Abstract
Cocoa contains more phenolic antioxidants than most foods. Flavonoids, including catechin, epicatechin, and procyanidins predominate in antioxidant activity. The tricyclic structure of the flavonoids determines antioxidant effects that scavenge reactive oxygen species, chelate Fe2+ and Cu+, inhibit enzymes, and upregulate antioxidant defenses. The epicatechin content of cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated through anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-κB. Antioxidant effects of cocoa may directly influence insulin resistance and, in turn, reduce risk for diabetes. Further, cocoa consumption may stimulate changes in redox-sensitive signaling pathways involved in gene expression and the immune response. Cocoa can protect nerves from injury and inflammation, protect the skin from oxidative damage from UV radiation in topical preparations, and have beneficial effects on satiety, cognitive function, and mood. As cocoa is predominantly consumed as energy-dense chocolate, potential detrimental effects of overconsumption exist, including increased risk of weight gain. Overall, research to date suggests that the benefits of moderate cocoa or dark chocolate consumption likely outweigh the risks. Antioxid. Redox Signal. 15, 2779–2811.
I. Introduction
A. History
Chocolate is best known as an indulgent confection, but historically it has also been consumed for its purported healing properties (60). Foods and beverages made from beans from the Theobroma cacao tree (cocoa, cacao) have been consumed by humans since at least as early as 460 AD (224). The medicinal uses of cacao or chocolate either as a primary remedy or as a vehicle to deliver other medicines originated in Mesoamerica, where it was consumed by indigenous peoples, and diffused to Europe in the mid-1500s. Between the 16th and 20th centuries, well over 100 uses for cacao or chocolate, as a medical treatment, have been documented (60). Among these, three applications are most common: (i) to induce weight gain in emaciated patients; (ii) to stimulate the nervous system; and (iii) to improve digestion and elimination (60).
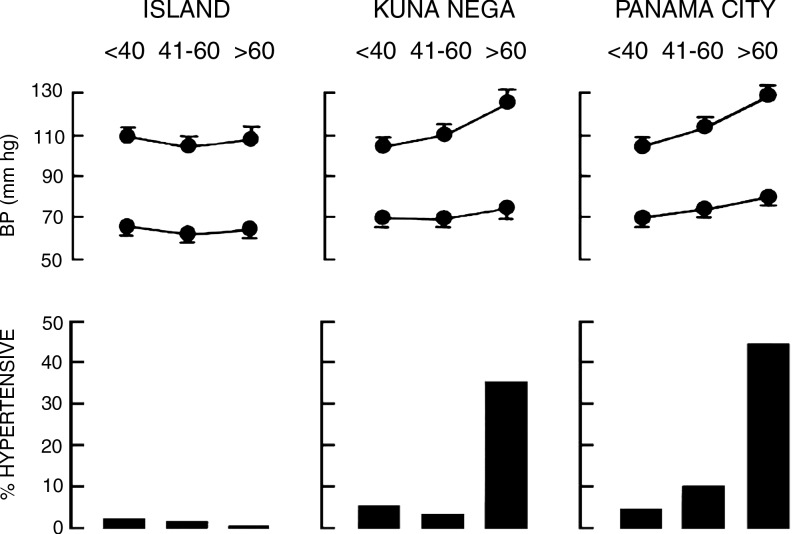
The Kuna Indians of the San Blas islands of Panama consume an average of three 10-ounce cups of cocoa beverage daily, ingesting approximately 1880 mg of procyanidins (39, 162). The prevalence of hypertension among the Kuna islanders is very low (2.2%) and blood pressure (BP) does not increase with age (104). The population also experiences lower rates of diabetes mellitus, myocardial infarction, stroke, and cancer than mainland Panamanians (103). Among Kuna who have migrated to urban areas on mainland Panama, the prevalence of hypertension is higher (10.7%) and reaches 45% among those over age 60 (Fig. 1) (104). McCullough et al. have hypothesized that the high intake of a traditional cocoa beverage may be partly responsible for the low incidence of cardiovascular disease among the Kuna islanders (162). Compared to Kuna living in a suburb of Panama City, those still living on the remote islands consume twice as much fruit, four times as much fish, and 10 times as much cocoa (162).
FIG. 1.
Blood pressure and prevalence of hypertension among island-dwelling and mainland Kuna Indians. Reprinted with permission from Hollenberg (102).
The majority of research on chocolate and cocoa has taken place over the last decade (41) and has primarily focused on the relationship between cocoa consumption and cardiovascular risk. More recent research has provided insights to the possible benefits of cocoa consumption on other organ systems. This review will discuss the reported physiologic effects of cocoa consumption and possible mechanisms by which they might occur.
B. Economic data
Worldwide, chocolate consumption ranges from 0.12 kg per person per year in China to 11.85 kg in Ireland. The United States falls somewhere in the middle of this range, with per capita annual consumption of 5.18 kg (33). Developed countries account for 64% of worldwide consumption (75a). The largest cocoa bean producing country in the world is Côte d'Ivoire, which produced 1.2 million tons of cocoa in 2006–2007 (113a).
C. Components of cocoa
Cocoa, or cacao, is the dried and fully fermented fatty seed of the fruit of the cocoa tree, Theobroma cacao (163). Cocoa liquor is the paste made from ground, roasted, shelled, and fermented cocoa beans, called nibs. It contains both nonfat cocoa solids and cocoa butter. Cocoa liquor is what is referred to as “percent cacao” on food packaging. Cocoa powder is made by removing some of the cocoa butter from the liquor. Chocolate is a solid food made by combining cocoa liquor with cocoa butter and sugar. The proportion of cocoa liquor in the final product determines how dark the chocolate is. Milk chocolate is made with the addition of condensed or powdered milk to the chocolate mixture (281). The type of chocolate consumed most in the United States is milk chocolate, which typically contains 10%–12% cocoa liquor (18). Semisweet or bittersweet chocolate is often referred to as dark chocolate and must contain no less than 35% by weight of cocoa liquor. White chocolate contains only cocoa butter (at least 20% by weight) combined with sweeteners and dairy ingredients (258).
Cocoa liquor is a complex food and contains many bioactive compounds. Cocoa butter contains significant amounts of fatty acids, whereas the nonfat cocoa solids contain vitamins, minerals, fiber, and polyphenols.
1. Lipids
The oil in cacao, referred to as cocoa butter, is a mixture of monounsaturated and saturated fatty acids. In the monounsaturated fraction, oleic acid predominates, as it does in olive oil (26). The majority of the saturated fatty acids are palmitic acid and stearic acid. In general, saturated fatty acid consumption has been associated with an increased risk of coronary heart disease as saturated fatty acids elevate total cholesterol and LDL (10, 280). A single meal containing relatively high levels of saturated fat may destabilize coronary plaque and impair endothelial function (128, 198, 275). Stearic acid is unusual in that it does not elevate serum lipid levels to the same degree that other saturated fatty acids do (112). Thus, whereas shorter-chain saturated fatty acids such as myristic acid (14:0) and palmitic acid (16:0) are associated with increased LDL and atherogenesis, stearic acid is not (32, 124, 223). Although the lipid content of chocolate is relatively high, one-third of the lipids in cocoa butter is stearic acid (18:0), which is believed to be nonatherogenic and to exert a neutral cholesterolemic response in humans (26). The 2010 Dietary Guidelines Advisory Committee specifically acknowledged stearic acid's unusual nature and has recommended that it be considered separately from cholesterol-raising fats (262).
2. Fiber
Although the bran of the cocoa bean is high in fiber, and its consumption has been shown to improve the LDL:HDL ratio, much of the bran is lost in processing (117). Still, some fiber remains in commercial cocoa products, though there is a wide range of fiber content. A 100-kcal portion of dark chocolate (70%–85% cacao) contains 1.7 g of fiber, whereas semisweet chocolate and milk chocolate contain 1.2 g and 0.6 g per 100 kcal, respectively. Unsweetened cocoa powder contains almost 2 g of fiber per tablespoon, and provides only 12 kcal (1). The majority of fiber in cocoa is insoluble (139). Although soluble fiber is noted for reducing serum cholesterol (19, 71), total dietary fiber is recognized as important for weight maintenance, and insoluble fiber has been associated with reduced risk of type 2 diabetes (270).
3. Minerals
The cocoa bean contains several minerals necessary for vascular function. Dietary magnesium, copper, potassium, and calcium all reduce risk of hypertension and atherosclerosis (248).
Dark chocolate (70%–85% cacao) provides 36 mg of magnesium per 100 kcal serving, which is 9% of the U.S. recommended dietary allowance (RDA) for middle-aged men—more than three times the amount provided by milk chocolate (11 mg) (1, 184). Magnesium is a cofactor in protein synthesis, muscle relaxation, and energy production (249). Magnesium is an antiarrhythmic and hypotensive hypotensive (4, 36, 53, 87, 237, 260).
Copper is a cofactor for a number of enzymes and is required for processes, including iron transport, glucose metabolism, infant growth, and brain development (190, 259). Copper deficiency can lead to anemia and pancytopenia, causing hypertension, inflammation, and myocardial hypertrophy (214). Copper deficiency has been linked to glucose intolerance, cardiac arrhythmia, and hypercholesterolemia in animals and humans (134); however, elevated copper status may also be harmful. High serum copper concentration is associated with an increased risk of cardiovascular death (206), all-cause, cancer, and cardiovascular mortality (142). Chocolate is a significant source of copper for Americans; milk chocolate provides 10% of the U.S. RDA for copper per 100-kcal serving, whereas dark chocolate provides 31%, and cocoa powder 23% per tablespoon (1, 184). Despite the potential detrimental effects of excess copper, the prevention of copper deficiency is, nevertheless, important for the maintenance of cardiovascular health. Because a 1000-kcal serving of chocolate would need to be consumed to reach the RDA for copper, it is unlikely that chocolate consumption would elevate serum copper concentrations to harmful levels.
Dietary potassium may protect against hypertension caused by excess sodium intake (8). Low potassium intake has been associated with increased risk of cardiovascular mortality (261). Chocolate is relatively low in potassium; dark chocolate contains 114 mg potassium (2% RDA) per 100 kcal, whereas unsweetened cocoa powder contains 82 mg per tablespoon, and milk chocolate contains 67 mg (1% RDA) (1, 184).
Iron (Fe) deficiency is one of the most important nutritional problems in the world (50). Milk chocolate contains 5% of the RDA for iron for adult men and postmenopausal women (0.42 mg) per 100 kcal; dark chocolate provides 25% of the RDA (1.90 mg). A tablespoon of unsweetened cocoa powder contains more iron than milk chocolate, but less than solid dark chocolate (0.75 mg) (1, 184).
4. Polyphenols and antioxidant activity
Cocoa powder contains up to 50 mg of polyphenols per gram. Single servings of cocoa and cocoa products contain more phenolic antioxidants than most foods (Table 1), and more procyanidins than the average amount consumed by Americans per day (86).
Table 1.
Flavan-3-ol Content and Antioxidant Capacity of Various Foods and Beveragesa
| Flavanols+procyanidins, mg | ORAC, mmol Trolox equivalents | |
|---|---|---|
| Cocoa liquor | ||
| Per 100 g | 1400.0 | 40.0 |
| Dark (semisweet) chocolate | ||
| Per 100 g | 170.0 | 13.1 |
| Per 100 kcal | 85.0 | 2.7 |
| Milk chocolate | ||
| Per 100 g | 70.0 | 6.7 |
| Per 100 kcal | 14.0 | 1.3 |
| Apples | ||
| Per 100 g | 106.0 | 0.2 |
| Per 100 kcal | 130.0 | 0.3 |
| Cranberry juice cocktail | ||
| Per 100 g | 12.6 | 0.2 |
| Per 100 kcal | 20.0 | 0.4 |
| Red wine | ||
| Per 100 g | 22.0 | 0.7 |
| Per 100 kcal | 25.0 | 0.9 |
| Brewed black tea | ||
| Per 2 g tea bag/200 ml waterb | 40.0 | 1.6 |
Reprinted with permission from Steinberg et al. (248).
Axtioxidant activity is reported as oxygen radical absorbance capacity (ORAC) and expressed as mmol Trolox equivalents. Data are provided on a per-weight and per-kcal basis to facilitate comparison among foods. Data from refs. (10, 12, 87, 89, 90, 123).
Data for tea are provided in an amount relevant to what might normally be consumed.
Cocoa contains a number of polyphenolic compounds, but it is particularly rich in flavonoids—specifically, flavanols, also called flavan-3-ols. Flavanols form complexes with salivary proteins and are responsible for the bitterness of cocoa (13, 150). Although flavanols impart a bitter astringent flavor to foods, the flavor is frequently masked in chocolates by aggressive processing and the addition of other flavors. Estimates vary, but in one study, the average total flavanol content of commercially available dark chocolate was more than five times that of milk chocolate (168). Table 2 summarizes the polyphenol content and antioxidant capacity of selected commercially available cocoa products.
Table 2.
Polyphenol Content and Antioxidant Capacity of Selected Commercially Available Cocoa Products
| Type of product | n | % NFCS | % fat | ORAC (μmol of TE) | Total polyphenolsa | Epicatechin (mg/g) | Catechin (mg/g) |
|---|---|---|---|---|---|---|---|
| Cocoa powder | 3 | 81.6 (8.2) | 15.0 (5.8) | 803.7 (78.2) | 52.4 (7.5) | 1.854 (0.849) | 0.578 (0.285) |
| Baking chocolate | 4 | 47.5 (2.2) | 52.6 (0.7) | 456.8 (50.7) | 27.7 (1.3) | 1.142 (0.103) | 0.491 (0.222) |
| Dark chocolate | 3 | 23.4 (5.3) | 34.7 (5.5) | 198.0 (47.0) | 13.0 (1.7) | 0.336 (0.031) | 0.164 (0.064) |
| Semisweet chocolate chips | 3 | 16.9 (1.7) | 28.9 (1.0) | 180.3 (8.5) | 12.4 (0.6) | 0.483 (0.085) | 0.194 (0.071) |
| Milk chocolate | 3 | 6.2 (1.2) | 32.6 (4.0) | 62.0 (17.6) | 4.4 (1.1) | 0.099 (0.067) | 0.043 (0.038) |
| Chocolate syrup | 3 | 6.2 (1.3) | 0.9 (0.3) | 63.4 (4.9) | 4.2 (0.6) | 0.074 (0.046) | 0.042 (0.015) |
Adapted from Miller et al.
Values are presented as means and standard deviations.
Total polyphenols expressed as gallic acid equivalents.
NFCS, nonfat cocoa solids.
The main flavanols found in cocoa are epicatechin and catechin, and procyanidins (Table 2). Procyanidins provide the majority of antioxidant activity in cocoa products (200).
In addition to polyphenols, cocoa contains methylxanthine compounds—predominantly theobromine—about 2% to 3% by weight. Caffeine is also present in small amounts (0.2%). Theobromine has antioxidant activity similar to caffeine (232) and relatively little stimulating effect on the central nervous system (272).
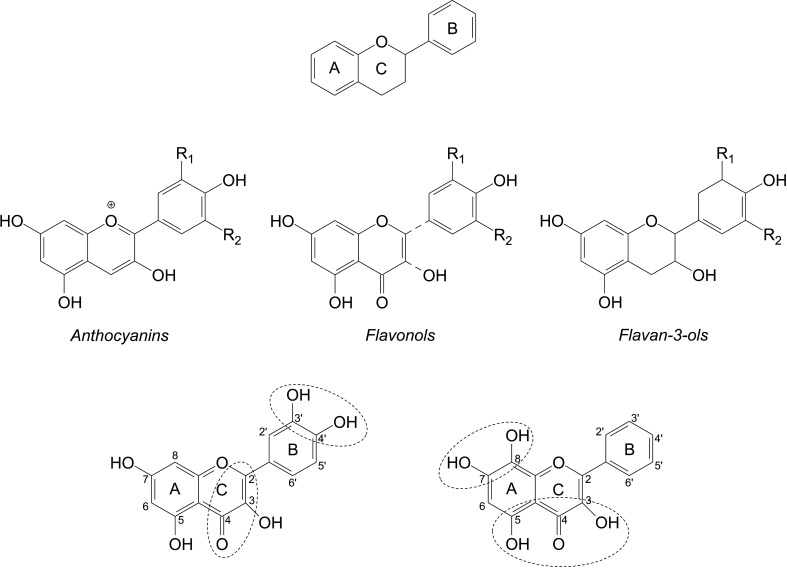
Flavonoids consist of two aromatic carbon rings, benzopyran (A and C rings) and benzene (B ring) (Fig. 2), and are subdivided into 13 classes based on the degree of hydroxylation and oxidation of the rings; they are anthocyanidins, flavonols, isoflavones, flavones, flavanones, and flavanols (150). Flavanols in cocoa are present as either the monomers (−) epicatechin and (+) catechin or oligomers of epicatechin and/or catechin, called proanthocyanidins or condensed tannins. A full listing of flavonoids and other phenols in cocoa is provided in Table 3.
FIG. 2.
C15 (C6-C3-C6) Flavonoid basic skeleton with high multifunctional activities including free radical scavenging, metal ion chelating, and enzyme inhibiting.
Table 3.
Cocoa Flavonoid and Nonflavonoid Phenols
| Class | Compounds |
|---|---|
| Cocoa nonflavonoid phenols | |
| Flavanols | |
| (−)-Epicatechin | |
| (+)-Catechin | |
| (−)-Epicetechin-3-O-gallate | |
| (−)-Epigallocatechin | |
| Procyanidin B1 (epicatechin-(4β(8)-catechin | |
| Procyanidin B2 (epicatechin-(4β(8)-epicatechin | |
| Procyanidin B2-O-gallate (epicatechin-3-O-gallate-(4β(8)-epicatechin | |
| Procyanidin B2-3,3-di-O-gallate (epicatechin-3-O-gallate-(4β(8)-epicatechin-3-O-gallate | |
| Procyanidin B3 (catechin-(4β(8)-catechin) | |
| Procyanidin B4 (catechin-(4β(8)-epicatechin) | |
| Procyanidin B4-3-O-gallate (catechin-(4β(8)-epicatechin-3-O-gallate | |
| Procyanidin C1 (epicatechin-(4β(8)-epicatechin-(4β(8)-epicatechin) | |
| Flavonols | |
| Quercetin | |
| Isoquercitin (quercetin-3-O-glucoside) | |
| Quercitin-3-O-arbinoside | |
| Quercitin-3-O-galactoside | |
| Anthocyanins | |
| 3-alpha-L-Arabinosidyl cyanidin | |
| 3-beta-D-Galactosidyl cyanidin | |
| Flavones | |
| Luteolin | |
| Luteolin-7-O-hyperoside | |
| Iso-orientin | |
| Vitexin | |
| Flavanones | |
| Naringenin | |
| Naringenin-7-O-glucoside | |
| Cocoa nonflavonoid phenols | |
| Phenolic acids | |
| Chlorogenic acid | |
| Vanillic acid | |
| Coumaric acid | |
| Phloretic acid | |
| Caffeic acid | |
| Ferulic acid | |
| Phenylacetic acid | |
| Syringic acid | |
| Others | |
| Clovamide | |
| Deoxyclovamide | |
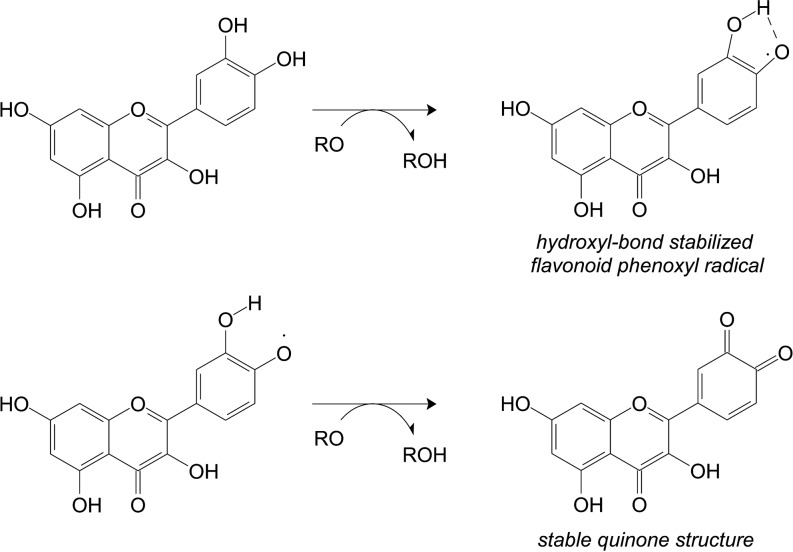
The tricyclic structure of the flavonoids determines their antioxidant (and possibly pro-oxidant) effects; phenolic-quinoid tautomerism and the delocalization of electrons over the aromatic system scavenge reactive oxygen species (Fig. 3). These aromatic rings directly neutralize free radicals, chelate metals (Fe2+ and Cu+) that enhance reactive oxygen species, inhibit enzymes, and upregulate antioxidant defenses (Fig. 3) (44, 216). Enzymes inhibited by cocoa flavonoids include xanthene oxidase, NADPH-oxidase, tyrosine kinases, and protein kinases (64). Cocoa intake increases serum antioxidant capacity, protecting the endothelium from oxidative stress and endogenous ROS (136).
