Abstract
Previous studies report that cavum septum pellucidum (CSP) is frequent among athletes with a history of repeated traumatic brain injury (TBI), such as boxers. Few studies of CSP in athletes, however, have assessed detailed features of the septum pellucidum in a case-control fashion. This is important because prevalence of CSP in the general population varies widely (2% to 85%) between studies. Further, rates of CSP among American pro-football players have not been described previously. We sought to characterize MRI features of the septum pellucidum in a series of retired pro-football players with a history of repeated concussive/subconcussive head traumas compared with controls. We retrospectively assessed retired American pro-football players presenting to our memory clinic with cognitive/behavioral symptoms in whom structural MRI was available with slice thickness ≤2 mm (n=17). Each player was matched to a memory clinic control patient with no history of TBI. Scans were interpreted by raters blinded to clinical information and TBI/football history, who measured CSP grade (0–absent, 1–equivocal, 2–mild, 3–moderate, 4–severe) and length according to a standard protocol. Sixteen of 17 (94%) players had a CSP graded ≥2 compared with 3 of 17 (18%) controls. CSP was significantly higher grade (p<0.001) and longer in players than controls (mean length±standard deviation: 10.6 mm±5.4 vs. 1.1 mm±1.3, p<0.001). Among patients presenting to a memory clinic, long high-grade CSP was more frequent in retired pro-football players compared with patients without a history of TBI.
Key words: : concussion, magnetic resonance imaging, septum pellucidum, traumatic brain injury
Introduction
In autopsy studies, cavum septum pellucidum (CSP) is a common neuropathological feature of chronic traumatic encephalopathy (CTE), a tauopathy that has been identified in some patients exposed to repeated traumatic brain injury (TBI) or subconcussive head trauma.1 In one large autopsy series of CTE in which a neuropathological staging system is proposed, there is a trend toward increasing frequency of abnormalities of the septum pellucidum with increasing neuropathological stage of CTE such that 0% of stage 1 CTE brains had a CSP while more than 50% of stage 4 CTE brains had a CSP.1
In neuropathology and neuroimaging studies, CSP has been reported to occur at high rates among boxers and other contact-sport athletes.2–5 These studies are challenging to interpret in the context of reported rates of CSP among the normal adult population that range from 2% to 85%.6 Few previous studies of athletes have systematically described detailed features of the septum pellucidum in a matched case-control fashion.7 Further, rates of CSP in retired professional American football players have not been described previously.
In this small, retrospective, clinic-based case-control series, we characterized neuroimaging features of the septum pellucidum in a cohort of retired American pro-football players presenting with cognitive/behavioral symptoms compared with similar memory clinic patients without a history of TBI. We outline a simple scoring system using structural MRI that has the potential for application in prospective studies.
Methods
Subjects
We identified all retired American pro-football players presenting to our center between January 2010 and January 2015 for evaluation of cognitive/behavioral symptoms who had a 1.5T or 3T structural MRI with at least one three-dimensional (3D) T1 or T2 series with slice thickness of ≤2 mm (n=17) suitable for evaluating the CSP. Details of lifetime TBI history and football exposure were derived from chart review. All players reported at least 1 year of American pro-football exposure.
Controls were identified by systematically reviewing the charts of all patients evaluated at our center for cognitive/behavioral symptoms, starting with the most recent cases, until an age/sex-matched patient was identified who had no documented history of any TBI, head trauma, falls, motor vehicle accidents, military service, boxing, or American football, and who had an appropriate MRI for review (1.5T or 3T with 3D T1 or T2 series with ≤2 mm slice thickness). All MRIs were obtained to evaluate the etiology of cognitive/behavioral symptoms and/or to assist in the differential diagnosis of a neurodegenerative syndrome.
This study was approved by the University of California, San Francisco (UCSF) human research committee. Written informed consent was obtained from patients evaluated in the UCSF Memory and Aging Center clinic (n=25) and the UCSF Alzheimer's Disease Research Center (n=9) allowing chart review and/or research data review for the purposes of anonymized research studies.
