, M.D., employees of Medtronic, for comments on an earlier draft of this manuscript. This study was funded by Medtronic, Inc. Paradigm, iPro, and Sof-sensor are trademarks of Medtronic MiniMed, Inc., Northridge, CA.
Author Disclosure Statement
J.B.B, Y.C.K., T.B., and S.N.D have received research support from Medtronic, Inc. J.S. and J.B.W. are employees of Medtronic, Inc.
References
- 1.Monnier L. Colette C. Owens D. The glycemic triumvirate and diabetic complications: is the whole greater than the sum of its component parts? Diabetes Res Clin Pract. 2012;95:303–311. doi: 10.1016/j.diabres.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch IB. Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–181. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Cameron FJ. Baghurst PA. Rodbard D. Assessing glycemic variation: why, when and how? Pediatr Endocrinol Rev. 2010;7(Suppl 3):S432–S444. [PubMed] [Google Scholar]
- 4.Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Peoples T. Perkins BA. Welsh JB. Willi SM. Wood MA. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 5.Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Perkins BA. Welsh JB. Willi SM. Wood MA. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011;34:2403–2405. doi: 10.2337/dc11-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis SN. Horton ES. Battelino T. Rubin RR. Schulman KA. Tamborlane WV. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther. 2010;12:249–255. doi: 10.1089/dia.2009.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyrot M. Rubin RR. Chen X. Frias JP. Associations between improved glucose control and patient-reported outcomes after initiation of insulin pump therapy in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:471–476. doi: 10.1089/dia.2010.0167. [DOI] [PubMed] [Google Scholar]
- 8.Su G. Mi S. Tao H. Li Z. Yang H. Zheng H. Zhou Y. Ma C. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monnier L. Wojtusciszyn A. Colette C. Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13:813–818. doi: 10.1089/dia.2011.0049. [DOI] [PubMed] [Google Scholar]
- 10.Brownlee M. Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 11.Patton SR. Midyett LK. Dolan LM. Powers SW. A comparison of average daily risk range scores for young children with type 1 diabetes mellitus using continuous glucose monitoring and self-monitoring data. Diabetes Technol Ther. 2012;14:239–243. doi: 10.1089/dia.2011.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao YQ. Zhou M. Zhou J. Lu W. Gao YC. Pan XP. Tang JL. Lu HJ. Jia WP. Relationship between serum osteocalcin and glycaemic variability in Type 2 diabetes. Clin Exp Pharmacol Physiol. 2011;38:50–54. doi: 10.1111/j.1440-1681.2010.05463.x. [DOI] [PubMed] [Google Scholar]
- 13.Alghothani N. Dungan KM. The effect of glycemic variability on counterregulatory hormone responses to hypoglycemia in young children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2011;13:1085–1089. doi: 10.1089/dia.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi M. Pathak D. Freedman JE. Chakrabarti S. CD40–CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med. 2008;14:530–538. doi: 10.1016/j.molmed.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Unek IT. Bayraktar F. Solmaz D. Ellidokuz H. Yuksel F. Sisman AR. Yesil S. Enhanced levels of soluble CD40 ligand and C-reactive protein in a total of 312 patients with metabolic syndrome. Metabolism. 2010;59:305–313. doi: 10.1016/j.metabol.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Peters AL. Stunz LL. Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lajer M. Tarnow I. Michelson AD. Jorsal A. Frelinger AL. Parving HH. Rossing P. Tarnow L. Soluble CD40 ligand is elevated in type 1 diabetic nephropathy but not predictive of mortality, cardiovascular events or kidney function. Platelets. 2010;21:525–532. doi: 10.3109/09537104.2010.500422. [DOI] [PubMed] [Google Scholar]
- 18.Wright RJ. Newby DE. Stirling D. Ludlam CA. Macdonald IA. Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010;33:1591–1597. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherney DZ. Scholey JW. Sochett E. Bradley TJ. Reich HN. The acute effect of clamped hyperglycemia on the urinary excretion of inflammatory cytokines/chemokines in uncomplicated type 1 diabetes: a pilot study. Diabetes Care. 2011;34:177–180. doi: 10.2337/dc10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]