FIG. 3.
Mechanism of antioxidant action of flavonoids (3′, 4′-diOH polyphenols).
Flavonoids, flavan-3-ols, and their oligomeric derivatives, procyanidins, have a variety of beneficial physiologic actions. Flavonoids have a number of properties that may contribute to their cardioprotective effects, including antioxidant and antiplatelet activity, immunoregulatory properties, and beneficial effects on the endothelium (42). The epicatechin content of cocoa is primarily responsible for its favorable impact on vascular endothelium, which is the result of both acute and chronic upregulation of nitric oxide production (222, 277). The combined catechin/epicatechin content in chocolate is 460–610 mg/kg (150). Epicatechins improve vascular function, reduce BP, improve insulin sensitivity, and reduce platelet activity (42). Epicatechin in cocoa quenches OH· 100 times more effectively than mannitol, a typical OH· scavenger (239).
Food products with significant antioxidant properties, such as green tea (79), degrade in antioxidant activity over time. Hurst et al. (113) assessed commercial preparations of chocolate and found that milk chocolate bars maintain oxygen radical absorbance capacity (ORAC), total polyphenols, and flavan-3-ol monomers for at least 50 weeks in commercial preparations, whereas cocoa powder and cocoa beans demonstrate stability in samples over 75 years old. Based on these results, Hurst posited that gallated flavan-3-ols (epigallocatechin-3-gallate [EGCG] and ECG; found in green tea) may be more susceptible to oxidation in relation to epicatechin (113).
a. Vascular effects mediated through NO
Independent of their antioxidant effects, plant polyphenols also promote the vasodilating factors nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor and inhibit pro-angiogenic factors endothelin-1 and vascular endothelial growth factor (VEGF) (217, 250). Cocoa is one of many food sources of substances that increase the production or bioavailability of endothelial nitric oxide. Other sources include grapes and berries, red wine, black and green tea, soy beans, pomegranates, olive oil, fish oil, and garlic (217). The vasodilatory response to cocoa flavanols is dependent on NO and can be reversed by blocking nitric oxide synthesis (74).
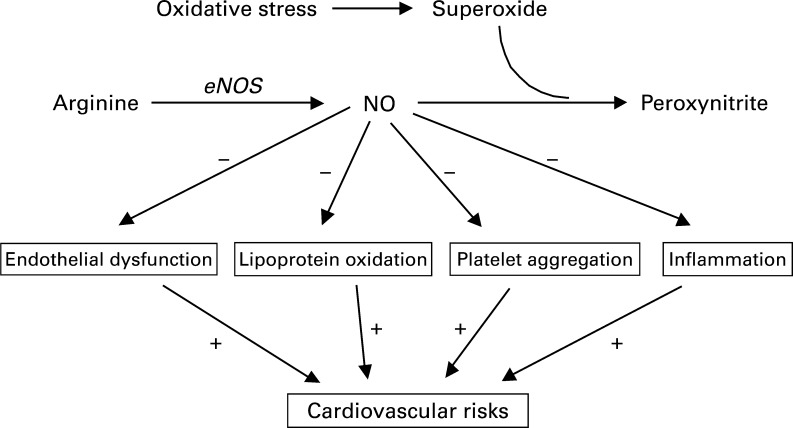
Endothelium-derived NO regulates vasodilation and the environment of the blood vessel wall (228), and is therefore critical for vascular function. Endothelial NO is produced by endothelial nitric oxide synthase (eNOS), which converts L-arginine to L-citrulline in the presence of necessary cofactors (143). Adequate production and bioavailability of eNOS-derived NO is necessary for the maintenance of healthy endothelium. A reduction in the bioavailability of eNOS-derived NO results in endothelial dysfunction, which is associated with all major risk factors for atherosclerosis. There is reason to believe that improving the function of the eNOS pathway may be effective in the prevention and treatment of atherosclerosis (Fig. 4) (143).
FIG. 4.
Diagram to show how the downstream effects of cocoa polyphenols might affect the vascular system, with nitric oxide (NO) as the target. Endothelial dysfunction, lipoprotein oxidation, platelet aggregation, and inflammation all increase cardiovascular risk (+), whereas vascular effects of cocoa polyphenols reduce risk (−) from these intermediary factors. Reprinted with permission from Cooper et al. (41). eNOS, endothelial nitric oxide synthase.
Endothelial NOS gene expression is regulated primarily by shear stress elicited by the circulating blood or endothelial cell proliferation, but its enzyme activity increases in response to receptor-operated substances such as acetylcholine, bradykinin, and serotonin (12).
The action of NO is mediated by the activation of the soluble guanylate cyclase in the smooth muscle cells and platelets, which increases the level of cyclic guanosine monophosphate (cGMP). The rise in cGMP inhibits calcium flux and decreases cytosolic calcium concentration, resulting in smooth muscle relaxation and platelet aggregation inhibition (173, 174). Prostacyclin is another vasodilator that works synergistically with NO to inhibit thrombosis. Increases in cGMP can increase cyclic adenosine monophosphate (cAMP) in the cell; cAMP is required for the activation of prostacyclin (171, 173, 174).
In addition to causing vasodilation, NO also prevents leukocyte adhesion and migration, smooth muscle cell proliferation, and platelet adhesion and aggregation (80). Nitric oxide and other endothelium-derived factors are important for the control of vascular biology, not only in the peripheral but also in the cerebral circulation (81). Deficiency of NO favors the development of atherosclerosis and is associated with increased cardiovascular risk in conditions such as type 2 diabetes, metabolic syndrome, hypertension, and atherosclerosis (31, 80). Pure epicatechin ingestion has been shown to acutely reduce the plasma levels of endothelin-1 in healthy men (144).
The specific mechanisms by which cocoa flavanols improve vascular function are the subject of ongoing research, but their effects on NO metabolism appear to be more substantial than their general antioxidant effects; NADPH oxidase may be the site of action.
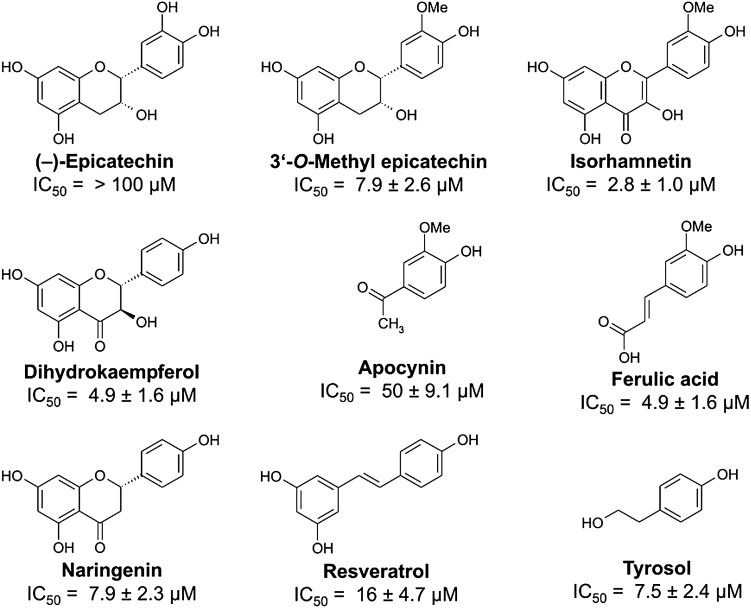
NADPH oxidase is implicated in vascular dysfunction; NADPH oxidase isoforms generate O2•– that scavenge NO•. Cocoa polyphenols may help maintain NADPH oxidase activity at levels low enough to not harm vascular endothelium. Epicatechin increases bioavailability of NO by inhibiting NADPH oxidase (216, 247). Schewe and colleagues demonstrated that exposure to (–)-epicatechin elevates cellular levels of NO• and cGMP and protects against oxidative stress elicited by oxidized LDL (216); however, these effects tend to be transient. Longer-term effects of cocoa flavonoids on endothelial cells may be an adaptive mechanism to long-term exposure to high-flavonoid foods. Schewe et al. postulate that the effect on endothelial function may be mediated by changes in gene expression and protein synthesis or breakdown, including an elevated level of endothelial NO• synthase (eNOS) in the vascular endothelium (216, 245). Figure 5 illustrates structures of selected polyphenols and half maximal inhibitory concentration (IC50) values for inhibition of NADPH oxidase activity. The 4-hydroxyphenyl group of apocynin is a determinant for NADPH oxidase inhibition by structurally similar flavonoids (216).
FIG. 5.
Structures of selected polyphenols and IC50 values for inhibition of NADPH oxidase activity. The noncatechol 4-hydroxyphenyl group of apocynin is a determinant for inhibition of NADPH oxidase by structurally related flavonoids and derivatives of cinnamic acid and silbenes. IC50 is the half-maximal inhibitory concentration, a measure of a compound's effectiveness in inhibiting a biochemical function. IC50 values are dependent on the specific conditions of the assay and cannot simply be translated to in vivo conditions. They permit, however, comparison of structurally related compounds. Reprinted with permission from Schewe et al. (216).
II. Epidemiology: Chocolate and Health Outcomes
The majority of research on cocoa and health has been in the form of clinical trials on surrogate markers. However, a few prospective cohort studies are notable. An early ecological study investigated coronary mortality in relation to consumption of coffee, tea, cocoa, alcohol, and tobacco in 20 countries, but found no significant associations (28). Later studies have mostly supported a protective association between cocoa or chocolate consumption and a variety of health indicators. These include total and cardiovascular mortality, serum C-reactive protein, psychological well-being, and risk of diabetes, myocardial infarction, and/or stroke (30, 59, 116, 189, 236).
The first cohort study of cocoa intake and cardiovascular outcomes was published in 2006 (29). The study population included 470 Dutch men free of cardiovascular disease and diabetes from the Zutphen Elderly Study cohort. Systolic and diastolic BPs were inversely associated with cocoa intake after adjustment for a wide range of possible confounders. This relationship was statistically significant for diastolic BP (p=0.03) and near significant for systolic BP (p=0.06). Cocoa consumption was associated with significantly lower cardiovascular and all-cause mortality. Those in the highest tertile of cocoa intake compared with those in the lowest tertile had 50% lower rates of all-cause mortality and cardiovascular disease. Individuals in the highest tertile consumed more than 2.30 g of cocoa daily, whereas those in the lowest tertile consumed less than 0.36 g/day. Consumption of other food groups, including confectionary, was not associated with mortality.
In 2009, a study in Stockholm, Sweden assessed cardiac mortality in a particularly high-risk group: nondiabetic patients hospitalized with a first myocardial infarction (116). After a mean 8.6 years of follow-up, patients who reported eating chocolate twice or more per week were 66% less likely to suffer a cardiac death compared to those who reported never eating chocolate. After adjustment for demographic and socioeconomic variables, coffee consumption, and intake of sweets, there was a significant, linear, inverse relationship between frequency of chocolate consumption and cardiac mortality (p=0.01). Total mortality, however, was not associated with chocolate consumption.
Other observational studies have identified a number of positive health outcomes associated with cocoa or chocolate consumption. In a Japanese study, risk of being found to have diabetes was reduced 35% among men who consumed “chocolate snack pieces” once per week or more compared with those who never or almost never ate chocolate (189). A similar but nonsignificant reduction in risk was observed for women (189). In another study, a J-shaped relationship was observed between dark chocolate consumption and serum C-reactive protein (CRP) levels in Italian men and women (59). Individuals consuming a 20-g serving of dark chocolate daily had the lowest CRP concentrations. A 2009 study suggested a relationship between psychological health and chocolate preference in which elderly men preferring chocolate reported feeling less lonely and depressed and happier than men preferring other types of candy (251).
A cross-sectional analysis of data from 2217 participants in the NHLBI Family Heart Study identified an inverse relationship between chocolate consumption and calcified atherosclerotic plaque in the coronary arteries (61). Individuals who reported consuming (any type of) chocolate two or more times per week were 32% less likely to have prevalent coronary artery calcification compared with those who never ate chocolate. The odds ratios decreased with increasing frequency of chocolate consumption and were adjusted for age, sex, energy intake, waist-to-hip ratio, education, smoking, alcohol consumption, total cholesterol:HDL ratio, nonchocolate candy intake, and diabetes mellitus.
In 2010, Buijsse and colleagues released results from the first cohort study to demonstrate a reduced risk of myocardial infarction and stroke associated with chocolate consumption (30). The study followed 19,357 men and women in Germany over a mean of 8 years. Compared with the bottom quartile of chocolate consumption, those in the highest quartile had a 39% reduced risk of myocardial infarction or stroke (p=0.014). The highest quartile reported a mean chocolate intake of 7.5 g/day. The food frequency questionnaire used in this study did not distinguish between white, milk, and dark chocolate. However, a more detailed analysis of intake among a subset of participants found that milk chocolate was the type most often consumed (57%), followed by dark chocolate (24%) and white chocolate (2%).
Another 2010 study partially supports the findings of Buijsse et al. In this study, Mostofsky and colleagues assessed the relationship between chocolate consumption and heart failure in 31,823 middle-aged and elderly women over 9 years of follow-up (175). They found that women consuming 1–3 servings per month or 1–2 servings per week of chocolate had significantly lower rates of heart failure hospitalization or heart failure death compared with those consuming no chocolate (OR, 0.74; 0.68, respectively). However, the odds of heart failure outcomes were slightly, but not significantly, higher among women who reported consuming 3–6 servings per week (OR, 1.09; 95% CI, 0.74–1.62) or one or more servings per day (OR, 1.23; 95% CI, 0.73–2.08).
III. Effects on Cardiovascular Disease
A. Pathogenesis
Cardiovascular disease is the leading cause of death and disability-adjusted life years globally and is associated with risk factors, including hypertension, smoking, hyperlipidemia, and diabetes mellitus (286). The vast majority (80%) of cardiovascular disease burden is now experienced in low- and middle-income countries (286). Atherosclerosis is the result of a series of inflammatory responses at the cellular and molecular level that leads to the development of lesions in medium-sized arteries. Chronic inflammation is responsible for the progression of atherosclerotic lesions through advancing stages. It has been proposed that endothelial dysfunction initiates the process of atherosclerosis. There are many possible etiological factors for endothelial dysfunction, including elevated LDL or homocysteine in the blood, infectious microorganisms, genetics, and oxidative stress. The injury caused by these varied agents initiates an inflammatory response that may continue indefinitely (209). The resulting chronic inflammatory state is characterized by increasing numbers of macrophages and lymphocytes and proliferation of smooth muscle cells. Unabated inflammation will further increase the concentration of macrophages and lymphocytes that promote the formation of fibrous tissue in the lesion through the release of hydrolytic enzymes, cytokines, chemokines, and growth factors. The resulting complicated lesion has the potential to narrow the lumen of the artery and block blood flow (209). Advanced plaques high in lipid content are particularly vulnerable to disruption, which can lead to the occlusive thrombosis that characterizes acute coronary syndromes (43).
Diet has been established as one of the most important lifestyle factors that can strongly influence the incidence of cardiovascular disease (51, 76, 125). Dietary flavonoids may decrease cardiovascular risk by protecting lipids, proteins, and nucleic acids from oxidative damage, as well as reducing inflammation and regulating vascular homeostasis (205). Flavonoid intake has been inversely associated with coronary artery disease (CAD) incidence and mortality (98).
A number of pro-inflammatory enzyme systems are involved in the pathogenesis of atherosclerosis, including xanthine oxidase and NADH/NADPH oxidase (159) that produce reactive oxygen species (ROS), which can cause endothelial dysfunction (265). Flavanols, particularly epicatechin (222), in cocoa have anti-inflammatory properties that inhibit the production of ROS, and have antihypertensive and vasoprotective effects (42).
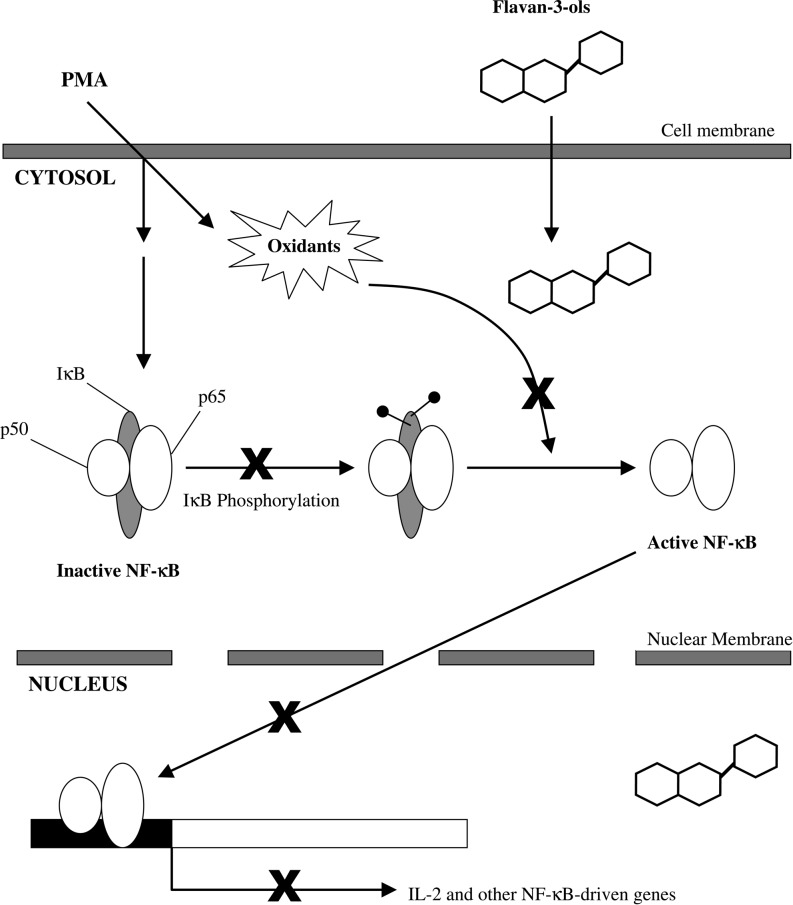
Cocoa polyphenols modulate the activity of NF-κB (141), a protein complex involved in DNA transcription that is a pivotal factor in a number of inflammatory processes (Fig. 6) (226). NF-κB activation in leukocytes (as well as in endothelial cells and macrophages) results in leukocyte adhesion to the endothelium, tissue invasion, and the secretion of mediating factors leading to tissue injury. Cocoa polyphenols can reduce the activity of NF-κB, downregulating leukocyte activation and attenuating the production of inflammatory mediators and ROS (226, 229). Further, polyphenols act synergistically with other nutrients, such as vitamin C and selenium, to increase endogenous antioxidant capacity (229).
FIG. 6.
Inhibition mechanisms of phorbol myristate acetate (PMA)-induced NF-kB activation by cocoa flavanols, including a dimer. The specific steps in the NF-kB activation cascade by which cocoa flavanols can interfere are indicated with an “x.” Reprinted with permission from Selmi et al. (226).
Cocoa flavanols can affect oxidant enzymes such as lipoxygenases, involved in arachidonic acid metabolism and the biosynthesis of leukotrienes. High-procyanidin chocolate was found to increase plasma prostacyclin and decrease plasma leukotrienes (220, 233), reflecting anti-inflammatory and vasoprotective properties (233).
B. Effects on endothelial function
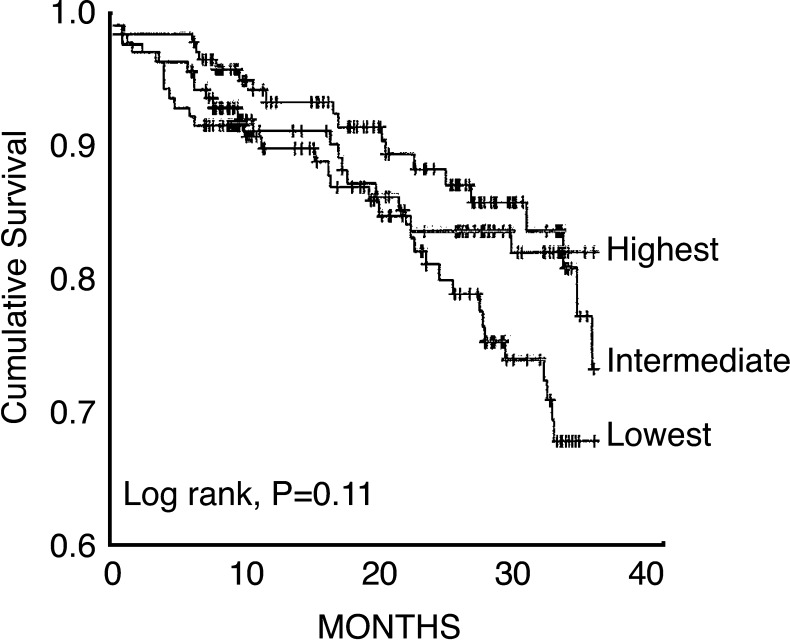
Endothelial function is recognized as an important measure of cardiac risk, as impaired endothelial function has been found to predict both recurring and incident cardiovascular events (7, 34, 52, 57, 68, 209, 215, 253, 284, 285) (Fig. 7).