Image acquisition, reformatting, and CSP rating
Nine patients (five players, four controls) underwent structural MRI through our research program on a Siemens 3-Tesla TrioTim MRI scanner with a 12-channel head coil using a volumetric magnetization prepared rapid gradient echo MRI sequence to obtain a T1-weighted image of the entire brain (repetition time, 2300 msec; echo time, 2.98 msec; inversion time, 900 msec; flip angle, 9) that was reconstructed at 1 mm3 spatial resolution. Twenty-five patients (12 players, 13 controls) had structural MRIs on a clinical basis using either a 1.5-Tesla (three players, one control) or 3-Tesla MRI scanner with either an 8- or 12-channel head coil using a protocol that included one 3D T1 or T2 FLAIR sequence acquired at less than 2 mm slice thickness. See the Supplementary Table 1 for full details of MRI parameters; see online supplementary material at ftp.liebertpub.com.
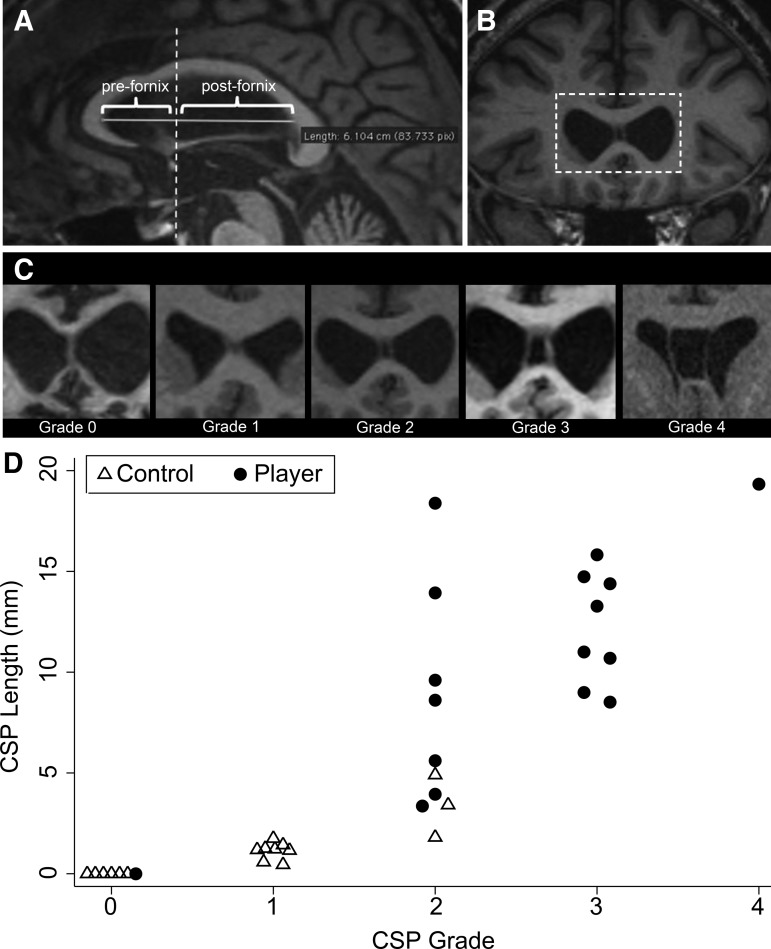
Images were de-identified and loaded into an OsiriX image viewer.8 In the midsagittal plane, the longest distance from genu to splenium of corpus callosum (termed “septal length”) was measured. The coronal plane was aligned and reformatted perpendicular to this septal-length axis to optimize measurements along the septum. A cyst within the pre-forniceal septum was termed “CSP”; a cyst within the post-forniceal septum, “cavum vergae” (CV). To grade the CSP, the pre-forniceal coronal slice with greatest evidence of CSP was identified and graded as 0 (absent), 1 (equivocal, septum unclear), 2 (mild, cyst less than septum thickness), 3 (moderate, cyst greater than septum thickness but less than half intraventricular width), or 4 (severe, cyst at least half intraventricular width) according to previously published criteria9 (Fig. 1, A–C).
FIG. 1.
Cavum septum pellucidum (CSP) grading diagrams and CSP grade and length for each patient. (A) The initial measurements that were taken in the midsagittal plane. The solid horizontal line depicts the longest intraventricular distance from genu to splenium of the corpus callosum (termed “septal length”). All images were then reformatted such that the coronal axis was perpendicular to this line to optimize coronal measurements along the septum. The dashed vertical line depicts the most anterior aspect of the columns of the fornix. This line was used to distinguish between the pre-fornix septum (where separations of the leaves of the septum pellucidum are termed CSP) and the post-fornix septum (where separations of the leaves of the septum pellucidum are termed “cavum vergae” [CV]). (B) A reformatted coronal MRI image for one patient that shows the greatest evidence of separation of the leaves of the septum pellucidum. This view was used to perform CSP grading for each patient. The dashed box depicts the region that is then enlarged to illustrate “Grade 2” in panel B. (C) Enlarged views of representative T1 coronal images for each CSP grade. Note that the Grade 0 septum appears crisp without any evidence of cyst (CSP absent). Grade 1 septum shows slight interior hypointensity that is not, however, clearly CSF signal intensity (septum unclear/CSP equivocal). Grades 2–4 show clear evidence of CSF signal between the separated leaves of the septum pellucidum. The degree of separation between the leaves of the septum pellucidum is then used to assign a grade of 2–4: Grade 2 CSP is not wider than the septum, Grade 3 CSP is wider than the septum but less than half the intraventricular width, and Grade 4 CSP is greater than half the intraventricular width. (D) CSP grade and length for each patient. Manual horizontal jitter was added to overlapping “grade” values in the graph to improve visibility.
By counting coronal slices and multiplying by slice thickness, the following measurements were taken: pre-forniceal septal length, post-forniceal septal length, CSP length, and CV length. To account for varied head/ventricular sizes, the following measurements were calculated: CSP ratio (CSP length/pre-forniceal septal length),6 CV ratio (CV length/post-forniceal septal length), total septal cyst length (CSP length+CV length), and total septal cyst ratio (total septal cyst length/total septal length). Given the potentially subjective nature of CSP grading, all scans were graded by two raters (MBR and RCG) who were blinded to clinical information, including TBI and football history, at the time of rating. Inter-rater agreement was excellent (weighted Cohen kappa=0.80). The final CSP grade used in the analyses was based on discussion between the raters until consensus was reached.
Statistical analysis
Summary statistics were generated for baseline characteristics, demographics, and septum pellucidum measurements of players and controls and compared using t tests for continuous variables, chi-square tests for binary variables, and nonparametric tests of trend for ordered categorical variables (e.g., CSP grade). In clinical-anatomical correlation analyses among players only, correlation between CSP length and Mini Mental Status Exam score, years of football exposure, age, and years since retirement from football was assessed using Spearman rho. CSP length and grade were also compared between players with a history of concussion with loss of consciousness (LOC) compared with players without a history of LOC using t tests (for CSP length) and nonparametric tests of trend (for CSP grade). Among players, Mini Mental Status Exam score, years of football exposure, age, and years since retirement were compared among players with CSP grade >2 versus ≤2 using t tests. The threshold for statistical significance was defined as p<0.05.
Results
Baseline clinical data are shown in Table 1. All individual patient CSP grades and lengths are shown in Figure 1D. CSP was significantly higher grade (p<0.001) and longer in players than controls (mean length±Standard deviation [SD]: 10.6 mm±5.4 vs. 1.1 mm±1.3, p<0.001). Results were similar for CSP length, total septal cyst length, and their ratios, suggesting that results were not confounded by larger head sizes among players. Ninety-four percent (16/17) of players had a CSP graded ≥2 (at least mild) compared with 18% (3/17) of controls (p<0.001). Forty-one percent (7/17) of players had a CV compared with 0% of controls (p<0.005). CSP ≥2 distinguished players from controls with 94% sensitivity (95% confidence interval [CI] 71–100%) and 82% specificity (95% CI 57–96%). CSP length ≥5 mm distinguished players from controls with 82% sensitivity (95% CI 57–96%) and 100% specificity (95% CI 81–100%).
Table 1.
Clinical Characteristics
| Controls (n=17) | Players (n=17) | p value | |
|---|---|---|---|
| Age, years; mean (SD) | 54.7 (15.8) | 54.6 (15.8) | 0.99 |
| Male | 17/17 | 17/17 | N/A |
| Education, years; mean (SD) | 17.3 (5.2) | 17.7 (3.3) | 0.79 |
| MMSE; mean (SD)* | 26.5 (4.5) | 26.5 (2.5) | 0.94 |
| Total lifetime football exposure (childhood, high-school, college, pro), years; mean (SD) | N/A | 17.3 (4.5) | N/A |
| Total pro-football exposure, years; mean (SD) | N/A | 7.7 (3.8) | N/A |
| Patient reported repeated concussions | 0/17 | 15/17 | N/A |
| Patient reported at least one concussion with LOC | 0/17 | 11/17 | N/A |
| Pro-football position played | N/A | Defensive back (2), defensive end (1), defensive linebacker (6), defensive safety (1), long-snapper (1), offensive lineman (1), offensive tackle (4), offensive wide-receiver (1) | N/A |
| Years since retired from pro-football; mean (SD) | N/A | 24.5 (15.5) | N/A |
| Clinical diagnoses (n) | MCI (9), AD (3), FTLD gene-carrier (2), bvFTD (1), HD (1), svPPA (1) | CPCS (5), MCI (6), HAND (1), cognitive disorder NOS (1), early-onset AD (1), mild dementia NOS (2), nfvPPA (1) | N/A |
SD, standard deviation; N/A, not applicable; LOC, loss of consciousness; MCI, mild cognitive impairment; AD, Alzheimer disease; FTLD, frontotemporal lobar degeneration; CPCS, chronic post-concussive syndrome; HAND, human immunodeficiency virus-associated neurocognitive disorder; bvFTD, behavioral variant frontotemporal dementia; HD, Huntington disease; svPPA, semantic variant of primary progressive aphasia; NOS, not otherwise specified; nfvPPA, nonfluent variant of primary progressive aphasia.