FIG. 7.
Kaplan-Meier curves for cumulative event-free survival associated with endothelial function measured as flow-mediated dilatation (FMD) (lowest tertile<2%, highest tertile>6.3%). Log-rank analysis revealed a significant difference between the low and intermediate tertiles (p=0.037). Further analysis revealed a significant difference in event rate between those with the most severe FMD abnormality (tertile 1) and the combined group of second and third tertiles (log-rank p=0.029). When this analysis was restricted to all-cause mortality alone, it remained significant (log-rank p=0.047). Adapted from Fathi et al. (68).
Endothelial function refers to arterial vasomotor responses mediated predominantly by the release of NO (vasodilating), and endothelin (vasoconstricting) from the vascular endothelium (145, 266), and plays an important role in the pathogenesis of atherosclerosis, hypertension, and cardiovascular disease (266). Endothelial dysfunction has been shown in the coronary and peripheral circulations before the development of atherosclerotic plaque (284, 285). Individuals with cardiac risk factors develop endothelial dysfunction via decreased NO production, abnormal signaling, and increased oxidative stress (266). Endothelial dysfunction has been shown to reverse in response to cardiac risk modification efforts (266). NO also has an anti-inflammatory effect, counteracting leukocyte recruitment and platelet aggregation at the site of inflammation (225) though other substances play important roles in modulating vascular tone, fibrinolysis, coagulation, and inflammation (274).
Human trials have demonstrated vasoprotective effects mediated by NO (92, 225, 256). A 2008 meta-analysis of randomized, controlled trials of flavonoid-containing foods concluded that cocoa was the only food to show significant effects acutely and chronically on flow-mediated dilatation (FMD) of the brachial artery, a noninvasive method to measure endothelial function (107). Data from two long-term studies and six acute studies indicated that chocolate or cocoa significantly increased FMD by 1.45% and 3.99%, respectively (107). Corti et al. reviewed 11 human trials of cocoa and endothelial function and found that all demonstrated significant improvement in FMD after cocoa consumption (42). Acute studies were 2 h in duration, whereas longer-term studies were 2 weeks long, with the exception of one 4-week study (17) and a 5-day study (73). Four of the trials were conducted on healthy individuals (65, 73, 222, 231) and two on smokers (95, 97). Other study populations included diabetic patients (17); hypertensives (85); hypertensives with impaired glucose tolerance (84); patients with at least one cardiovascular risk factor (92); and heart transplant recipients (75). In five of the studies, a cocoa beverage was administered (17, 73, 92, 95, 222); the other six used dark chocolate (65, 75, 84, 85, 97, 231).
1. Cocoa beverage consumption in healthy subjects
Fisher and colleagues, in their 5-day trial in 27 healthy individuals, found that consumption of flavanol-rich cocoa (821 mg flavanols/day) induced vasodilation, as measured by pulse wave amplitude, which was reversible by the NO synthase inhibitor NG-nitro-L-arginine methyl ester, indicating that the vasodilating effect was attributable to activation of the NO system. The effects of low-flavanol cocoa were significantly smaller (p=0.005) (73).
Schroeter et al. conducted a series of studies to determine the acute vascular effects of cocoa and the role of cocoa flavanols in mediating those effects. In one of these studies, the group assessed the effects of a single dose of high-flavanol cocoa beverage (917 mg total flavanols) compared to a low-flavanol cocoa (37 mg flavanols) in 10 healthy men. In a subgroup of three participants, the dependence of endothelial function improvements on NO synthase was tested by inhibiting NOS before consumption of the high-flavanol cocoa beverage (222). To determine the effects of flavanols, isolated from other cocoa components, the researchers used a randomized, cross-over design to test two different doses of pure epicatechin (1 or 2 mg/kg of body weight) mixed with water, compared to water only in six individuals (three per treatment arm). Further, a cross-sectional study was completed to compare urinary flavanol metabolites in 18 mainland-dwelling Kuna Indians and 16 of those still living on the San Blas Islands, where cocoa intake is very high. Key findings from these studies include the following: (i) FMD was significantly increased at 1–4 h after ingestion paralleled by increases in plasma flavanols and metabolites; (ii) peripheral arterial tonometry (PAT, a measure of microvascular circulation) responses increased after high-flavonol cocoa consumption; (iii) FMD response was significantly attenuated by NO synthase inhibition; (iv) FMD and PAT increased significantly 2 h after ingestion of either 1 or 2 mg of pure epicatechin per kg of body weight, whereas no changes were associated with water alone—these changes were similar to those observed after high-flavanol cocoa ingestion; (v) urinary flavanol metabolites were more than six times higher in Kuna Islanders than in the mainland Kuna sample.
Fisher and colleagues also reported significantly greater improvements in endothelial function (FMD) after 4–6 days of flavanol-rich cocoa consumption in older (≥50 years) than in younger (<50 years) individuals (72). Consistent with the findings of Heiss et al. (93) endothelial function was also further improved by acute ingestion of cocoa at the end of the period of sustained consumption.
Our lab has conducted two studies to investigate the effects of cocoa or chocolate on endothelial function. A randomized, placebo-controlled, single-blind crossover design was utilized to test the acute effects of dark chocolate, sugared cocoa and sugar-free cocoa, and the long-term effects of sugared and sugar-free cocoa. The placebos for both studies were white chocolate and white cocoa, as appropriate. The test products contained between 805 and 821 mg total flavanols, whereas the placebos contained no flavanols. The dark chocolate and white chocolate products were similar in energy, fat, and carbohydrate content, as were the sugared cocoa and white cocoa (66). The composition of test products used in this study is provided in Table 4.
Table 4.
Composition of Test Productsa
| Content | Placebo chocolate | Solid dark chocolate | Sugar-free cocoa | Sugared cocoa | Placebo cocoa |
|---|---|---|---|---|---|
| Weight (g)b | 74 | 74 | 23.4 | 114.9 | 125.3 |
| Cocoa powder (g) | 0 | 22 | 22 | 22 | 0 |
| Energy (kcal) | 389 | 327 | 90 | 460 | 500 |
| Total fat (g) | 22 | 27 | 2 | 2 | 2 |
| Carbohydrates (g) | 44 | 39 | 12 | 104 | 110 |
| Protein (g) | 6 | 6 | 6 | 6 | 8 |
| Sodium (mg) | 97 | 4 | 110 | 110 | 410 |
| Potassium (mg) | 306 | 366 | 334 | 334 | 512 |
| Calcium (mg) | 215 | 33 | 33.4 | 33.4 | 314 |
| Magnesium (mg) | 19 | 119 | 133.6 | 133.6 | 38 |
| Catechin (mg) | 0 | 10.4 | 20.9 | 20.9 | 0 |
| Epicatechin (mg) | 0 | 21.5 | 48.4 | 48.4 | 0 |
| Procyanidin dimer (mg) | 0 | 81.4 | 92.0 | 92.0 | 0 |
| Procyanidin trimer (mg) | 0 | 67.3 | 98.1 | 98.1 | 3.3 |
| Procyanidin tetramer (mg) | 0 | 37.0 | 30.6 | 30.6 | 0 |
| Procyanidin pentamer and hexamer (mg) | 0 | 67.0 | 54.8 | 54.8 | 5.5 |
| Total procyanidins (total flavanols) (mg) | 0 | 821 | 805.2 | 805.2 | 8.8 |
| Theobromine (mg) | 1.5 | 525 | 436 | 436 | 0 |
| Caffeine (mg) | 3.7 | 44 | 28.1 | 28.1 | 0 |
Reprinted with permission from Faridi et al. (66).
Energy and nutrient data of the tested products are provided by the Hershey Company.
Refers to total product weight.
The acute study had two phases. In the first, participants received single doses of 74 g dark chocolate and 74 g white chocolate, in random sequence and with a 7-day washout period between treatments (66). FMD and BP were measured after an 8-h fast and 2 h after ingestion of each treatment. Compared with placebo, dark chocolate improved FMD from baseline (4.3±3.4% compared with −1.8±3.3%; p<0.001) and reduced BP (systolic: −3.2±5.8 mm Hg compared with 2.7±6.6 mm Hg; p<0.001; diastolic: −1.4±3.9 mm Hg compared with 2.7±6.4 mm Hg; p<0.001).
In the second phase of the study, participants were randomized to one of six possible permutations of treatment sequences (188). All received each treatment (two cups of sugared, sugar-free, or placebo cocoa providing 805, 805, and 0 mg flavanols, respectively) with 7-day washout periods between each treatment assignment. Single doses of sugared and sugar-free cocoa improved FMD significantly from baseline compared with placebo (5.7%±2.6% and 2.0±1.8% compared with −1.5%±2.8%; p<0.001), but the effect was significantly greater after sugar-free cocoa consumption compared with sugared cocoa (p<.001). Significant reductions in systolic and diastolic BPs were also observed after consumption of sugar-free, but not sugared, cocoa compared with placebo (systolic: −2.1±7.0 mm Hg compared with 3.2±5.6 mm Hg; p<0.001; diastolic: −1.2±8.7 mm Hg compared with 2.8±5.6; p=0.014).
In the long-term study (188) 44 subjects were enrolled and 37 completed the study. Using the same cocoa preparations from the acute study (sugared cocoa, sugar-free cocoa, and placebo cocoa), we assigned subjects to consume each of the cocoa preparations daily (two cups per day providing 805 mg flavanols or no flavanols) for 6 weeks, in random sequence. Both flavanol-containing cocoas improved FMD compared to placebo (2.4 and 1.5% for sugar-free and sugared cocoa, respectively, compared with −0.8%; p<0.01). Although improvement was greater after sugar-free cocoa than sugared cocoa, the difference was not significant (p=0.15). BP did not improve significantly in either intervention period compared with placebo.
2. Cocoa beverage consumption in subjects at risk for cardiovascular disease
Heiss and colleagues have contributed much to the study of cocoa. In a 2003 study, they assessed the effects of high- and low-flavanol cocoa drinks on FMD and nitrosylated and nitrosated species (RNO) in 20 participants with at least one cardiovascular risk factor (92). In the double-blind, crossover trial, participants received 100 ml of high-flavanol cocoa drink (176 mg) and a low-flavanol cocoa (<10 mg) on 2 consecutive days, in random sequence. The high-flavanol and low-flavanol cocoas contained 73 and 66 kcal, respectively. In an initial pilot test, the high-flavanol cocoa increased FMD maximally 2 h after ingestion, whereas the low-flavanol drink had no effect. Based on these results, outcome measures were assessed 2 h after ingestion of the test products in the full study. Principal findings from this study include the following: (i) flavanol-rich cocoa, but not low-flavanol cocoa, significantly increased RNO and FMD (from 3.4% to 6.3%); (ii) changes in RNO and FMD were correlated (r=0.42, p=0.02); (iii) there were no significant changes in other vascular variables. These findings were replicated in a subgroup of 11 smokers with no other cardiovascular risk factors (95).
In a 2007 study series, Heiss et al. investigated the effects of acute (up to 6 h after ingestion) and chronic (daily ingestion for 7 days) high-flavanol cocoa consumption on FMD in healthy male smokers (93). In the 7-day study, high-flavanol cocoa contained 306 mg total flavanols and 80 kcal per dose and was consumed three times per day (93). Two acute studies were conducted using a randomized, double-blind crossover design to test the effects of three different doses of cocoa flavanols for 1–6 h. In the first, five subjects received cocoa drinks containing 36, 330, and 918 mg on different days in random order. In the second acute study, a separate group of six subjects received cocoa with 28, 179, and 483 mg of flavanols. The drinks were closely matched for other nutrients. The acute studies found that maximum increases in FMD occurred 2 h after ingestion of high-flavanol cocoa, regardless of dose, but the magnitude of FMD response and the time to return to baseline was dose dependent. For example, ingestion of cocoa containing 36 mg of flavanols did not significantly change FMD, whereas cocoa containing 330 mg increased FMD at 1, 2, and 3 h after ingestion and cocoa containing 918 mg increased FMD at 1, 2, 3, 4, and 6 h after ingestion. Peak FMD was also significantly different across all three groups. In the chronic study, fasting FMD increased progressively during 1 week of daily high-flavanol cocoa consumption. FMD responses on days 1, 3, 5, and 8 were 3.7%, 5.2%, 6.1%, and 6.6%, respectively. Further, the degree of acute FMD response after cocoa ingestion on each day was maintained, so that both fasting and post-cocoa ingestion measures were higher on successive days. FMD returned to baseline levels after a 1-week washout period. These findings suggest that although cocoa's effects on the vascular endothelium occur primarily in the short-term, chronic consumption may result in sustained benefits.
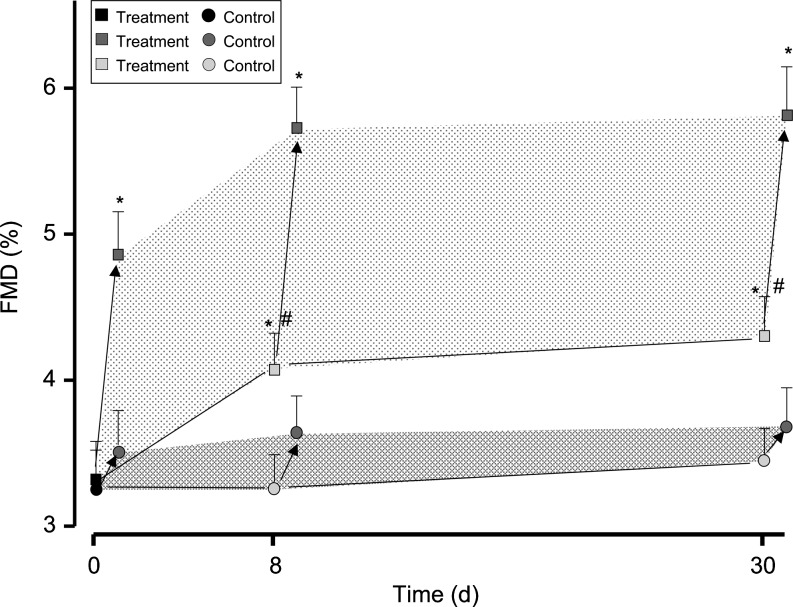
In a randomized, controlled, double-blind study by Balzer et al., FMD increased by 30% among 21 medicated diabetics who consumed cocoa containing 321 mg of flavanols per dose three times per day for 30 days, but not in the 20 subjects who consumed a nutrient-matched low-flavanol cocoa (17). No significant differences in BP, heart rate, or glycemic control were observed between the groups. The test products provided between 160 and 170 kcal, approximately, per day. Like Heiss et al., Balzer and colleagues also observed maximal increases in FMD 2 h after ingestion of cocoa and greater increases among participants ingesting cocoa containing higher amounts of flavanols. Consistent with Heiss et al., baseline FMD improved after 8–30 days of sustained cocoa consumption compared with control, and acute FMD responses to cocoa ingestion also increased relative to baseline (Fig. 8).
FIG. 8.
Acute and sustained effects of flavanol-containing cocoa. At study entry, baseline values for flow-mediated dilation (FMD) were similar in both groups. In the treatment group (squares), FMD was significantly augmented over time. On top of sustained FMD increases, acute improvements were observed at 2 h after ingestion of flavanol-containing cocoa. No significant changes could be observed in the control group (circles). Data are given as mean ± standard deviation. *Indicates significant differences in FMD compared with baseline differences within each group, p < 0.001; #Indicates significant differences in FMD between the control group and the treatment group, p < 0.05. Reprinted with permission from Balzer et al. (17).
3. Solid dark chocolate consumption in healthy subjects
Engler et al. investigated the effects of sustained dark chocolate consumption on endothelial function, as well as measures of oxidative stress, blood lipids, and BP in 21 healthy men and women using a randomized, double-blind, placebo-controlled design (65). The test products were 46 g of high-flavonoid (21 mg procyanidins, 46 mg epicatechin) or low-flavonoid (trace flavanols) dark chocolate, consumed once daily for 2 weeks. A nonsignificant decrease in FMD from baseline was observed in the low-flavonoid group (p=0.17), whereas a near-significant increase was observed in the high-flavonoid group (p=0.05). The change in FMD between the two groups was significantly different, however (p=0.024). No significant changes in any other outcome measures were observed. In a separate study with two individuals, consumption of a single 120 g dose of high-flavonoid chocolate did not increase plasma epicatechin levels further compared with the 46 g dose used in the larger study (65).
Hermann and colleagues also studied the effects of dark chocolate on endothelial function in smokers (97). They randomly assigned 20 men to receive a single dose of either 40 g of dark chocolate or 50 g of white chocolate. FMD and shear stress-dependent platelet function were assessed before and 2 h after chocolate ingestion. Consistent with other studies, dark chocolate improved FMD response significantly from 4.4% to 7.0% (p=0.026), whereas white chocolate had no effect. Platelet function was also reduced in the dark chocolate group (5.0%–3.2%, p=0.03) but not the white chocolate group. Accordingly, antioxidant status increased after dark chocolate ingestion only.
Two endothelial function studies involving dark chocolate were conducted by Grassi et al. (84, 85). In the first, 20 patients with essential hypertension received 100 g of dark chocolate (88 mg flavanols) and 90 g of white chocolate (no flavanols) daily for 15 days, in random sequence with a 7-day washout period between treatments (85). Outcome measures included FMD, 24-h ambulatory BP, 2-h glucose, serum cholesterol, and markers of vascular inflammation. Measures of insulin sensitivity were also calculated. Dark chocolate significantly improved FMD, systolic (−11.9 mm Hg) and diastolic (−8.5 mm Hg) BPs, insulin sensitivity, and serum LDL (−0.4 mM). All measures remained unchanged after white chocolate consumption.
In their second study, Grassi and colleagues used the same research design and chocolate dose to test the effects of regular dark chocolate consumption in 19 hypertensive subjects who were also glucose intolerant (84). In addition to the variables measured in the first study, serum CRP, plasma homocysteine, and β-cell function (corrected insulin response) (120) were also assessed. In this population, dark chocolate again significantly improved FMD, insulin sensitivity, 24-h ambulatory systolic and diastolic BP, and LDL. Dark chocolate also significantly decreased total cholesterol (-6.5%, p<0.0001) and improved β-cell function (p=0.035).
4. Dark chocolate and coronary circulation
Another dark chocolate study by Flammer et al. provides support for the extension of cocoa's vascular effects to coronary arteries (75). In this double-blind, randomized trial, 22 heart transplant recipients received a single dose of either 40 g of dark chocolate (15.6 mg epicatechin equivalents per gram) or a flavonoid-free control chocolate. Coronary artery diameter and endothelium-dependent coronary vasomotion increased significantly and platelet adhesion decreased significantly 2 h after ingestion of the flavonoid-rich dark chocolate, but not flavonoid-free chocolate. These changes were paralleled by increases in serum epicatechin concentrations.
A 2009 study by Shiina et al. also found improvement in coronary circulation associated with dark chocolate ingestion (231). In this randomized, single-blind study, 39 healthy men were assigned to consume either 45 g of flavonoid-rich dark chocolate (550 mg polyphenols) or 35 g flavonoid-free white chocolate daily for 2 weeks. Coronary flow velocity reserve (CFVR) measured by echocardiography, BP, serum lipids, and markers of oxidative stress were assessed at baseline and at the end of the study. Dark chocolate, but not white chocolate, significantly improved CFVR, independent of changes in other variables.
5. Cocoa and chocolate consumption in CAD patients
In contrast to the positive findings of studies in healthy individuals and those with cardiovascular risk factors, the results of a study in 40 subjects with CAD were null (67). In this randomized, double-blind, placebo-controlled study, the active treatment was a chocolate bar and cocoa beverage, containing a total of 444 mg of flavanols. Isocaloric placebo chocolate bars and cocoa drinks containing only 19.6 mg of flavanols but matched for macronutrient, caffeine, and theobromine content were provided to the control group. Each treatment was consumed daily for 6 weeks and provided approximately 275 kcal and 18g fat per day. Endothelial function, high-sensitivity CRP, oxidized LDL, lipids, glucose, and insulin were assessed. No significant differences in any measure were observed between the treatment groups.