Missing MMSE data on one player and two controls.
Among players, CSP length was not significantly correlated with Mini Mental Status Exam score, years of football exposure (total or pro-only), age, or years since retirement from football. Similarly, among players with CSP grade >2 versus ≤2, there was no significant difference in Mini Mental Status Exam score (mean score±SD 27.1±1.7 vs. 25.9±3.3, p=0.3), total years of football exposure (mean years±SD 18.2±5.3 vs. 16.4±3.6, p=0.4), years of pro-football exposure (mean years±SD 8.8±4.1 vs. 6.5±3.1, p=0.2) age (mean years±SD 57.2±15.8 vs. 51.6±16.2, p=0.5), or years since retirement from football (mean years±SD 25.9±14.1 vs. 22.9±17.7, p=0.7). CSP length was not greater in players with a history of at least one concussion with LOC compared to players without any history of LOC (mean length±SD 11.6 mm±5.2 mm vs. 8.8 mm±5.6 mm, p=0.3). Similarly, CSP grade was not higher among players with a history of LOC compared with players without a history of LOC (p=0.6).
Eight players were followed longitudinally for 6–36 months. All remained stably impaired (n=3) or declined cognitively/behaviorally (n=4, including the player with CSP grade 0) except one player (CSP grade 3, age 29) who improved at 11-month follow-up.
Discussion
We demonstrate that prevalence, grade, and length of CSP are higher among symptomatic retired American pro-football players compared with memory clinic patients without TBI.
The septum pellucidum is composed of two sheaths of white matter derived from the commissural plate, a thickening of the lamina terminalis that appears during week 5 of embryonic development. During the gradual anterior-posterior extension of the corpus callosum, the underlying portion of the commissural plate thins out forming the septum pellucidum.6 Initially, there is a physiological CSP that fuses from posterior to anterior during the first 3 months of life, often leaving a small triangular cavity behind the callosal genu.10
Some have hypothesized that the CSP seen in patients after TBI may result from acceleration-deceleration forces that shear the septum pellucidum at the time of a traumatic hit9,11 or from transient increases in intracranial pressure.12 Others have hypothesized that CSP arises as a result of cerebral atrophy and ex-vacuo ventricular dilation.5 Our finding that memory clinic control patients with a variety of neurodegenerative syndromes have very low rates of CSP, however, may argue against this atrophy-based hypothesis.
Lastly, a minority have proposed that CSP in boxers is a neurodevelopmental abnormality that correlates with the tendency to engage in boxing.13 High rates of CSP in an autopsy series of victims of fatal motor vehicle accidents,11 as well as preliminary evidence for development of new CSPs on serial neuroimaging in professional boxers,14 renders this latter explanation less plausible among patients subjected to head trauma.
There is a growing body of literature reporting high rates of CSP among patients with a range of psychiatric and neurodevelopmental disorders including opiate abuse, bipolar disorder, Tourette syndrome, obsessive-compulsive disorder, and schizophrenia. It is unclear, however, to what degree these lesions are congenital versus acquired. Functionally, lesions of the septum pellucidum have been associated with abnormal reward processing and volume/fiber loss in adjacent limbic structures.9,15
This study is limited by its small size and potential for confounding if, for example, pro-football players have different brain structure than memory clinic controls for reasons independent of head trauma. Lastly, this and other previous studies raise the hypothesis that CSP may be a neuroimaging biomarker of previous brain trauma of sufficient magnitude to produce chronic neurological symptoms. Given the small sample size and the lack of an asymptomatic pro-football player control group, however, we are unable to fully assess the relationship of CSP characteristics with clinical features or specific types of TBI/football exposure (e.g., position played) in these players. Indeed, our negative clinical-anatomical correlation analyses (assessing the relationship between CSP length, CSP grade, and certain clinical characteristics of players) may have been underpowered.
Our findings suggest, however, that more research is warranted in larger cohorts of aging contact-sport athletes comparing characteristics of CSP in those who are symptomatic versus those who are asymptomatic. Such a future study could determine whether large high-grade CSP may be a neuroimaging biomarker of chronic post-TBI neurological symptoms or CTE.
Supplementary Material
Acknowledgments
We thank our patients and their families for contributing to research on TBI. We acknowledge administrative and technical support from Trishna Subas and Shirley Reeder. Funding: Department of Veterans Affairs Advanced Fellowship in Mental Illness Research/Treatment (RCG); UCSF Pepper Center Research Career Development Core (RCG); NIA K24-AG031155 (KY), NIA R01-AG045611 (GDR), Alzheimer's Association (GDR), Avid Radiopharmaceuticals (GDR), John Douglas French Alzheimer's Foundation (GDR), Hellman Family Foundation (GDR), Tau Consortium (GDR), UCSF ADRC (P50 AG023501) (GDR, BM), and State Center grants (BM), NIA K23-AG037566 (KP).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabanis E.A., Perez G., Tamraz J.C., Iba-Zizen M.T., Roger B., Alfonso J.M., and Rougemont D. (1986). Cephalic magnetic resonance imaging of boxers. Preliminary results. Acta Radiol. Suppl. 369, 365–366 [PubMed] [Google Scholar]
- 3.Orrison W.W., Hanson E.H., Alamo T., Watson D., Sharma M., Perkins T.G. and Tandy R.D. (2009). Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J. Neurotrauma 26, 689–701 [DOI] [PubMed] [Google Scholar]
- 4.Bogdanoff B., and Natter H.M. (1989). Incidence of cavum septum pellucidum in adults: a sign of boxer's encephalopathy. Neurology 39, 991–992 [DOI] [PubMed] [Google Scholar]
- 5.Smith D.H., Johnson V.E. and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nature reviews. Neurology 9, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Born C.M., Meisenzahl E.M., Frodl T., Pfluger T., Reiser M., Moller H.J., and Leinsinger G.L. (2004). The septum pellucidum and its variants. An MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 254, 295–302 [DOI] [PubMed] [Google Scholar]
- 7.Corsellis J.A., Bruton C.J., and Freeman-Browne D. (1973). The aftermath of boxing. Psychol. Med. 3, 270–303 [DOI] [PubMed] [Google Scholar]
- 8.Rosset A., Spadola L., and Ratib O. (2004). OsiriX: an open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 17, 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silk T., Beare R., Crossley L., Rogers K., Emsell L., Catroppa C., Beauchamp M., and Anderson V. (2013). Cavum septum pellucidum in pediatric traumatic brain injury. Psychiatry Res. 213, 186–192 [DOI] [PubMed] [Google Scholar]
- 10.Raybaud C. (2010). The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 52, 447–477 [DOI] [PubMed] [Google Scholar]
- 11.Pittella J.E., and Gusmao S. (2005). Cleft cavum of the septum pellucidum in victims of fatal road traffic accidents: a distinct type of cavum associated with severe diffuse axonal injury. Surg. Neurol. 63, Suppl 1, S30–S35 [DOI] [PubMed] [Google Scholar]
- 12.Mawdsley C., and Ferguson F.R. (1963). Neurological disease in boxers. Lancet 2, 795–801 [DOI] [PubMed] [Google Scholar]
- 13.Bodensteiner J.B., and Schaefer G.B. (1997). Dementia pugilistica and cavum septi pellucidi: born to box? Sports Med. 24, 361–365 [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman R.D., Jordan B. D. (1993). Neuroradiology of boxing injuries, in: Medical Aspects of Boxing. Jordan B.D. (ed). CRC Publishing: Boca Raton, FL, pps. 188–196 [Google Scholar]
- 15.Hwang J., Kim J.E., Kaufman M.J., Renshaw P.F., Yoon S., Yurgelun-Todd D.A., Choi Y., Jun C., and Lyoo I.K. (2013). Enlarged cavum septum pellucidum as a neurodevelopmental marker in adolescent-onset opiate dependence. PloS One 8, e78590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.