However, another study in CAD patients published in July 2010 had different results (94). This study used a randomized, double-blind, cross-over design to investigate whether cocoa flavanols improve endothelial function in this population and whether improvement involves an increase in number and function of circulating angiogenic cells (CACs). All 16 subjects consumed each of the test beverages—high-flavanol cocoa (375 mg) and low-flavanol cocoa (9 mg)—twice daily for 30 days, in random sequence. FMD improved significantly from baseline in both groups, but high-flavanol cocoa increased FMD significantly more than low-flavanol cocoa (3.8% vs. 1.3%, respectively, p=0.001). High-flavanol cocoa also significantly increased the mobilization of functional CACs and reduced BP compared with low-flavanol cocoa. CAC numbers more than doubled after the high-flavanol cocoa intervention, a finding the authors suggest is clinically relevant and comparable to effect sizes reported for other interventions including statins, estrogen, exercise, and smoking cessation.
The conflicting results of this study and the study by Farouque et al. are noteworthy. Flavanol intake and sample size per treatment arm or phase were similar in both studies, as were age, gender, and BMI. Some differences in baseline characteristics of the study populations include higher proportions of diabetics and hypertensives in the Heiss et al. study (38% vs. 7.5% and 88% vs. 55%, respectively). Fewer subjects in the Farouque et al. study were using beta blockers (45% vs. 88%). The Farouque et al. study was also longer in duration (6 weeks vs. 30 days). The form in which cocoa flavanols were delivered did differ between the studies, and may have affected the results. In the Heiss et al. trial, the active treatment was a cocoa beverage and provided 50 kcal and <1 g fat per day. In comparison, the cocoa beverage/solid chocolate combination used in the Farouque et al. study provided 275 kcal and 18 g fat. Additional research is needed in CAD patients to determine whether there is a clinical benefit of cocoa flavanol consumption and to identify the most beneficial way to deliver them.
C. Effects on platelets
When the endothelium is damaged and the underlying fibrous matrix exposed, as it is when atherosclerotic plaque ruptures, platelets can adhere to the matrix and become activated (9). Once activated, platelets can recruit leukocytes and endothelial progenitor cells, and interact with these cells to induce inflammatory responses and promote thrombus formation (88, 158).
Dietary flavonoids, and flavanols in particular, have been associated with decreased platelet reactivity. Although the specific mechanisms responsible for this association have not yet been identified, it has been theorized that flavanols may affect the activity of platelets by inducing changes in membrane fluidity, ligand-receptor affinity, and intracellular signaling pathways (105). It has also been suggested that platelet-derived nitric oxide may be a target of flavanols (204). Although many plant foods contain flavanols, cocoa is an especially concentrated source (150), and has been recognized as having significant effects on platelets in humans. A systematic review of 25 intervention studies of polyphenol-rich diets and platelet function noted cocoa's platelet-inhibiting effects as the only consistent finding among the trials (194). In 2007, Bordeaux and colleagues found that, among healthy participants in a platelet function study, those who had consumed chocolate before testing (n=141) had reduced platelet activity compared to nonconsumers (23). A small study of 30 healthy subjects found that platelet activation decreased 2 and 6 h after cocoa consumption (205). In another trial, cocoa consumption inhibited platelets in vivo, whereas dealcoholized red wine consumption did not (204). Hamed and colleagues demonstrated that these short-term effects could also be seen after 1 week of solid dark chocolate consumption (containing 700 mg of flavonoids per day) in 28 healthy subjects (89). Flammer and colleagues found that dark chocolate consumption decreased platelet adhesion 2 h after consumption in 22 heart transplant patients (75). A study by Heptinstall et al. evaluated the effects of cocoa beverage consumption on platelets and leukocytes in vitro and ex vivo supporting previous findings of platelet inhibition, and identifying a potential role for cocoa flavanols in suppressing leukocyte activation (96).
Existing evidence strongly supports a beneficial effect of cocoa consumption on platelet activation, an important contributor to the inflammation and thrombosis that leads to advanced cardiovascular disease. The effects are likely due mostly to the actions of flavanols, though the minerals (potassium, magnesium, and calcium) in cocoa or stearic acid present in in chocolate may also play a role (196).
D. Effects on lipids
Cocoa's effects on serum lipids are not as clear as its effects on endothelial function and platelet activity. Evidence for a lipid-lowering effect is limited and inconclusive.
Numerous studies have investigated the effects of cocoa consumption on the lipid profile, with conflicting results. In one trial in patients with hypertension, daily consumption of 100 g flavonoid-rich chocolate over 2 weeks significantly reduced serum total cholesterol by 7% and LDL by 12% (85). Consumption of cocoa and dark chocolate increased HDL by 4% in one study (267).
Mellor et al. found that HDL increased in subjects consuming a high-polyphenol chocolate, but not in persons consuming a low-polyphenol chocolate over the course of 16 weeks (166).
In contrast, Engler et al. found no difference in serum lipids between subjects consuming 46 g of high-flavonoid dark chocolate and those consuming low-flavonoid chocolate daily for 2 weeks (65). Almoosawi et al. also did not observe significant changes in total cholesterol among overweight and obese subjects after 2 weeks of daily consumption of dark chocolate containing either 500 mg or 1000 mg polyphenols (6).
Feeding studies of cocoa beverages containing only cocoa powder have also had mixed results. Wang-Polagruto and colleagues studied the effects of consuming a high flavanol (446 mg total flavanols) cocoa beverage relative to a low flavanol (43 mg) cocoa beverage daily for 6 weeks in hypercholesterolemic postmenopausal women (269). In this study, HDL levels increased 6.6% in the high flavanol group, whereas HDL decreased 9.6% in the low flavanol group (p<0.05). Another study investigated the effects of consuming a cocoa beverage containing four different levels of polyphenols (0 g, 13 g, 19.5 g, or 26 g) daily for 4 weeks on the lipid panel in normocholesterolemic and mildly hypercholesterolemic individuals (14). Although none of the polyphenol-containing cocoa treatments differed significantly from the placebo cocoa, all three significantly improved LDL and HDL levels from baseline in subjects with high LDL at the start of the study. However, these improvements were small. Baba et al. observed a greater increase in HDL (24%) after daily ingestion of 12 g sugar plus 26 g cocoa powder for 12 weeks. This increase was significantly different than the small increase (5%) observed in the control group, which only received 12 g sugar daily (15). At least three studies found no effect of cocoa beverage consumption on blood lipids (45, 178, 188), including one which tested a combination of liquid cocoa and solid dark chocolate (45).
Because of their high flavonoid content, the cocoa solids present in chocolate are typically hypothesized to affect measures of cardiovascular health, whereas other components of chocolate products (e.g., cocoa butter) are thought to have little or no effect. Given this reasonable assumption, the results of a study by Kris-Etherton et al. are surprising (135). In their study, consumption of milk chocolate as a substitute for a high-carbohydrate snack bar improved levels of serum HDL and triglycerides. This improvement occurred despite the fact that the milk chocolate bar increased the total fat and saturated fat in the diet. Because milk chocolate contains a relatively small proportion of cocoa solids, this finding might suggest that a component in chocolate other than flavonoids (possibly stearic acid) was responsible. However, in another trial, HDL increased by 11.4% and 13.7% when subjects consumed dark chocolate and polyphenol-enriched dark chocolate, respectively, but not when they consumed white chocolate (181). One possible explanation for the results of Kris-Etherton et al. is that the chocolate bar's displacement of a high-carbohydrate snack, rather than some active compound in the chocolate bar itself, led to the change in lipid levels.
A 2010 meta-analysis of eight trials summarized the short-term impact of cocoa consumption on blood lipids (119). The data from these trials indicate that cocoa may significantly reduce LDL cholesterol, and may also reduce total cholesterol when consumed in low doses by individuals with cardiovascular risk factors. This meta-analysis supports a lipid-lowering effect of cocoa, but it is limited by the small total sample (n=215), the paucity of well-designed trials, and the heterogeneity of the studies included. Whether cocoa products substantially improve lipid levels in the blood remains unclear. There is, however, convincing evidence that consumption of chocolate in most forms has at worst, a neutral effect on the lipid profile. This evidence should allay fears that the high saturated fat content of chocolate would negate the effects of its other health-promoting compounds. Further, although cocoa may not appreciably change the quantity of lipids in the blood, it may change their quality, that is, their ability to cause blood vessel damage.
Lipids, n-6 fatty acids in particular, are susceptible to peroxidation by reactive oxygen species (ROS) produced as a result of normal metabolic processes or pathological events. To a certain extent, cells can compensate for the activity of ROS with endogenous antioxidant defenses, but when pro-oxidants exceed antioxidants, cells experience oxidative stress (287). Exogenous antioxidants from food sources are therefore important in maintaining a favorable balance between ROS and the antioxidants that can inactivate them (287).
Levels of oxidized LDL in the blood have been shown to predict CAD better than total cholesterol, triglycerides, HDL, and LDL (63, 110). In general, intervention studies suggest that cocoa can inhibit LDL oxidation. Decreased levels of plasma-oxidized LDL have been observed in subjects after long-term daily consumption of cocoa powder (14, 15) and dark chocolate (267). These effects might be attributed to epicatechin, which attenuates LDL oxidation and protects the endothelium from the actions of oxidized LDL (246). However, some studies suggest that consumption of chocolate low in flavonoids may also be beneficial. In two studies, milk chocolate (135) and white chocolate (181) inhibited LDL oxidation. In the latter study, a marker of lipid peroxidation decreased 11.9% after consumption of white chocolate, dark chocolate, or dark chocolate enriched with polyphenols (181). These results indicate that the fatty acids in chocolate may play an important role in LDL oxidation.
Although one study failed to find an effect of either flavonoid-rich dark chocolate or low-flavonoid chocolate on LDL oxidation (65), most available evidence supports the hypothesis that consumption of cocoa or chocolate protects lipids from oxidation.
E. Effects on BP
A relationship between cocoa consumption and reduced BP was first observed in the Zutphen Elderly Study (29). Subsequent randomized, controlled trials have confirmed the association. A meta-analysis of five such trials found that cocoa consumption was associated with significant reductions in systolic and diastolic BPs of 4.7 and 2.0 mm Hg, respectively. Other intervention studies have had similar findings (6, 66, 84) but a few have found no effect of cocoa on BP (45, 65, 178, 180). A 2010 study found that a daily dose of 1052 mg cocoa flavanols was required to reduce 24-h ambulatory BP. There was no effect on seated BP or on either measure at lower doses of flavanols (48). Another study found that a dark chocolate bar containing 500 mg polyphenols lowered BP as effectively as a bar containing 1000 mg (6). Crews et al. did not observe any change in BP among healthy men and women over the age of 60 who consumed both a cocoa beverage and dark chocolate daily for 6 weeks (providing a total of 754.71 mg proanthocyanins), relative to those consuming low-flavanol control products (45). However, Berry and colleagues demonstrated that consumption of a high-flavanol (701 mg) cocoa beverage could significantly improve BP response to exercise compared to a low-flavanol (22 mg) beverage (21).
Although some studies have not observed significant changes in BP associated with consumption of cocoa products containing average levels of flavanols, the majority of the research conducted in this area indicates that regular cocoa/chocolate ingestion can reduce BP. A meta-analysis of 10 trials published in 2010 confirmed the findings of the previous 2007 meta-analysis. Mean change in systolic and diastolic BP across the trials was −4.5±1.35 and −2.5±1.36 mm Hg, respectively. There was a wide range of flavanol intake across these trials, with epicatechin intake as low as 5 mg/day or as high as 174 mg/day (56). Of note, in the trial reporting the lowest level of flavanols, subjects, nevertheless, experienced significant improvement in BP (256). Similar to the trials of flow-mediated dilatation, Hooper and colleagues' meta-analysis of flavonoid-rich food trials highlighted cocoa as the sole food capable of significantly reducing BP in human studies (107). In addition to a reduction in risk for MI and stroke, Buijsse et al. reported significantly lower systolic (p=0.0008) and diastolic (p<0.0001) BPs among individuals consuming higher amounts of chocolate (30).
A number of mechanisms have been proposed to explain cocoa's effects on BP (83). Because of their importance in BP maintenance, the improvements in nitric oxide availability and endothelial function associated with cocoa consumption may explain much, if not all, of its antihypertensive effects (183). However, there is some evidence that flavanols and flavanol-rich foods, including cocoa, can inhibit angiotensin-converting enzyme (ACE) activity in vitro (2, 3). Angiotensin-converting enzyme regulates the renin–angiotensin system; it cleaves angiotensin-I into angiotensin-II, which stimulates the release of vasopressin or aldosterone and antidiuretic hormone, increasing sodium and water retention. It also inactivates vasodilators bradykinin and kallidin (138). Whether ACE inhibition mediates the antihypertensive activity of cocoa flavanols in humans is not yet known.
F. Cardiovascular effects: conclusion
Rimbach et al. noted that beneficial effects on BP, FMD, and platelet aggregation have not been found in all human trials (67, 73). Further, improvements are often small when they are observed (165, 256). The authors also raise the concern about chocolate's caloric density and its potential to contribute to weight gain, which may counteract any small benefits of cocoa's polyphenol content. This is a legitimate concern, as evidenced by a study by Taubert et al., in which daily dark chocolate consumption providing an additional 480 kcal per day decreased systolic BP by 5 mm Hg (255). However, in a subsequent study, Taubert and colleagues found that a mere 30 kcal per day of dark chocolate for 18 weeks reduced systolic BP by 2.9 mm Hg (256). Their argument also ignores the fact that the bioactive components of cocoa (i.e., flavanols) are found in the nonfat portion of the cocoa bean and can be readily isolated in a low-energy-dense form, that is, cocoa powder. For example, in their study in CAD patients, Heiss et al. used an active treatment that provided a substantial daily dose of 375 mg flavanols but only 50 kcal (94).
Rimbach et al. also questioned the rigor of cocoa feeding trials, noting their small sample sizes and acknowledging the need for randomized, placebo-controlled, and cross-over studies. However, the majority of the endothelial function studies discussed in this review, including those from our lab, are randomized and placebo-controlled, and many are cross-over designs and/or double-blinded. It is true that most cocoa and chocolate studies have used small sample sizes, increasing variance and risk of type 2 error, biasing toward the null. Nevertheless, when they detect significant differences in cardiovascular measures that favor treatment, this is a testament to the strength of the observed effect. Table 5 summarizes results from these human trials investigating cardiovascular effects of cocoa or chocolate.
Table 5.
Human Trials Investigating Cardiovascular Effects of Cocoa or Chocolate
| Author, year | Population | N | Treatment | Duration | Control | Outcomes |
|---|---|---|---|---|---|---|
| Balzer, 2008 | Medicated diabetics | 41 | High-flavanol cocoa (321 mg flavanols per dose), three times daily | 30 days | Low-flavanol cocoa (25 mg per dose), three times daily | FMD ↑ BP Ø HR Ø glycemic control Ø |
| Farouque, 2006 | CAD patients | 40 | High-flavanol chocolate bar and cocoa (444 mg total flavanols) | 6 weeks | Low-flavanol chocolate bar and cocoa (19.6 mg total flavanols) | FMD Ø SAC Ø Soluble CAM Ø FBF Ø |
| Faridi, 2008 | Overweight adults | 45 | High-flavanol dark chocolate (821 mg), sugar-free cocoa (805 mg), and sugared cocoa (805 mg) | Single dose | Flavanol-free white chocolate bar or white cocoa | All treatments: FMD ↑ BP ↓ |
| Fisher, 2003 | Healthy individuals | 27 | High-flavanol cocoa (205 mg flavanols per dose), four times/day | 5 days | Low-flavanol cocoa (<10.25 mg per dose); single day pre-post evaluation in seven subjects | FPWA ↑ |
| Fisher, 2006 | Older (>50) and younger (<50) healthy individuals | 24 | High-flavanol cocoa (205 mg flavanols per dose), four times/day | 4–6 days | No control | FPWA ↑a |
| Engler, 2004 | Healthy adults | 21 | High-flavanol dark chocolate (259 mg) | 2 weeks | Low-flavanol dark chocolate (trace) | FMD ↑ LDL oxidation Ø TAC Ø 8-isoprostanes Ø BP Ø Serum lipids Ø BMI Ø |
| Flammer, 2007 | Heart transplant recipients | 22 | High-flavanol dark chocolate (624 mg) | Single dose | Flavanol-free chocolate | CAD ↑ EDCV ↑ Platelet adhesion ↓ |
| Grassi, 2008 | Hypertensive, glucose intolerant individuals | 19 | High-flavanol dark chocolate (1008 mg total phenols) | 15 days | Flavanol-free white chocolate | FMD ↑ SBP ↓ DBP ↓ Total chol ↓ LDL ↓ |
| Grassi, 2005 | Hypertensive patients | 20 | High-flavanol dark chocolate (88 mg flavanols) | 15 days | Flavanol-free white chocolate | FMD ↑ Ambulatory BP ↓ LDL ↓ |
| Heiss, 2003 | Outpatients with ≥1 cardiovascular risk factor | 20 | High-flavanol cocoa (176 mg flavanols) | 2 days | Low-flavanol cocoa (<10 mg flavanols) | FMD ↑ RNO ↑ Plasma nitrite Ø Plasma nitrate Ø |
| Heiss, 2007 | Individuals with smoking-related endothelial dysfunction | 6 | High-flavanol cocoa (306 mg per dose), three times/day | 7 days | Low-flavanol cocoa (12 mg per dose), three times/day | FMD ↑ Plasma nitrite ↑ Plasma nitrate Ø |
| Heiss, 2010 | Patients with CAD | 16 | High-flavanol cocoa (375 mg) | 30 days | Low-flavanol cocoa (9 mg) | FMD ↑ CACs ↑ CAC functions Ø SBP ↓ Plasma nitrite ↑ |
| Heiss, 2005 | Smokers | 11 | High-flavanol cocoa (176–185 mg) | Single dose | Low-flavanol cocoa (<11 mg) | FMD ↑ RNO ↑ |
| Hermann, 2006 | Male smokers | 20 | 40g dark chocolate | Single dose | 40g white chocolate | FMD ↑ SSDPF ↓ |
| Njike, 2009 | Overweight adults | 44 | Sugar-free cocoa (805 mg), and sugared cocoa (805 mg) | 6 weeks | Flavanol-free white cocoa | Both treatments: FMD ↑ BP Ø Total chol, HDL, LDL, TG Ø CRP Ø LDL oxidation Ø Lipid hydroperoxide Ø Endothelin Ø |
| Schroeter, 2006 | Healthy men | 16 | High-flavanol cocoa (917 mg) | Single dose | Low-flavanol cocoa (37 mg) | FMD ↑ RNO ↑ PAT ↑ |
| Shiina, 2009 | Healthy men | 39 | High-flavanol dark chocolate (550 mg polyphenols) | 2 weeks | Flavanol-free white chocolate | CFVR ↑ BP Ø HR Ø Total chol, HDL, LDL, TG Ø |
Unless otherwise specified, treatment refers to daily dose.
FPWA increased to a greater extent in older subjects.
HR, heart rate; SAC, systemic arterial compliance; CAM, cellular adhesion molecules; FBF, forearm blood flow; FPWA, finger pulse wave amplitude; TAC, total antioxidant capacity; CAD, coronary artery disease; EDCV, endothelium-dependent coronary vasomotion; RNO, sum of nitrosylated and nitrosated species; CAC, circulating angiogenic cell; SSDPF, shear stress dependent platelet function; PAT, peripheral arterial tonometry index; CFVR, coronary flow velocity reserve.
IV. Effects on Insulin Resistance
There is reason to believe that the flavanols in cocoa may ameliorate insulin resistance by reducing oxidative stress, improving endothelial function, and/or altering glucose metabolism. Ceriello and Motz have proposed that oxidative stress is the underlying mechanism for both insulin resistance and cardiovascular disease (35). This hypothesis is supported by the observation that many antidiabetic drugs and drugs used to treat cardiovascular disease demonstrate antioxidant effects. This effect may be direct—as in the case of calcium channel blockers, statins, ACE inhibitors, and AT-1 receptor antagonists—or indirect—as in acarbose and glinides, which prevent oxidative stress caused by postprandial hyperglycemia (35). If this hypothesis is correct, the demonstrated antioxidant activity of cocoa flavanols (170) could theoretically also protect against insulin resistance. However, better evidence exists for an insulin-sensitizing effect mediated by changes in endothelial function and/or glucose metabolism.
Many polyphenols, including catechin and epicatechin, have been found to alter glucose metabolism in animal and in vitro studies (90). In studies, catechin has inhibited alpha-glucosidase activity (114) and inhibited absorption of glucose from the intestine (121, 273). Studies in diabetic rats support an insulin-sensitizing effect of cocoa. In two such studies, epicatechin regenerated pancreatic β-cells (37) and increased insulin secretion (37, 100). In a 2005 study, supplementation of diabetic rats with cocoa extract for 4 weeks was dose-dependently associated with reduced serum glucose and LDL, and increased HDL (213). A similar study by Jalil and colleagues found that cocoa extract reduced postprandial hyperglycemia, plasma free fatty acids, and 8-isoprostane, a biomarker of oxidative stress (115). However, no change was observed in fasting glucose or insulin.
The well-documented effects of cocoa on endothelial function also point to a possible effect on insulin sensitivity. The relationship between endothelial function and insulin resistance is a reciprocal one. Increased insulin sensitivity improves endothelial function; conversely, improvement in endothelial function can increase insulin sensitivity (131). In healthy individuals, insulin increases blood flow to skeletal muscles and glucose uptake by muscle cells through vasodilation. In contrast, in insulin-resistant individuals, insulin-mediated vasodilation is impaired and glucose disposal is inhibited. Insulin resistance has been associated with reduced activity of endothelium-derived NO synthase and with increased plasma levels of asymmetric dimethylarginine, an endogenous NOS inhibitor (254). Thus, the availability of NO likely plays a role in mediating cells' response to insulin. Because of cocoa's ability to increase bioavailability of NO, the possible relationship between cocoa consumption and insulin resistance has been investigated.
Although low rates of hypertension may be the most striking of the Kuna population's unique characteristics (102), their comparatively low rates of diabetes are also intriguing (103). Grassi and colleagues reported that ingestion of 100 g of flavonoid-rich dark chocolate for 15 days was associated with not only reduced BP and improved endothelial function, but also improved insulin sensitivity in hypertensive patients (84). These results have also been observed in healthy individuals and in hypertensives with impaired glucose tolerance (84). In the former study, subjects received oral glucose tolerance tests (OGTT) after consuming either dark chocolate or white chocolate for 15 days. Compared with white chocolate, ingestion of dark chocolate was associated with higher homeostasis model insulin resistance (HOMA-IR) values, and lower quantitative insulin-sensitivity check index values. In the latter study, hypertensive, glucose-intolerant subjects also received OGTT after 15 days of daily consumption of either flavanol-rich dark chocolate or white chocolate. Dark chocolate decreased HOMA-IR, increased insulin sensitivity, and increased β-cell function compared to white chocolate.
Insulin sensitivity improved significantly in overweight and obese adults consuming high-flavanol cocoa (902 mg flavanols) for 12 weeks compared to low-flavanol cocoa (49). Cocoa's effects on insulin resistance may be dependent on its continual consumption over a long period of time. In one study, daily cocoa (900 mg flavanols) ingestion for 2 weeks was not sufficient to improve measures of insulin resistance, though it did improve insulin-mediated vasodilation (178). Almoosawi et al. found in their study, that fasting capillary whole blood glucose was significantly reduced after 2 weeks of daily dark chocolate (500 or 1000 mg polyphenols) consumption (6).
The evidence from these studies suggests that cocoa may be useful in ameliorating insulin resistance in metabolic syndrome and slowing the progression to type 2 diabetes. Results from other studies indicate that cocoa may also have therapeutic potential in preventing cardiovascular complications in diabetic patients. In one such study, flavanol-rich cocoa consumption three times daily for 30 days increased flow-mediated dilatation by 30% in medicated diabetics. Further, acute improvement in FMD after a single dose of cocoa was found to be dose-dependent (17). Although studies in humans with diabetes are lacking, a number of animal studies support beneficial effects of cocoa on glucose control (115, 213). Additional studies to confirm these effects in humans are needed, though there is biologic plausibility and preliminary evidence for an insulin-sensitizing effect of cocoa.
In summary, there are plausible mechanisms for the antioxidant effects of cocoa polyphenols to directly influence insulin resistance and, in turn, reduce risk for diabetes. Cocoa may induce pancreatic β-cell regeneration and stimulate insulin secretion, have a hypoglycemic effect, and improve glucose tolerance. The vasodilatory effects of cocoa can also improve insulin sensitivity mediated by endothelial function. Sustained consumption of cocoa over long periods may affect insulin resistance to a greater degree than single doses of cocoa products.
V. Effects on Immune Function and Carcinogenesis
There are no clinical trials documenting immune effects of cocoa or cocoa extracts in humans. However, preclinical evidence in mice and rats demonstrates that cocoa may exhibit some immunomodulatory effects. Ramiro-Puig et al. (202) demonstrated that a diet consisting of 10% cocoa can enhance antioxidant defenses in the thymus and influences differentiation of thymocytes. Other studies have found that 3 weeks of a 10% cocoa diet increases the percentage of B cells and decreases the percentage of T-helper cells in the spleen of young rats (200, 201). Further, gut-associated lymphoid tissue is also affected by cocoa intake—specifically, Peyer's patches and mesenteric lymph nodes that show changes in lymphocyte composition and T-helper cell percentages. It is believed that flavonoids most likely represent the active ingredients responsible for immunomodulatory effects (127).
In vitro, cocoa has been found to reduce secretion of TNF-α, monocyte chemoattractant protein-1, and NO by lipopolysaccharide-stimulated macrophages (127), with differential effects, based on fraction size, on IL-5 secretion in peripheral blood mononuclear cells (152). Cocoa procyanidins have also been shown to affect signaling pathways of polymorphonuclear cells, white blood cells involved in inflammation and injury (127). Kenny et al. demonstrated that flavanol fractions can enhance secretion of the cytokines TNF-α, IL-1, IL-6, and IL-10 from stimulated human peripheral blood mononuclear cells (126). Cocoa procyanidins decrease IL-2 at the transcriptional level. Ramiro et al. demonstrated that IL-4 release can be enhanced in the presence of cocoa flavonoids, thus downregulating T-lymphocyte activation and the acquired immune response (199).
Chronic inflammation and oxidative stress are significant contributing factors to carcinogenesis. Reactive oxygen (ROI) and nitrogen intermediates (RNI) can damage DNA or interfere with DNA repair, leading to mutations necessary for cells to avoid controls on growth and replication. Inflammation increases the production of ROI and RNI stimulated by cytokines and chemokines, and angiogenesis (69). Cocoa constituents, such as procyanidins and catechins, influence immune responses by modulating activation of the transcription factor NF-kB, involved in inflammatory responses, cellular proliferation, cell adhesion, and regulating cytokine production (149).
The antioxidant activity of cocoa flavanols is of particular interest for its potential influence on the initiation stage of carcinogenesis. In a study examining the radical scavenging capacity of 10 beverages containing antioxidants, cocoa mix ranked fifth, behind coffee, prune juice, and green tea, but ahead of grape juice and other types of tea (137). In another study, dark chocolate consumption significantly improved DNA resistance to oxidative stress in the short-term. In this study, Spadafranca et al. (239) assigned 10 healthy subjects to consume 45 g of dark chocolate or white chocolate for 14 days. Oxidative damage to DNA was reduced in the dark chocolate group 2 h after consumption; 22 h later the effects were not seen (239).
It has been suggested that foods containing antioxidant polyphenols, including cocoa, may have anticancer properties (271). In particular, the polyphenols epicatechin gallate and EGCG in green tea appear to have significant anticancer effects (38, 197, 283). Intake of soy, an important source of the isoflavone genistein, has been associated with up to 49% reduced risk of endometrial and ovarian cancers in women (182). Epidemiologic studies of cocoa intake and cancer risk are few, and those assessing overall mortality provide only weak support for a benefit of cocoa. However, human intervention trials indicate that cocoa favorably affects intermediary factors in cancer progression—specifically, markers of antioxidant status (155). In vitro and ex vivo studies support an anti-inflammatory effect of cocoa flavanols, but these effects have not been universally replicated in vivo (225). One study, a cross-over feeding trial in men and women at high risk for atherosclerosis, found that cocoa consumption was associated with reduced expression of the adhesion molecules VLA-4, CD40, and CD36 on monocyte surfaces and reduced circulating levels of the inflammatory markers soluble P-selectin and ICAM-1. However, other biomarkers of inflammation, including IL-6 and high sensitivity-CRP, were unaffected (172). The observed decrease in adhesion molecules is consistent with a previous cocoa study in postmenopausal hypercholesterolemic women (269).
Cocoa polyphenols have also been found to inhibit the mutagenic activity of heterocyclic amines in vitro and ex vivo (157). Kim et al. found that a cocoa polyphenol extract inhibited TNF-α (a pro-inflammatory cytokine involved in the pathogenesis of cancer) as well as VEGF expression in JB6 mouse epidermal cells. This extract reduced TNF-α-induced upregulation of VEGF by directly inhibiting phosphoinositide 3-kinase and mitogen-activated protein kinase kinase-1 activities (132).
There is growing evidence that polyphenols may play a role in regulating apoptosis (203). Apoptosis may be triggered intrinsically, through the mitochondrial pathway (118) or extrinsically by death ligands and receptors (99). It is the external pathway that may potentially be modulated by bioactive food components. Numerous dietary agents have been found to induce apoptosis in cancer cells in vitro; they include compounds in green tea, cruciferous vegetables, red pepper, grapes, and many other fruits, vegetables, herbs, and spices (130). Flavanols found in cocoa have also exhibited pro-apoptotic effects. Proanthocyanadins inhibited growth of human lung cancer cells in vitro and in vivo (230), and epicatechin synergistically enhanced apoptosis in lung cancer cells treated with EGCG (252). Phenol-rich cocoa extracts were found to prevent apoptosis in MLP29 liver cells induced by Celecoxib, suggesting that upstream components of the apoptotic pathway (such as the Bax gene) are targets of cocoa phytochemicals (11).
In summary, cocoa exhibits regulatory effects on immune cells involved in innate and acquired immunity. Cocoa is a rich source of flavonoid antioxidants, which might promote changes in redox-sensitive signaling pathways involved in the expression of many genes and consequently in several cell functions, such as the immune response. Procyanidins and catechins may prevent inflammatory and oxidative damage to DNA and thus affect carcinogenesis. No human trials demonstrate benefit in cancer treatment, though cocoa has consistently demonstrated an ability to increase serum antioxidant status, and enhance apoptosis of cancer cells, thus theoretically reducing cancer risk.
VI. Effects on the Central Nervous System
Flavonoids in cocoa have demonstrated a variety of effects in central nervous processes, and there is promising preliminary evidence for protection from neurodegradation, increased perfusion, decreased neuroinflammation, and modulation of neuronal function through interaction with a number of signaling pathways.
The pathogenesis of Parkinson's disease involves the death of neurons in the substantia nigra. This process is believed to be mediated by the formation of the endogenous neurotoxin 5-S-cysteinyl-dopamine (5-S-cys-DA) and its oxidation product, dihydrobenzothiazine, that may be produced through a mechanism involving ROS (242). Neuronal injury by 5-S-cys-DA is attenuated by a number of derivatives of catechin and epicatechin, including quercetin, hesperetin, and caffeic acid (242).
Neuroinflammation is involved in the pathogenesis of Parkinson's disease, Alzheimer's disease, and neuronal injury associated with stroke (111). Chronic responses in glial cells, occupying the majority of brain volume (133), can lead to progressive neuronal degeneration (5). Flavonoids may modulate this response; quercetin structurally resembles a number of kinase inhibitors that have anti-inflammatory effects in glial cells (240). Further, flavonoids interact with a variety of neuronal protein kinase and lipid kinase signaling cascades (240), and can possibly prevent excitotoxic death in neurons (122).
The consumption of flavonoid-enriched cocoa affects cerebral blood flow; functional magnetic resonance imaging studies demonstrate increased blood flow to gray matter 3 h after consuming cocoa as well as other changes to regional blood flow (77). Increased blood flow to the cerebral gray matter induces angiogenesis and new nerve cell growth in the hippocampus, a key region involved in the processing of memory (242). Increased blood flow velocity in the middle cerebral artery has been demonstrated, providing insights to possible protective effects against dementia and stroke (238).
Epicatechin induces both extracellular signal regulated kinase and cyclic AMP response element binding protein (CREB) activation in cortical neurons increasing CREB-regulated gene expression (221) involved in the formation of long-term memory (24). Flavonoids and their metabolites may interact within MAPK signaling pathways involved in mitogenesis, differentiation, apoptosis, and various forms of cellular plasticity (241), as well as, serine/threonine kinase, one of the key downstream effectors of phosphatidylinositol-3 kinase involved in neuronal survival (243).
In summation, the antioxidant properties of catechin and epicatechin derivatives can protect from neuronal injury and neuroinflammation implicated in the pathogenesis a number of neurological conditions. Cocoa can also increase cerebral blood flow, which can be neuroprotective. Further, epicatechin is involved in the processes of formation of long-term memory. These results are preliminary; clinical trials are necessary to confirm cocoa's neuroprotective effects in humans.
VII. Effects on Skin
Cocoa butter is a common ingredient in skin moisturizers, but cocoa's beneficial effects on skin may extend beyond its use as a topical agent. A number of studies have identified a role of cocoa flavanols in protecting skin from damage from UV light, believed to be mediated through factors, such as generation of ROS and inflammatory markers such as prostagladins, NO, leukotrienes, and histamine (276). Twelve weeks of high-flavanol cocoa consumption decreased erythema induced by UV light by 25% in one study (91) and specially produced high-flavanol chocolate (>600 mg flavanols and >10 000 ORAC units per 20 g portion) more than doubled the dose of UV light necessary to produce erythema in another. However, commercially available chocolate did not have this effect (276). The antioxidant actions of cocoa flavanols are one possible mechanism by which skin protection could be conferred. There is also some evidence that their vasodilatory effects may be important in nutrient delivery, and thermoregulation is dependent on cutaneous microcirculation (22). In support of this hypothesis is a study that found that consumption of high-flavanol cocoa for 12 weeks increased dermal blood flow and oxygen saturation by 70% and 80%, respectively (187). Though the volume of research in this area is limited, there is reason to believe that skin protection may be another possible health benefit of cocoa.
VIII. Effects on Obesity
Although chocolate may be perceived as an indulgent and fattening food because of its energy density, randomized trials have not found an increase in weight after sustained consumption of small amounts of cocoa (17, 49) or dark chocolate (256). In one study, a daily dose of 25 g (125 kcal) of dark chocolate slightly increased body weight after 3 months, but a 6 g dose (30 kcal) was not associated with any weight change. In both doses, the 24-h mean ambulatory BP decreased (55).
Nitric oxide (NO) has been shown to increase uptake of glucose, increase oxidation of fatty acids and glucose, inhibit fat synthesis, and enhance lipolysis in adipose tissue (120). Cocoa has been found to decrease visceral adipose tissue in rats, possibly by changing the expression of genes for enzymes and transport molecules involved in fatty acid synthesis and thermogenesis in liver and white adipose tissue (157).
Massolt and colleagues (156) demonstrated that the smell of chocolate could suppress appetite in humans. In their study, 12 females were given chocolate to eat and then randomized to either smell chocolate or to serve as a control (no eating or smelling). At the start of these sessions, insulin, glucagon-like peptide-1 (GLP-1), and cholecystokinin (CCK), but not glucose, were found to correlate with appetite levels. They also found that ghrelin levels correlated inversely with appetite. Chocolate eating and smelling both resulted in appetite suppression with no relationship seen between appetite levels and insulin, GLP-1, or CCK levels. Of note, the smell of dark chocolate (85% cocoa) resulted in a satiation response that was inversely correlated with ghrelin levels. As higher levels of ghrelin increase food intake and increase fat mass (257), these provocative findings suggest that regular cocoa and chocolate consumption may reduce appetite and possibly weight gain.
Although research to date has not specifically addressed whether cocoa may actually reduce adiposity, there is reason to believe that cocoa consumption may induce favorable metabolic changes through its effects on NO availability or lipid metabolism. The olfactory properties of cocoa may increase satiety and thus reduce appetite. Given the potential beneficial effects of cocoa on conditions frequently associated with obesity and the negligible risk of weight gain associated with effective doses of chocolate, moderate chocolate consumption appears to have a favorable risk/benefit profile for obese individuals.
IX. Psychoactive Effects
A. Chocolate craving
In addition to the wide range of somatic effects of cocoa, possible psychological effects on mood, cravings, and cognitive function have also been suggested. Much of the research on cocoa's effects on psychological variables has focused on explaining chocolate cravings, likely because chocolate is such a widely craved food. In one study, chocolate accounted for 49% of all food cravings reported by a sample of 25 healthy women (101). Greater chocolate craving has been reported among women than among men (193, 212, 289) particularly during the perimenstrual period (27, 212, 288), and a significant but small decline in cravings may occur after menopause (108). However, this association may be at least partially explained by cultural factors, as suggested by the work of Zellner et al. (288). They found a large and significant difference between the proportions of American and Spanish women reporting perimenstrual chocolate cravings when asked an open-ended question about timing of cravings (40% vs. 4%) or when asked directly if they experienced perimenstrual cravings (60% vs. 24%).
Cravings or increases in chocolate consumption also appear to be associated with negative mood (208). In a cross-sectional study, chocolate consumption was associated with higher depression scores (indicating more depressive symptoms), but whether the relationship is a causal one is still unclear (208). Further, individuals classified as “emotional eaters” have reported greater craving for and consumption of chocolate (148). Willner and colleagues investigated the relationship between depressed mood and chocolate craving in an experimental study (278). In this trial, 120 female college students who liked chocolate were randomly assigned to hear music intended to induce either elated or depressed mood. Before and after listening to the music, subjects completed questionnaires measuring mood and attitudes to chocolate. They were then given the opportunity to complete a computer task to earn reinforcers of chocolate or carob. Afterward, subjects completed the questionnaires once more. Subjects in the depressed condition rated their craving for chocolate significantly higher than those in the elated condition and provided significantly more responses in the computer task to receive more reinforcers when chocolate, but not carob was given. The results of this study suggest that depressed mood may increase craving for chocolate. However, the reason that chocolate cravings occur during times of emotional distress is still not clear (278).
Parker et al. reviewed the psychoactive properties and mood effects of cocoa in 2006, with some noteworthy conclusions (195). They contend that a psychoactive component of cocoa is not likely to be the cause of chocolate cravings because milk chocolate, which contains a lower proportion of cocoa solids than dark chocolate or cocoa powder, is typically preferred. Rather, the unique orosensory properties of chocolate, which are responsible for its pleasurable taste, are the most likely explanation (195).
The findings of Rozin and colleagues provide strong evidence that it is not the bioactive compounds in cocoa solids, but rather the particular taste and mouth-feel of solid chocolate products that satisfy chocolate cravings (167). In their 1993 study, a total of 34 individuals who reported experiencing chocolate cravings at least once per week were given six sequentially numbered opaque boxes and instructed to open one at each occasion of a craving, in order. The boxes contained the following treatments, in random order: (i) a 44 g milk chocolate bar; (ii) six opaque capsules containing 3.8 g cocoa powder; (iii) six opaque capsules containing 4.5g white flour; (iv) 42.7 g white chocolate; (v) 39.9 g white chocolate plus six cocoa-containing capsules; and (vi) nothing. The boxes also contained craving rating scales, which the subjects were asked to complete before, immediately after, and 90 min after consuming the given treatment. The doses of the treatments were varied according to reported usual quantity of chocolate intake. Subjects who reported typically eating one chocolate bar when they experienced a craving received the doses described above, and those who reported eating more than one chocolate bar to satisfy a craving received double the amounts for all treatments. Milk chocolate was associated with the greatest reduction in craving (61 points on the 70-point scale), whereas white chocolate and white chocolate plus cocoa capsules were associated with significantly smaller decreases (42 and 43 points, respectively). The cocoa capsules had only a minimal decrease (16 points), which was similar to the reductions observed after consumption of flour capsules and no treatment. The results of this study strongly suggest that sensory properties are responsible for the satiation of chocolate cravings.
B. Mood and cognitive function
Two studies by Macht have also supported the view that chocolate's psychoactive properties are solely taste-related (146, 147). These studies found that chocolate consumption can improve a negative mood state in the short-term, but that the improvement is likely attributable to chocolate's palatability (146, 147). In Macht's 2007 study, ingestion of a palatable chocolate improved mood significantly more than a nonpalatable chocolate, whose effects were not significantly different from the control condition (nothing eaten) (147). In a 2006 study, Macht found that a chocolate bar elevated mood and elicited joy to a greater extent than an apple, but these effects were most pronounced at 5 and 30 min after consumption. This suggests that the sensory experience of eating chocolate improved mood, rather than neurochemical effects, which would be expected to manifest later (146).
On the other hand, there is some evidence to support a psychoactive effect of flavanols or methylxanthine compounds in cocoa—effects that may extend beyond mood to cognitive function. Scholey and colleagues conducted a randomized, controlled, double-blind crossover trial in 30 healthy adults to investigate the effects of cocoa flavanol ingestion on cognitive performance, state anxiety, and mental fatigue (218). The three treatments tested were cocoa beverages containing 46 mg (control), 520 mg, and 994 mg of total flavanols. Both of the flavanol-rich preparations significantly increased cognitive performance and reduced mental fatigue relative to the control beverage. Smit et al. looked to cocoa's methylxanthine compounds, caffeine and theobromine, as potential mood-altering agents (236) in two studies. The first was designed to compare the effects on mood, alertness, and cognitive performance of encapsulated cocoa powder (CP) equivalent to a 50 g chocolate bar, isolated caffeine and theobromine (CA+TB) equivalent to a 50 g chocolate bar, and a placebo. In a second study, the treatments were 60 g portions of chocolate prepared to look identical, but contain differing amounts of methylxanthines (zero, low-, and high-methylxanthines [MX]). Cocoa powder and CA+TB both significantly decreased reaction time and increased energetic arousal compared to placebo. CA+TB also significantly increased scores on a rapid visual information processing (RVIP) task and increased hedonic tone (a measure of positive mood) compared to placebo, whereas CP was associated with a nonsignificant increase. The chocolate treatments of varying methylxanthine levels produced similar results. High MX chocolate significantly decreased reaction time, whereas both low and high MX chocolates increased RVIP scores relative to placebo. Both MX chocolates increased energetic arousal and hedonic tone more than placebo, but these differences were not significant. In a follow-up study, fondness for a novel drink increased over repeated exposures when paired with theobromine and caffeine, but not when paired with a placebo, suggesting that the methylxanthines in cocoa may also contribute to partiality for chocolate (235). In all three of these studies, the effect of chocolate's orosensory characteristics was controlled for.
In a randomized, double-blind, placebo-controlled trial that investigated the effects of cocoa and dark chocolate on BP, blood lipids, and C-reactive protein (all null), Crews and colleagues also assessed neuropsychological functioning in a healthy, older (≥60 years) population (45). Ninety participants completed the trial, which was 6 weeks in duration. During the intervention period, subjects consumed either a polyphenol-rich artificially sweetened cocoa beverage and a dark chocolate bar (754.71 mg total proanthocyanidins) or nutrient-matched placebo cocoa and chocolate daily. Eight neuropsychological tests were administered as was a self-report questionnaire to assess subjects' perceptions of changes in their cognitive functioning. There were no significant differences in any of the cognitive test measures between the intervention and placebo groups.
Martin et al. conducted a comprehensive assessment of the metabolic impact of dark chocolate consumption in 30 subjects who consumed 40 g of dark chocolate daily for 2 weeks (154). They found that dark chocolate had significant effects only in subjects who reported higher anxiety at the beginning of the study. In these individuals, chocolate consumption reduced urine cortisol and catecholamines and partially normalized other metabolic parameters associated with the high-anxiety trait.
More research is needed to make sense of the inconsistencies in the current literature on cocoa's psychoactive effects. Nevertheless, cocoa products are unique among potentially health-promoting foods, and they are generally considered pleasurable and may positively affect mood. Currently, research suggests that any mood improvement is likely short-lived and due to chocolate's palatability, rather than its flavanol content, concentration of methylxanthines, or other bioactive compound.
X. Bioavailability of Cocoa Polyphenols
A. Metabolism of cocoa polyphenols
Because little is known about the absorption and metabolism of polyphenols, there is increasing interest and much speculation in this area (46, 150, 234). Cocoa has a high concentration of proanthocyanidins, which is large relative to that of other polyphenols (150) and can be detected in serum 30 min after consumption of cocoa (106). A dose–response effect has been shown between cocoa intake and serum epicatechin levels where 100 mg catechins increased serum levels increased by 200 nM 2 h after consuming cocoa products (151). Wang et al. found a similar dose–response effect in plasma epicatechin associated with dose–response increases in plasma antioxidant capacity and decreases in plasma lipid oxidation products (268).
Some studies have indicated that oligomers larger than trimers may not be able to be absorbed in the small intestine (16, 54, 62). Although it is possible that procyanidin oligomers are broken down into monomers and dimers, which may be absorbed, this does not appear to occur in the stomach (207). Therefore, the effects of proanthocyanidins in cocoa on plasma concentrations are much lower than those of epicatechin, the predominant monomeric flavanol in cocoa (106). However, the antioxidant activity of proanthocyanidins may be beneficial in the colon (150).
Urpi-Sarda et al. found that epicatechin metabolites from cocoa accumulated in the thymus, testicles, liver, lymph nodes, and spleen in Wistar rats fed a high cocoa diet compared to control rats, suggesting that these sites are important sites of oxidation (264).
B. Effects of food processing on flavonoids
The caveat of consuming chocolate for its health benefits is the requirement that the chocolate or cocoa contains an effective dose of the active components. Flavonoid content varies by the proportion of cocoa liquor present in different types of chocolate (white, milk, and dark), but even within categories there may be significant variation due to differences in cocoa bean processing (Table 2). For example, cocoa liquor that has undergone more fermentation has higher concentrations of flavonoids (164). The extent to which cocoa beans are roasted also has a substantial impact on the antioxidant capacity of the cocoa's phenolic compounds; longer roasting time is associated with reduced antioxidant activity (191). However, radical-scavenging Maillard reaction products such as melanoidins are also produced during roasting, partially replacing the lost antioxidant activity of cocoa polyphenols (191).
Variation in antioxidant capacity can also be seen in cocoa powder (169). Cocoa powder is often treated with alkali to neutralize the natural acetic acid in cocoa in a process called “Dutching.” This process substantially reduces the flavanol content of the cocoa. In one study of commercially available cocoa powders, the flavanol content of heavily alkalized cocoas was 78.5% lower than that of natural cocoas (Fig. 9) (164, 169). With the exception of products that have undergone Dutching, most cocoa and chocolate products will contain amounts of polyphenols that are reflective of the proportion of nonfat cocoa solids present (170).
FIG. 9.
Average flavanol content of natural cocoa powders and cocoa powders undergoing light, medium, or heavy alkalization. Reprinted with permission from Miller et al. (169).
With the assurance that a particular cocoa product contains a biologically relevant dose of flavanols, concern about the bioavailability may remain. The addition of milk, sugar, and other substances to cocoa powder or cocoa liquor in the production of commercial cocoa drink mixes and chocolate candy may impact the bioavailability of cocoa flavanols if not their concentration. A number of studies have sought to determine whether milk affects flavanol metabolism, with somewhat mixed results (129, 176, 210, 211, 227). In 2003, Serafini and colleagues found that consumption of dark chocolate with 200 ml of milk attenuated the increase in plasma antioxidant capacity observed after consumption of dark chocolate alone (227). They hypothesized that proteins in the milk may bind to cocoa flavanols, reducing their absorption. This study was followed by a string of other studies, which found no effect of milk on the bioavailability of cocoa flavanols (129, 210, 211). In a 2009 study, however, consumption of 250 ml of commercial cocoa with milk was associated with reduced urinary epicatechin metabolites, though plasma metabolites were not substantially affected (176). Cooper et al. found that (−)-catechin is the most affected by manufacturing conditions and cocoa origin compared to other cocoa polyphenols (40). Belscak-Cvitanović et al. found that cocoa powder mixtures containing lower fat (10%–12%) had higher total polyphenol, total flavonoid, flavan-3-ol, and proanthocyanidin contents compared to cocoa prepared from higher fat content (16%–18%) cocoa powder (20).
The impact of sugar on the biological effects of cocoa has not been investigated to a great extent. Studies in humans (219) and rats (186) indicate that the consumption of cocoa with carbohydrates, including sugar, may actually increase the bioavailability of cocoa flavanols. Whether the potential negative effects of over-consumption of sugar—namely, endothelial dysfunction (140), insulin resistance, and weight gain (109)—negate any benefit of increased plasma flavanol concentrations is not yet understood. In one trial, a sugar-free cocoa beverage produced greater improvements in BP and endothelial function than a sugar-sweetened cocoa; however, the differences were not statistically significant (188). If artificially sweetened cocoa is determined to have greater benefit than cocoa with sugar, palatability of such products will be an important consideration. Interestingly, subjects in another study reported preferring the taste of cocoa mixtures sweetened with nonnutritive sweeteners (aspartame/acesulfame K and stevia extract) to those sweetened with sugar or glucose, suggesting that sugar-free cocoas are acceptable to consumers (20).
Concurrent milk consumption can affect the metabolism of phenolic acid constituents of cocoa. Urpi-Sarda and colleagues found that urinary excretion of vanillic acid and phenylacetic acid increased after milk and cocoa were consumed together, whereas urinary excretion of other components (3,4-dihydroxyphenylacetic acid, protocatechuic acid, 4-hydroxybenzoic acid, 4-hydroxyhippuric acid, and hippuric acid) was reduced (263).
Often cocoa products are not consumed as purchased, but rather used to make other foods, beverages, or confections. In one study, polyphenol content of cocoa powder was not substantially affected by preparation techniques used to make chocolate frosting, hot cocoa beverage, or chocolate cookies; however, after undergoing baking in a chocolate cake, natural cocoa powder retained only 5% of its epicatechin content and 54% of its antioxidant activity measured by ORAC. The reason for this is likely the increase in pH caused by the addition of baking soda (244).
XI. Detrimental Health Effects
Chocolate has been implicated in conditions, such as acne (47) and heart burn or gastroesophageal reflux disease (GERD) (179, 282). There is also a common perception that chocolate can be a trigger for migraines (279). As discussed elsewhere in this review, weight gain is also a concern when energy-dense solid chocolate is consumed regularly.
Some population-based studies suggest that dairy intake may be positively associated with acne, and clinical trials indicate that a high glycemic index or glycemic load diet may also play a role in acne severity and duration (25, 70). Currently, evidence does not support a link between chocolate or any other specific food and acne, though few well-designed studies have been undertaken (47).
The American College of Gastroenterology names chocolate as one of the foods that may contribute to GERD symptoms, though it acknowledges a lack of rigorous testing of this hypothesis (58). Chocolate has been found to reduce lower esophageal sphincter pressure (179, 282). Yet, no studies have investigated whether eliminating chocolate from the diet can improve symptoms. There is currently not sufficient evidence to suggest that dietary modifications of any kind can improve GERD symptoms or pathological measures (123). Interestingly, one study found that chocolate was the most often cited constipation-causing food among patients with constipation-predominant irritable bowel syndrome (177), but no other studies have investigated this reported effect.
It has been hypothesized that phenylethylamine, present in chocolate, could provoke a migraine (160, 161). The perceived association between chocolate and migraines appears to be a widely held misconception. In one study investigating trigger factors of migraines, the proportion of patients who reported having heard that chocolate could be a migraine trigger (61.7%) was significantly and substantially greater than the proportion who identified chocolate as a trigger based on their own experience (14.3%, p<0.001) (279). Although one small study in 1991 found that chocolate ingestion was nearly significantly associated with migraine compared with placebo (p=0.051) (82), one trial conclusively found no association (153).
The majority of the research discussed in this review has discussed protective or health-promoting effects of cocoa or chocolate consumption. It is certainly biologically plausible for cocoa to cause detrimental effects on human health. Chocolate consumption may exacerbate symptoms of GERD or trigger migraine headaches in sensitive persons, though human trials establishing a causal relationship are scant and inconclusive. The real possibility of weight gain when overindulging in chocolate or cocoa-based foods is real, but certainly not attributable to the cocoa content.
XII. Conclusions and Implications
Cacao, the quintessential ingredient in all true chocolate and cocoa products, is a highly complex food source. As this review indicates, cacao imparts to chocolate a rich endowment of nutritional properties, from minerals, to antioxidants, to vasoactive, and even psychoactive compounds.
The tendency in Western science when a food appears to have specific health effects is to seek the active ingredient. Indeed, specific effects of chocolate may be attributable to specific constituents, such as flavonoids. However, just as the active ingredient making a food such as spinach or broccoli highly nutritious may be nothing less than spinach or broccoli, the same may be true of chocolate. The active ingredient in chocolate may be chocolate, or at least cacao, with overall health effects representing at least the sum of diverse parts.
There is, however, much interest in, and potential gain from, elucidating those parts and their mechanisms of action. Table 6 begins to suggest an array of mechanistic pathways, enumerating various constituents of chocolate, and their actions at diverse sites. Numerous cytokines and enzyme systems are influenced by various polyphenols in chocolate. Some of these pathways clearly involve direct antioxidant effects, whereas others likely do not. Among the unknowns is which of the many polyphenolic compounds in chocolate exert specific effects, and this area invites further investigation.
Table 6.
Effects of Cocoa Polyphenols on Enzyme Systems and Molecular Targets
| Compound | Target | Function | Reference |
|---|---|---|---|
| Catechin | ↓ a-glucosidase | Catalyze hydrolysis of polysaccharides | 114 |
| Epicatechin | ↓ Xanthene oxidase | Catalyzes the oxidation of hypoxanthine to xanthine, generates ROS | 64, 222 |
| Epicatechin | ↓ NADPH oxidase | Scavenge NO | 216, 247 |
| Epicatechin | ↑ cGMP | Activates intracellular protein kinases | 216 |
| Epicatechin | ↑ endothelial NO• synthase (eNOS) | Vasodilating factor | 216, 222, 277 |
| Epicatechin | ↑ extracellular signal regulated kinase and cyclic AMP response element binding protein activation in cortical neurons | Involved in long-term memory formation | 221 |
| Epicatechin | ↓ plasma levels of endothelin-1 | Constrict blood vessels; raise blood pressure | 144 |
| Procyanidins | ↓ lipoxygenases | Arachidonic acid metabolism and the biosynthesis of leukotrienes (inflammatory pathways) | 220, 233 |
| Procyandins | ↑ IL-2 transcription | Cytokine immune system signaling molecule | 199 |
| Quercetin, hesperetin, and caffeic acid | ↓ 5-S-cysteinyl-dopamine and its oxidation product, dihydrobenzothiazine | Endogenous neurotoxin | 242 |
| Cocoa polyphenols (unspecified) | ↑ prostacyclin | Vasodilating factor | 217, 250 |
| Cocoa polyphenols (unspecified) | ↑ endothelium-derived hyperpolarizing factor | Vasodilating factor | 217, 250 |
| Cocoa polyphenols (unspecified) | ↓ endothelin-1 | Angiogenic factor | 217, 250 |
| Cocoa polyphenols (unspecified) | ↓ VEGF | Angiogenic factor | 217, 250 |
| Cocoa polyphenols (unspecified) | ↓ NF–κB activity | DNA transcription; factor in inflammatory processes | 149, 226 |
| Cocoa polyphenols (unspecified) | ↓ angiotensin-converting enzyme | Regulates renin–angiotensin system | 2, 3 |
| Cocoa polyphenols (unspecified) | ↓ 8-isoprostane | Biomarker of oxidative stress | 115 |
| Cocoa polyphenols (unspecified) | ↓ monocyte chemoattractant protein-1 | Recruitment of monocytes and lymphocytes to sites of cellular immune reactions | 127 |
| Cocoa polyphenols (unspecified) | ↓ TNF-α in lipopolysaccharide-stimulated macrophages | Cytokine involved in systemic inflammation | 127 |
| Cocoa polyphenols (unspecified) | ↑ IL-1 secretion from stimulated human peripheral blood mononuclear cells | Cytokine, involved in regulation of immune responses | 126 |
| Cocoa polyphenols (unspecified) | ↑ IL-6 secretion from stimulated human peripheral blood mononuclear cells | Pro-inflammatory and anti-inflammatory cytokine | 126 |
| Cocoa polyphenols (unspecified) | ↑ IL-10 secretion from stimulated human peripheral blood mononuclear cells | Anti-inflammatory cytokine | 126 |
| Cocoa polyphenols (unspecified) | ↑ IL-4 release | Acquired immune response | 199 |
| Cocoa polyphenols (unspecified) | ↓ expression of CD40 on monocyte surfaces | Adhesion molecule | 172 |
| Cocoa polyphenols (unspecified) | ↓ expression of VLA-4 on monocyte surfaces | Adhesion molecule | 172 |
| Cocoa polyphenols (unspecified) | ↓ expression of CD36 on monocyte surfaces | Adhesion molecule | 172 |
| Cocoa polyphenols (unspecified) | ↓ circulating levels of P-selectin | Inflammatory marker | |
| Cocoa polyphenols (unspecified) | ↓ circulating levels of ICAM-1 | Inflammatory marker | |
| Cocoa polyphenols (unspecified) | ↓ phosphoinositide 3-kinase | Upregulates VEGF, mediated through TNF-α | 132 |
| Cocoa polyphenols (unspecified) | ↓ mitogen-activated protein kinase kinase-1 | Upregulates VEGF, mediated through TNF-α | 132 |
| Cocoa polyphenols (unspecified) | serine/threonine kinase | Involved in neuronal survival | 243 |
VEGF, vascular endothelial growth factor.
The implications of this review—an indication of both how much is known, and how much is unknown about specific metabolic and molecular effects of chocolate—are apt to vary with professional orientation and perspective. For the public health nutritionist, the operative questions are: is there a benefit, of what kind, and for whom? There is a strong body of accumulating evidence pointing to a net health benefit from routine consumption of dark chocolate, but further epidemiologic study before such evidence is widely acknowledged as truly decisive.
For the clinician/nutritionist dealing with patients individually, the operative questions are apt to be: what dose do I prescribe, what frequency, and for whom? The notion that chocolate can serve, quite literally, as “medicine” has been espoused in the peer-reviewed literature (78). Before such an assertion can migrate from postulate to practice, however, the prescriptive details are required. Short-term intervention studies utilizing such outcomes as endothelial function as a proxy for likely effects on cardiovascular endpoints are eminently feasible, and needed to clarify how the potentially medicinal effects of dark chocolate might best be translated into clinical counseling.
For the nutritional biochemist, a likely focus is: what are the specific constituents of chocolate responsible for observed effects in vivo and in vitro? As suggested by Table 6, diverse constituents of chocolate are known to exert a wide array of metabolic effects of potential clinical importance. Among the compounds in chocolate with promising effects, epicatechin stands out to some degree. Although molecular, metabolic, and overall medical effects of chocolate may owe something more to the whole than to any given part, food science would, nonetheless, benefit from further study of specific parts, or compounds, both in isolation and in various combination. Insights generated might have implications for fortification and functional foods, and help food scientists answer the questions most germane to their efforts: what additions, deletions, and/or modifications enhance or attenuate favorable health effects of chocolate?
Finally, for molecular biologists, and perhaps the dedicated readers of this journal, the focus may be less on the active compounds in chocolate, than on their actions. Namely, a question of general interest is apt to be: what are the mechanistic pathways involving oxidation and reduction that mediate effects of chocolate? As noted (see pp. 9–11), the means by which chocolate ingestion results in even as well-studied an effect as enhanced endothelial function is far from certain. Polyphenols, and in particular epicatechin, are known to reduce levels of endothelin, and increase levels of nitric oxide. However, these compounds also influence gene expression, and may help preserve eNOS. Which of these effects predominates, and which has the most direct clinical significance is unknown. Molecular and genetic studies are required to elucidate further the mechanisms of chocolate's diverse metabolic effects.
In general, this review invites the question: is dark chocolate legitimately considered a “health food”? The weight of evidence reviewed here would seem to support a qualified “yes.” The qualification relates both to what is known, and what is not. What is known is that chocolate, unlike spinach, carries with it the liabilities of processing, added ingredients (notably sugar), and energy density. In Western societies prone to obesity, a concentrated source of calories represents at least a potential health threat.
Thus, the benefits of chocolate consumption must be weighed against the at-least theoretical risk of weight gain. Little actual evidence of that harm exists, however, at least for dark chocolate per se.
What is not known is how best to make use of chocolate for purposes of health promotion per se. While specific quantities of fruits and vegetables may be recommended, and even alcohol prescribed with some degree of confidence, a health-enhancing dose of chocolate remains a matter of conjecture. How much of what kind of chocolate is ideal for overall health? What frequency is optimal? Are there populations in which chocolate is of particular benefit, and populations in which it is less so- or even detrimental? The differential effects of chocolate observed in those with and without established coronary disease is an area clearly warranting further examination. Perhaps constituents of chocolate can help forestall, but not treat, coronary atherosclerosis. This is as yet unknown.
What is known, however, is that apparent health benefits of chocolate consumption relate to diverse constituents of chocolate and pertain to a wide array of health outcomes. While the case is strongest for cardiovascular benefits, the immunomodulating effects of chocolate hint at potential benefits in both infectious disease and cancer. Metabolic and psychological benefits are also strongly suggested, and mechanistically plausible.
The voluminous literature addressing health effects of cocoa and chocolate, only a portion of which is represented here, is overall quite compelling. The probability of a net health benefit for most people from habitual intake of dark chocolate is high. The translation of this general theme in the literature into specific public health guidance would seem the next great challenge for the research community. The recommendations, of course, must consider the potential for weight gain and increased cardiovascular risk by consuming chocolate products containing high amounts of sugar, possibly countering some of the documented benefits of cocoa. If a dose range for alcohol intake can be established, doing so for chocolate would seem perfectly feasible. Food can be medicine, and chocolate may be a noteworthy example. However, potential in this area will not be fully realized until or unless parameters for prescription are established.
Abbreviations Used
- BP
blood pressure
- CAC
circulating angiogenic cell
- CAD
coronary artery disease
- CCK
cholecystokinin
- CFVR
coronary flow velocity reserve
- CP
cocoa powder
- CREB
cyclic AMP response element binding protein
- CRP
C-reactive protein
- EGCG
epigallocatechin-3-gallate
- FMD
flow-mediated dilatation
- GERD
gastroesophageal reflux disease
- GLP-1
glucagons-like peptide-1
- HOMA-IR
homeostasis model insulin resistance
- MX
methylxanthines
- OGTT
oral glucose tolerance test
- PAT
peripheral arterial tonometry
- RDA
recommended dietary allowance
- RNI
reactive nitrogen intermediates
- RNO
nitrosylated and nitrosated species
- ROI
reactive oxygen intermediates
- RVIP
rapid visual information processing
- TB
theobromine
- VEGF
vascular endothelial growth factor
Acknowledgments
The authors thank Lisa Rosenberger, ND, LAc, Aruna Kotla, MD, and Valentine Yanchou Njike, MD, MPH, for technical assistance. This publication was supported by Cooperative Agreement #U48 DP000053 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
References
- 1. This reference has been deleted.
- 2.Actis-Goretta L. Ottaviani JI. Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem. 2006;54:229–234. doi: 10.1021/jf052263o. [DOI] [PubMed] [Google Scholar]
- 3.Actis-Goretta L. Ottaviani JI. Keen CL. Fraga CG. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003;555:597–600. doi: 10.1016/s0014-5793(03)01355-3. [DOI] [PubMed] [Google Scholar]
- 4.Al-Delaimy WK. Rimm EB. Willett WC. Stampfer MJ. Hu FB. Magnesium intake and risk of coronary heart disease among men. J Am Coll Nutr. 2004;23:63–70. doi: 10.1080/07315724.2004.10719344. [DOI] [PubMed] [Google Scholar]
- 5.Allan SM. Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358:1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almoosawi S. Fyfe L. Ho C. Al-Dujaili E. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br J Nutr. 2010;103:842–850. doi: 10.1017/S0007114509992431. [DOI] [PubMed] [Google Scholar]
- 7.Anderson TJ. Uehata A. Gerhard MD. Meredith IT. Knab S. Delagrange D. Lieberman EH. Ganz P. Creager MA. Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 8.Ando K. Matsui H. Fujita M. Fujita T. Protective effect of dietary potassium against cardiovascular damage in salt-sensitive hypertension: possible role of its antioxidant action. Curr Vasc Pharmacol. 2010;8:59–63. doi: 10.2174/157016110790226561. [DOI] [PubMed] [Google Scholar]
- 9.Andrews RK. Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ. Sacks FM. Carey VJ. Obarzanek E. Swain JF. Miller ER., 3rd Conlin PR. Erlinger TP. Rosner BA. Laranjo NM. Charleston J. McCarron P. Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 11.Arlorio M. Bottini C. Travaglia F. Locatelli M. Bordiga M. Coisson JD. Martelli A. Tessitore L. Protective activity of Theobroma cacao L. phenolic extract on AML12 and MLP29 liver cells by preventing apoptosis and inducing autophagy. J Agric Food Chem. 2009;57:10612–10618. doi: 10.1021/jf902419t. [DOI] [PubMed] [Google Scholar]
- 12.Arnal JF. Dinh-Xuan AT. Pueyo M. Darblade B. Rami J. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell Mol Life Sci. 1999;55:1078–1087. doi: 10.1007/s000180050358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arts IC. van de Putte B. Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J Agric Food Chem. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 14.Baba S. Natsume M. Yasuda A. Nakamura Y. Tamura T. Osakabe N. Kanegae M. Kondo K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436–1441. doi: 10.1093/jn/137.6.1436. [DOI] [PubMed] [Google Scholar]
- 15.Baba S. Osakabe N. Kato Y. Natsume M. Yasuda A. Kido T. Fukuda K. Muto Y. Kondo K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr. 2007;85:709–717. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 16.Baba S. Osakabe N. Natsume M. Terao J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats. Free Radic Biol Med. 2002;33:142–148. doi: 10.1016/s0891-5849(02)00871-7. [DOI] [PubMed] [Google Scholar]
- 17.Balzer J. Rassaf T. Heiss C. Kleinbongard P. Lauer T. Merx M. Heussen N. Gross HB. Keen CL. Schroeter H. Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Barry Callebaut USA, Inc. Barry Callebaut survey finds Americans love milk chocolate but are experimenting with other varieties. 2007. www.barry-callebaut.com/56?release=3400. [May 12;2010 ]. www.barry-callebaut.com/56?release=3400
- 19.Bazzano LA. Effects of soluble dietary fiber on low-density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep. 2008;10:473–477. doi: 10.1007/s11883-008-0074-3. [DOI] [PubMed] [Google Scholar]
- 20.Belscak-Cvitanovic A. Benkovic M. Komes D. Bauman I. Horzic D. Dujmic F. Matijasec M. Physical properties and bioactive constituents of powdered mixtures and drinks prepared with cocoa and various sweeteners. J Agric Food Chem. 2010;58:7187–7195. doi: 10.1021/jf1005484. [DOI] [PubMed] [Google Scholar]
- 21.Berry NM. Davison K. Coates AM. Buckley JD. Howe PR. Impact of cocoa flavanol consumption on blood pressure responsiveness to exercise. Br J Nutr. 2010;103:1–5. doi: 10.1017/S0007114509993382. [DOI] [PubMed] [Google Scholar]
- 22.Boelsma E. van de Vijver LP. Goldbohm RA. Klopping-Ketelaars IA. Hendriks HF. Roza L. Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003;77:348–355. doi: 10.1093/ajcn/77.2.348. [DOI] [PubMed] [Google Scholar]
- 23.Bordeaux B. Yanek LR. Moy TF. White LW. Becker LC. Faraday N. Becker DM. Casual chocolate consumption and inhibition of platelet function. Prev Cardiol. 2007;10:175–180. doi: 10.1111/j.1520-037x.2007.06693.x. [DOI] [PubMed] [Google Scholar]
- 24.Bourtchuladze R. Frenguelli B. Blendy J. Cioffi D. Schutz G. Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 25.Bowe WP. Joshi SS. Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124–141. doi: 10.1016/j.jaad.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Bracco U. Effect of triglyceride structure on fat absorption. Am J Clin Nutr. 1994;60:1002S–1009S. doi: 10.1093/ajcn/60.6.1002S. [DOI] [PubMed] [Google Scholar]
- 27.Bruinsma K. Taren DL. Chocolate: food or drug? J Am Diet Assoc. 1999;99:1249–1256. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]
- 28.Brummer P. Coronary mortality and living standard. II. Coffee, tea, cocoa, alcohol and tobacco. Acta Med Scand. 1969;186:61–63. [PubMed] [Google Scholar]
- 29.Buijsse B. Feskens EJ. Kok FJ. Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen elderly study. Arch Intern Med. 2006;166:411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 30.Buijsse B. Weikert C. Drogan D. Bergmann M. Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31:1616–1623. doi: 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- 31.Busse R. Fleming I. Endothelial dysfunction in atherosclerosis. J Vasc Res. 1996;33:181–194. doi: 10.1159/000159147. [DOI] [PubMed] [Google Scholar]
- 32.Buyken AE. Flood V. Rochtchina E. Nestel P. Brand-Miller J. Mitchell P. Modifications in dietary fat quality are associated with changes in serum lipids of older adults independently of lipid medication. J Nutr. 2010;140:88–94. doi: 10.3945/jn.109.110486. [DOI] [PubMed] [Google Scholar]
- 33.CAOBISCO. Ranking of Consumption (Chocolate Confectionery) www.caobisco.com/doc_uploads/Charts/ranking_of_consumption_chocolate_confectionery_2007.pdf. [Jan 5;2010 ]. www.caobisco.com/doc_uploads/Charts/ranking_of_consumption_chocolate_confectionery_2007.pdf
- 34.Celermajer DS. Sorensen KE. Bull C. Robinson J. Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 35.Ceriello A. Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborti S. Chakraborti T. Mandal M. Mandal A. Das S. Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002;238:163–179. doi: 10.1023/a:1019998702946. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarthy BK. Gupta S. Gode KD. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (-)-epicatechin. Life Sci. 1982;31:2693–2697. doi: 10.1016/0024-3205(82)90713-5. [DOI] [PubMed] [Google Scholar]
- 38.Chen D. Daniel KG. Kuhn DJ. Kazi A. Bhuiyan M. Li L. Wang Z. Wan SB. Lam WH. Chan TH. Dou QP. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004;9:2618–2631. doi: 10.2741/1421. [DOI] [PubMed] [Google Scholar]
- 39.Chevaux KA JL. Villar ME, et al. Proximate, mineral and procyanidin content of certain foods and beverages consumed by the Kuna Amerinds of Panama. J Food Comp Anal. 2001;14:553–563. [Google Scholar]
- 40.Cooper KA. Campos-Gimenez E. Jimenez Alvarez D. Nagy K. Donovan JL. Williamson G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J Agric Food Chem. 2007;55:2841–2847. doi: 10.1021/jf063277c. [DOI] [PubMed] [Google Scholar]
- 41.Cooper KA. Donovan JL. Waterhouse AL. Williamson G. Cocoa and health: a decade of research. Br J Nutr. 2008;99:1–11. doi: 10.1017/S0007114507795296. [DOI] [PubMed] [Google Scholar]
- 42.Corti R. Flammer AJ. Hollenberg NK. Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 43.Corti R. Hutter R. Badimon JJ. Fuster V. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. J Thromb Thrombolysis. 2004;17:35–44. doi: 10.1023/B:THRO.0000036027.39353.70. [DOI] [PubMed] [Google Scholar]
- 44.Cotelle N. Role of flavonoids in oxidative stress. Curr Top Med Chem. 2001;1:569–590. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- 45.Crews WD., Jr. Harrison DW. Wright JW. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am J Clin Nutr. 2008;87:872–880. doi: 10.1093/ajcn/87.4.872. [DOI] [PubMed] [Google Scholar]
- 46.D'Archivio M. Filesi C. Vari R. Scazzocchio B. Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidovici BB. Wolf R. The role of diet in acne: facts and controversies. Clin Dermatol. 2010;28:12–16. doi: 10.1016/j.clindermatol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Davison K. Berry NM. Misan G. Coates AM. Buckley JD. Howe PR. Dose-related effects of flavanol-rich cocoa on blood pressure. J Hum Hypertens. 2010;24:568–576. doi: 10.1038/jhh.2009.105. [DOI] [PubMed] [Google Scholar]
- 49.Davison K. Coates AM. Buckley JD. Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32:1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 50.De Benoist B. Mclean E. Egli I. Cogswell M. Worldwide Prevalence of Anaemia 1993–2005. Geneva: World Health Organization; 2008. [DOI] [PubMed] [Google Scholar]
- 51.De Caterina R. Zampolli A. Del Turco S. Madonna R. Massaro M. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83:421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
- 52.Deanfield JE. Halcox JP. Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 53.Delva P. Magnesium and coronary heart disease. Mol Aspects Med. 2003;24:63–78. doi: 10.1016/s0098-2997(02)00092-4. [DOI] [PubMed] [Google Scholar]
- 54.Deprez S. Mila I. Huneau JF. Tome D. Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid Redox Signal. 2001;3:957–967. doi: 10.1089/152308601317203503. [DOI] [PubMed] [Google Scholar]
- 55.Desch S. Kobler D. Schmidt J. Sonnabend M. Adams V. Sareban M. Eitel I. Bluher M. Schuler G. Thiele H. Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients. Am J Hypertens. 2010;23:694–700. doi: 10.1038/ajh.2010.29. [DOI] [PubMed] [Google Scholar]
- 56.Desch S. Schmidt J. Kobler D. Sonnabend M. Eitel I. Sareban M. Rahimi K. Schuler G. Thiele H. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 57.Desjardins F. Balligand JL. Nitric oxide-dependent endothelial function and cardiovascular disease. Acta Clin Belg. 2006;61:326–334. doi: 10.1179/acb.2006.052. [DOI] [PubMed] [Google Scholar]
- 58.DeVault KR. Castell DO. American College of G. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 59.di Giuseppe R. Di Castelnuovo A. Centritto F. Zito F. De Curtis A. Costanzo S. Vohnout B. Sieri S. Krogh V. Donati MB. de Gaetano G. Iacoviello L. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J Nutr. 2008;138:1939–1945. doi: 10.1093/jn/138.10.1939. [DOI] [PubMed] [Google Scholar]
- 60.Dillinger TL. Barriga P. Escarcega S. Jimenez M. Salazar Lowe D. Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr. 2000;130:2057S–7072S. doi: 10.1093/jn/130.8.2057S. [DOI] [PubMed] [Google Scholar]
- 61.Djousse L. Hopkins PN. Arnett DK. Pankow JS. Borecki I. North KE. Curtis Ellison R. Chocolate consumption is inversely associated with calcified atherosclerotic plaque in the coronary arteries: The NHLBI Family Heart Study. Clin Nutr. 2011;30:38–43. doi: 10.1016/j.clnu.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donovan JL. Manach C. Rios L. Morand C. Scalbert A. Remesy C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br J Nutr. 2002;87:299–306. doi: 10.1079/bjnbjn2001517. [DOI] [PubMed] [Google Scholar]
- 63.Ehara S. Ueda M. Naruko T. Haze K. Itoh A. Otsuka M. Komatsu R. Matsuo T. Itabe H. Takano T. Tsukamoto Y. Yoshiyama M. Takeuchi K. Yoshikawa J. Becker AE. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 64.Engler MB. Engler MM. The emerging role of flavonoid-rich cocoa and chocolate in cardiovascular health and disease. Nutr Rev. 2006;64:109–118. doi: 10.1111/j.1753-4887.2006.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 65.Engler MB. Engler MM. Chen CY. Malloy MJ. Browne A. Chiu EY. Kwak HK. Milbury P. Paul SM. Blumberg J. Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 66.Faridi Z. Njike VY. Dutta S. Ali A. Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr. 2008;88:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 67.Farouque HM. Leung M. Hope SA. Baldi M. Schechter C. Cameron JD. Meredith IT. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study. Clin Sci (Lond) 2006;111:71–80. doi: 10.1042/CS20060048. [DOI] [PubMed] [Google Scholar]
- 68.Fathi R. Haluska B. Isbel N. Short L. Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 2004;43:616–623. doi: 10.1016/j.jacc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 69.Federico A. Morgillo F. Tuccillo C. Ciardiello F. Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 70.Ferdowsian HR. Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1–2, 5. [PubMed] [Google Scholar]
- 71.Fernandez ML. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol. 2001;12:35–40. doi: 10.1097/00041433-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Fisher ND. Hollenberg NK. Aging and vascular responses to flavanol-rich cocoa. J Hypertens. 2006;24:1575–1580. doi: 10.1097/01.hjh.0000239293.40507.2a. [DOI] [PubMed] [Google Scholar]
- 73.Fisher ND. Hughes M. Gerhard-Herman M. Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 74.Fisher ND. Sorond FA. Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S210–S214. doi: 10.1097/00005344-200606001-00017. [DOI] [PubMed] [Google Scholar]
- 75.Flammer AJ. Hermann F. Sudano I. Spieker L. Hermann M. Cooper KA. Serafini M. Luscher TF. Ruschitzka F. Noll G. Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 75a.Food and Agriculture Organization of the United Nations. Medium-Term Prospects for Agricultural Commodities: Projections to the Year 2010. 2003. www.fao.org/docrep/006/y5143e/y5143e0x.htm#bm33. [May 10;2010 ]. www.fao.org/docrep/006/y5143e/y5143e0x.htm#bm33
- 76.Ford ES. Bergmann MM. Kroger J. Schienkiewitz A. Weikert C. Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169:1355–1362. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]
- 77.Francis ST. Head K. Morris PG. Macdonald IA. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S215–S220. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]
- 78.Franco OH. Bonneux L. de Laet C. Peeters A. Steyerberg EW. Mackenbach JP. The Polymeal: a more natural, safer, and probably tastier (than the Polypill) strategy to reduce cardiovascular disease by more than 75% BMJ. 2004;329:1447–1450. doi: 10.1136/bmj.329.7480.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedman M. Levin CE. Lee SU. Kozukue N. Stability of green tea catechins in commercial tea leaves during storage for 6 months. J Food Sci. 2009;74:H47–H51. doi: 10.1111/j.1750-3841.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 80.Gewaltig MT. Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc Res. 2002;55:250–260. doi: 10.1016/s0008-6363(02)00327-9. [DOI] [PubMed] [Google Scholar]
- 81.Ghosh D. Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res. 2009;53:322–331. doi: 10.1002/mnfr.200800182. [DOI] [PubMed] [Google Scholar]
- 82.Gibb CM. Davies PT. Glover V. Steiner TJ. Clifford Rose F. Sandler M. Chocolate is a migraine-provoking agent. Cephalalgia. 1991;11:93–95. doi: 10.1046/j.1468-2982.1991.1102093.x. [DOI] [PubMed] [Google Scholar]
- 83.Grassi D. Desideri G. Ferri C. Blood pressure and cardiovascular risk: what about cocoa and chocolate? Arch Biochem Biophys. 2010;501:112–115. doi: 10.1016/j.abb.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 84.Grassi D. Desideri G. Necozione S. Lippi C. Casale R. Properzi G. Blumberg JB. Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 85.Grassi D. Necozione S. Lippi C. Croce G. Valeri L. Pasqualetti P. Desideri G. Blumberg JB. Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 86.Gu L. House SE. Wu X. Ou B. Prior RL. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J Agric Food Chem. 2006;54:4057–4061. doi: 10.1021/jf060360r. [DOI] [PubMed] [Google Scholar]
- 87.Gums JG. Magnesium in cardiovascular and other disorders. Am J Health Syst Pharm. 2004;61:1569–1576. doi: 10.1093/ajhp/61.15.1569. [DOI] [PubMed] [Google Scholar]
- 88.Gurbel PA. Serebruany VL. Adhesion molecules, platelet activation, and cardiovascular risk. Am Heart J. 2002;143:196–198. doi: 10.1067/mhj.2002.120304. [DOI] [PubMed] [Google Scholar]
- 89.Hamed MS. Gambert S. Bliden KP. Bailon O. Singla A. Antonino MJ. Hamed F. Tantry US. Gurbel PA. Dark chocolate effect on platelet activity, C-reactive protein and lipid profile: a pilot study. South Med J. 2008;101:1203–1208. doi: 10.1097/SMJ.0b013e31818859eb. [DOI] [PubMed] [Google Scholar]
- 90.Hanhineva K. Torronen R. Bondia-Pons I. Pekkinen J. Kolehmainen M. Mykkanen H. Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heinrich U. Neukam K. Tronnier H. Sies H. Stahl W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J Nutr. 2006;136:1565–1569. doi: 10.1093/jn/136.6.1565. [DOI] [PubMed] [Google Scholar]
- 92.Heiss C. Dejam A. Kleinbongard P. Schewe T. Sies H. Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 93.Heiss C. Finis D. Kleinbongard P. Hoffmann A. Rassaf T. Kelm M. Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol. 2007;49:74–80. doi: 10.1097/FJC.0b013e31802d0001. [DOI] [PubMed] [Google Scholar]
- 94.Heiss C. Jahn S. Taylor M. Real WM. Angeli FS. Wong ML. Amabile N. Prasad M. Rassaf T. Ottaviani JI. Mihardja S. Keen CL. Springer ML. Boyle A. Grossman W. Glantz SA. Schroeter H. Yeghiazarians Y. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:218–224. doi: 10.1016/j.jacc.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 95.Heiss C. Kleinbongard P. Dejam A. Perre S. Schroeter H. Sies H. Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 96.Heptinstall S. May J. Fox S. Kwik-Uribe C. Zhao L. Cocoa flavanols and platelet and leukocyte function: recent in vitro and ex vivo studies in healthy adults. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S197–S205. doi: 10.1097/00005344-200606001-00015. ; discussion S206–S209. [DOI] [PubMed] [Google Scholar]
- 97.Hermann F. Spieker LE. Ruschitzka F. Sudano I. Hermann M. Binggeli C. Luscher TF. Riesen W. Noll G. Corti R. Dark chocolate improves endothelial and platelet function. Heart. 2006;92:119–120. doi: 10.1136/hrt.2005.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hertog MG. Kromhout D. Aravanis C. Blackburn H. Buzina R. Fidanza F. Giampaoli S. Jansen A. Menotti A. Nedeljkovic S, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 99.Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- 100.Hii CS. Howell SL. Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. J Endocrinol. 1985;107:1–8. doi: 10.1677/joe.0.1070001. [DOI] [PubMed] [Google Scholar]
- 101.Hill AJ. Heaton-Brown L. The experience of food craving: a prospective investigation in healthy women. J Psychosom Res. 1994;38:801–814. doi: 10.1016/0022-3999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 102.Hollenberg N. Vascular action of cocoa flavanols in humans: the roots of the story. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S99–S102. doi: 10.1097/00005344-200606001-00002. discussion S119–S121. [DOI] [PubMed] [Google Scholar]
- 103.Hollenberg NK. Fisher ND. McCullough ML. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J Am Soc Hypertens. 2009;3:105–112. doi: 10.1016/j.jash.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hollenberg NK. Martinez G. McCullough M. Meinking T. Passan D. Preston M. Rivera A. Taplin D. Vicaria-Clement M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension. 1997;29:171–176. doi: 10.1161/01.hyp.29.1.171. [DOI] [PubMed] [Google Scholar]
- 105.Holt RR. Actis-Goretta L. Momma TY. Keen CL. Dietary flavanols and platelet reactivity. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S187–S196. doi: 10.1097/00005344-200606001-00014. ; discussion S206–S209. [DOI] [PubMed] [Google Scholar]
- 106.Holt RR. Lazarus SA. Sullards MC. Zhu QY. Schramm DD. Hammerstone JF. Fraga CG. Schmitz HH. Keen CL. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 107.Hooper L. Kroon PA. Rimm EB. Cohn JS. Harvey I. Le Cornu KA. Ryder JJ. Hall WL. Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 108.Hormes JM. Rozin P. Perimenstrual chocolate craving. What happens after menopause? Appetite. 2009;53:256–259. doi: 10.1016/j.appet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Hu FB. Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100:47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang H. Mai W. Liu D. Hao Y. Tao J. Dong Y. The oxidation ratio of LDL: a predictor for coronary artery disease. Dis Markers. 2008;24:341–349. doi: 10.1155/2008/371314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hunot S. Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S49–S58. doi: 10.1002/ana.10481. ; discussion S58–S60. [DOI] [PubMed] [Google Scholar]
- 112.Hunter JE. Zhang J. Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 113.Hurst WJ. Payne MJ. Miller KB. Stuart DA. Stability of cocoa antioxidants and flavan-3-ols over time. J Agric Food Chem. 2009;57:9547–9550. doi: 10.1021/jf901457s. [DOI] [PubMed] [Google Scholar]
- 113a.International Cocoa Organization. Annual Forecasts of Production and Consumption and Estimates of Production Levels to Achieve Equilibrium in the World Cocoa Market. 2008. www.icco.org/Attachment.aspx?Id=fgn64825. [May 10;2010 ]. www.icco.org/Attachment.aspx?Id=fgn64825
- 114.Ishikawa A. Yamashita H. Hiemori M. Inagaki E. Kimoto M. Okamoto M. Tsuji H. Memon AN. Mohammadio A. Natori Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo) 2007;53:166–173. doi: 10.3177/jnsv.53.166. [DOI] [PubMed] [Google Scholar]
- 115.Jalil AM. Ismail A. Pei CP. Hamid M. Kamaruddin SH. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (Ob-db) rats. J Agric Food Chem. 2008;56:7877–7884. doi: 10.1021/jf8015915. [DOI] [PubMed] [Google Scholar]
- 116.Janszky I. Mukamal KJ. Ljung R. Ahnve S. Ahlbom A. Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. J Intern Med. 2009;266:248–257. doi: 10.1111/j.1365-2796.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 117.Jenkins DJ. Kendall CW. Vuksan V. Vidgen E. Wong E. Augustin LS. Fulgoni V., 3rd Effect of cocoa bran on low-density lipoprotein oxidation and fecal bulking. Arch Intern Med. 2000;160:2374–2379. doi: 10.1001/archinte.160.15.2374. [DOI] [PubMed] [Google Scholar]
- 118.Jeong SY. Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- 119.Jia L. Liu X. Bai YY. Li SH. Sun K. He C. Hui R. Short-term effect of cocoa product consuption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218–225. doi: 10.3945/ajcn.2009.28202. [DOI] [PubMed] [Google Scholar]
- 120.Jobgen WS. Fried SK. Fu WJ. Meininger CJ. Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 121.Johnston K. Sharp P. Clifford M. Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005;579:1653–1657. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 122.Joseph JA. Shukitt-Hale B. Denisova NA. Bielinski D. Martin A. McEwen JJ. Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaltenbach T. Crockett S. Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965–971. doi: 10.1001/archinte.166.9.965. [DOI] [PubMed] [Google Scholar]
- 124.Katan MB. Zock PL. Mensink RP. Effects of fats and fatty acids on blood lipids in humans: an overview. Am J Clin Nutr. 1994;60:1017S–1022S. doi: 10.1093/ajcn/60.6.1017S. [DOI] [PubMed] [Google Scholar]
- 125.Katz DL. Life and death, knowledge and power: why knowing what matters is not what's the matter. Arch Intern Med. 2009;169:1362–1363. doi: 10.1001/archinternmed.2009.238. [DOI] [PubMed] [Google Scholar]
- 126.Kenny TP. Keen CL. Schmitz HH. Gershwin ME. Immune effects of cocoa procyanidin oligomers on peripheral blood mononuclear cells. Exp Biol Med (Maywood) 2007;232:293–300. [PubMed] [Google Scholar]
- 127.Kenny TP. Shu SA. Moritoki Y. Keen CL. Gershwin ME. Cocoa flavanols and procyanidins can modulate the lipopolysaccharide activation of polymorphonuclear cells in vitro. J Med Food. 2009;12:1–7. doi: 10.1089/jmf.2007.0263. [DOI] [PubMed] [Google Scholar]
- 128.Keogh JB. Grieger JA. Noakes M. Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25:1274–1279. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- 129.Keogh JB. McInerney J. Clifton PM. The effect of milk protein on the bioavailability of cocoa polyphenols. J Food Sci. 2007;72:S230–S233. doi: 10.1111/j.1750-3841.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 130.Khan N. Afaq F. Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 131.Kim JA. Montagnani M. Koh KK. Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 132.Kim JE. Son JE. Jung SK. Kang NJ. Lee CY. Lee KW. Lee HJ. Cocoa polyphenols suppress TNF-alpha-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br J Nutr. 2010;104:957–964. doi: 10.1017/S0007114510001704. [DOI] [PubMed] [Google Scholar]
- 133.Kim YS. Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 134.Klevay LM. Cardiovascular disease from copper deficiency—a history. J Nutr. 2000;130:489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 135.Kris-Etherton PM. Derr JA. Mustad VA. Seligson FH. Pearson TA. Effects of a milk chocolate bar per day substituted for a high-carbohydrate snack in young men on an NCEP/AHA Step 1 Diet. Am J Clin Nutr. 1994;60:1037S–1042S. doi: 10.1093/ajcn/60.6.1037S. [DOI] [PubMed] [Google Scholar]
- 136.Kris-Etherton PM. Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 137.Kwon do Y. Choi KH. Kim SJ. Choi DW. Kim YS. Kim YC. Comparison of peroxyl radical scavenging capacity of commonly consumed beverages. Arch Pharm Res. 2009;32:283–287. doi: 10.1007/s12272-009-1234-x. [DOI] [PubMed] [Google Scholar]
- 138.Lavoie JL. Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 139.Lecumberri E. Mateos R. Ramos S. Alia M. Ruperez P. Goya L. Izquierdo-Pulido M. Bravo L. Characterization of cocoa fiber and its effect on the antioxidant capacity of serum in rats] Nutr Hosp. 2006;21:622–628. [PubMed] [Google Scholar]
- 140.Lee IK. Kim HS. Bae JH. Endothelial dysfunction: its relationship with acute hyperglycaemia and hyperlipidemia. Int J Clin Pract Suppl. 2002;129:59–64. [PubMed] [Google Scholar]
- 141.Lee KW. Kundu JK. Kim SO. Chun KS. Lee HJ. Surh YJ. Cocoa polyphenols inhibit phorbol ester-induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 and activation of NF-kappaB and MAPKs in mouse skin in vivo. J Nutr. 2006;136:1150–1155. doi: 10.1093/jn/136.5.1150. [DOI] [PubMed] [Google Scholar]
- 142.Leone N. Courbon D. Ducimetiere P. Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17:308–314. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 143.Li H. Forstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr Pharm Des. 2009;15:3133–3145. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 144.Loke WM. Hodgson JM. Proudfoot JM. McKinley AJ. Puddey IB. Croft KD. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 145.Luscher TF. Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:II-3–II-10. [PubMed] [Google Scholar]
- 146.Macht M. Dettmer D. Everyday mood and emotions after eating a chocolate bar or an apple. Appetite. 2006;46:332–336. doi: 10.1016/j.appet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 147.Macht M. Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite. 2007;49:667–674. doi: 10.1016/j.appet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 148.Macht M. Mueller J. Interactive effects of emotional and restrained eating on responses to chocolate and affect. J Nerv Ment Dis. 2007;195:1024–1026. doi: 10.1097/NMD.0b013e31815c0878. [DOI] [PubMed] [Google Scholar]
- 149.Mackenzie GG. Carrasquedo F. Delfino JM. Keen CL. Fraga CG. Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18:167–169. doi: 10.1096/fj.03-0402fje. [DOI] [PubMed] [Google Scholar]
- 150.Manach C. Scalbert A. Morand C. Remesy C. Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 151.Manach C. Williamson G. Morand C. Scalbert A. Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 152.Mao TK. Van de Water J. Keen CL. Schmitz HH. Gershwin ME. Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J Med Food. 2002;5:17–22. doi: 10.1089/109662002753723188. [DOI] [PubMed] [Google Scholar]
- 153.Marcus DA. Scharff L. Turk D. Gourley LM. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia. 1997;17:855–862. doi: 10.1046/j.1468-2982.1997.1708855.x. ; discussion 800. [DOI] [PubMed] [Google Scholar]
- 154.Martin FP. Rezzi S. Pere-Trepat E. Kamlage B. Collino S. Leibold E. Kastler J. Rein D. Fay LB. Kochhar S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J Proteome Res. 2009;8:5568–5579. doi: 10.1021/pr900607v. [DOI] [PubMed] [Google Scholar]
- 155.Maskarinec G. Cancer protective properties of cocoa: a review of the epidemiologic evidence. Nutr Cancer. 2009;61:573–579. doi: 10.1080/01635580902825662. [DOI] [PubMed] [Google Scholar]
- 156.Massolt ET. van Haard PM. Rehfeld JF. Posthuma EF. van der Veer E. Schweitzer DH. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul Pept. 2010;161:81–86. doi: 10.1016/j.regpep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 157.Matsui N. Ito R. Nishimura E. Yoshikawa M. Kato M. Kamei M. Shibata H. Matsumoto I. Abe K. Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 158.May AE. Seizer P. Gawaz M. Platelets: inflammatory firebugs of vascular walls. Arterioscler Thromb Vasc Biol. 2008;28:s5–s10. doi: 10.1161/ATVBAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 159.Maytin M. Leopold J. Loscalzo J. Oxidant stress in the vasculature. Curr Atheroscler Rep. 1999;1:156–164. doi: 10.1007/s11883-999-0012-z. [DOI] [PubMed] [Google Scholar]
- 160.McCulloch J. Harper AM. Phenylethylamine and cerebral blood flow. Possible involvement of phenylethylamine in migraine. Neurology. 1977;27:817–821. doi: 10.1212/wnl.27.9.817. [DOI] [PubMed] [Google Scholar]
- 161.McCulloch J. Harper AM. Factors influencing the response of the cerebral circulation to phenylethylamine. Neurology. 1979;29:201–207. doi: 10.1212/wnl.29.2.201. [DOI] [PubMed] [Google Scholar]
- 162.McCullough ML. Chevaux K. Jackson L. Preston M. Martinez G. Schmitz HH. Coletti C. Campos H. Hollenberg NK. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S103–S109. doi: 10.1097/00005344-200606001-00003. ; discussion 119–121. [DOI] [PubMed] [Google Scholar]
- 163.McShea A. Leissle K. Smith MA. The essence of chocolate: a rich, dark, and well-kept secret. Nutrition. 2009;25:1104–1105. doi: 10.1016/j.nut.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 164.McShea A. Ramiro-Puig E. Munro SB. Casadesus G. Castell M. Smith MA. Clinical benefit and preservation of flavonols in dark chocolate manufacturing. Nutr Rev. 2008;66:630–641. doi: 10.1111/j.1753-4887.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 165.Mehrinfar R. Frishman WH. Flavanol-rich cocoa: a cardioprotective nutraceutical. Cardiol Rev. 2008;16:109–115. doi: 10.1097/CRD.0b013e31815d95e2. [DOI] [PubMed] [Google Scholar]
- 166.Mellor DD. Sathyapalan T. Kilpatrick ES. Beckett S. Atkin SL. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318–1321. doi: 10.1111/j.1464-5491.2010.03108.x. [DOI] [PubMed] [Google Scholar]
- 167.Michener W. Rozin P. Pharmacological versus sensory factors in the satiation of chocolate craving. Physiol Behav. 1994;56:419–422. doi: 10.1016/0031-9384(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 168.Miller KB. Hurst WJ. Flannigan N. Ou B. Lee CY. Smith N. Stuart DA. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J Agric Food Chem. 2009;57:9169–9180. doi: 10.1021/jf901821x. [DOI] [PubMed] [Google Scholar]
- 169.Miller KB. Hurst WJ. Payne MJ. Stuart DA. Apgar J. Sweigart DS. Ou B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J Agric Food Chem. 2008;56:8527–8533. doi: 10.1021/jf801670p. [DOI] [PubMed] [Google Scholar]
- 170.Miller KB. Stuart DA. Smith NL. Lee CY. McHale NL. Flanagan JA. Ou B. Hurst WJ. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J Agric Food Chem. 2006;54:4062–4068. doi: 10.1021/jf060290o. [DOI] [PubMed] [Google Scholar]
- 171.Mitchell JA. Ali F. Bailey L. Moreno L. Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 172.Monagas M. Khan N. Andres-Lacueva C. Casas R. Urpi-Sarda M. Llorach R. Lamuela-Raventos RM. Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr. 2009;90:1144–1150. doi: 10.3945/ajcn.2009.27716. [DOI] [PubMed] [Google Scholar]
- 173.Moncada S. Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 174.Moncada S. Palmer RM. Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 175.Mostofsky E. Levitan EB. Wolk A. Mittleman MA. Chocolate intake and incidence of heart failure: a population-based, prospective study of middle-aged and elderly women. Circ Heart Fail. 2010;3:612–616. doi: 10.1161/CIRCHEARTFAILURE.110.944025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mullen W. Borges G. Donovan JL. Edwards CA. Serafini M. Lean ME. Crozier A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am J Clin Nutr. 2009;89:1784–1791. doi: 10.3945/ajcn.2008.27339. [DOI] [PubMed] [Google Scholar]
- 177.Muller-Lissner SA. Kaatz V. Brandt W. Keller J. Layer P. The perceived effect of various foods and beverages on stool consistency. Eur J Gastroenterol Hepatol. 2005;17:109–112. doi: 10.1097/00042737-200501000-00020. [DOI] [PubMed] [Google Scholar]
- 178.Muniyappa R. Hall G. Kolodziej TL. Karne RJ. Crandon SK. Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. 2008;88:1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Murphy DW. Castell DO. Chocolate and heartburn: evidence of increased esophageal acid exposure after chocolate ingestion. Am J Gastroenterol. 1988;83:633–636. [PubMed] [Google Scholar]
- 180.Murphy KJ. Chronopoulos AK. Singh I. Francis MA. Moriarty H. Pike MJ. Turner AH. Mann NJ. Sinclair AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 181.Mursu J. Voutilainen S. Nurmi T. Rissanen TH. Virtanen JK. Kaikkonen J. Nyyssonen K. Salonen JT. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic Biol Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 182.Myung SK. Ju W. Choi HJ. Kim SC. Soy intake and risk of endocrine-related gynaecological cancer: a meta-analysis. BJOG. 2009;116:1697–1705. doi: 10.1111/j.1471-0528.2009.02322.x. [DOI] [PubMed] [Google Scholar]
- 183.Napoli C. Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 184.National Academy of Sciences. Institute of Medicine. Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Vitamins. 2004. http://iom.edu/en/Global/News%20Announcements/∼/media/Files/Activity%20Files/Nutrition/DRIs/DRISummaryListing2.ashx. [May 12;2010 ]. http://iom.edu/en/Global/News%20Announcements/∼/media/Files/Activity%20Files/Nutrition/DRIs/DRISummaryListing2.ashx
- 185. This reference has been deleted.
- 186.Neilson AP. Sapper TN. Janle EM. Rudolph R. Matusheski NV. Ferruzzi MG. Chocolate matrix factors modulate the pharmacokinetic behavior of cocoa flavan-3-ol phase II metabolites following oral consumption by Sprague-Dawley rats. J Agric Food Chem. 2010;58:6685–6691. doi: 10.1021/jf1005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Neukam K. Stahl W. Tronnier H. Sies H. Heinrich U. Consumption of flavanol-rich cocoa acutely increases microcirculation in human skin. Eur J Nutr. 2007;46:53–56. doi: 10.1007/s00394-006-0627-6. [DOI] [PubMed] [Google Scholar]
- 188.Njike VY. Faridi Z. Shuval K. Dutta S. Kay CD. West SG. Kris-Etherton PM. Katz DL. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.12.010. [Epub ahead of print]; [DOI] [PubMed] [Google Scholar]
- 189.Oba S. Nagata C. Nakamura K. Fujii K. Kawachi T. Takatsuka N. Shimizu H. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br J Nutr. 2010;103:453–459. doi: 10.1017/S0007114509991966. [DOI] [PubMed] [Google Scholar]
- 190.Olivares M. Uauy R. Copper as an essential nutrient. Am J Clin Nutr. 1996;63:791S–796S. doi: 10.1093/ajcn/63.5.791. [DOI] [PubMed] [Google Scholar]
- 191.Oliviero T. Capuano E. Cammerer B. Fogliano V. Influence of roasting on the antioxidant activity and HMF formation of a cocoa bean model systems. J Agric Food Chem. 2009;57:147–152. doi: 10.1021/jf802250j. [DOI] [PubMed] [Google Scholar]
- 192. This reference has been deleted.
- 193.Osman JL. Sobal J. Chocolate cravings in American and Spanish individuals: biological and cultural influences. Appetite. 2006;47:290–301. doi: 10.1016/j.appet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 194.Ostertag LM. O'Kennedy N. Kroon PA. Duthie GG. de Roos B. Impact of dietary polyphenols on human platelet function—a critical review of controlled dietary intervention studies. Mol Nutr Food Res. 2010;54:60–81. doi: 10.1002/mnfr.200900172. [DOI] [PubMed] [Google Scholar]
- 195.Parker G. Parker I. Brotchie H. Mood state effects of chocolate. J Affect Disord. 2006;92:149–159. doi: 10.1016/j.jad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 196.Pearson DA. Holt RR. Rein D. Paglieroni T. Schmitz HH. Keen CL. Flavanols and platelet reactivity. Clin Dev Immunol. 2005;12:1–9. doi: 10.1080/10446670410001722140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Philips BJ. Coyle CH. Morrisroe SN. Chancellor MB. Yoshimura N. Induction of apoptosis in human bladder cancer cells by green tea catechins. Biomed Res. 2009;30:207–215. doi: 10.2220/biomedres.30.207. [DOI] [PubMed] [Google Scholar]
- 198.Plotnick GD. Corretti MC. Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278:1682–1686. [PubMed] [Google Scholar]
- 199.Ramiro E. Franch A. Castellote C. Andres-Lacueva C. Izquierdo-Pulido M. Castell M. Effect of Theobroma cacao flavonoids on immune activation of a lymphoid cell line. Br J Nutr. 2005;93:859–866. doi: 10.1079/bjn20051443. [DOI] [PubMed] [Google Scholar]
- 200.Ramiro-Puig E. Castell M. Cocoa: antioxidant and immunomodulator. Br J Nutr. 2009;101:931–40. doi: 10.1017/S0007114508169896. [DOI] [PubMed] [Google Scholar]
- 201.Ramiro-Puig E. Perez-Cano FJ. Ramirez-Santana C. Castellote C. Izquierdo-Pulido M. Permanyer J. Franch A. Castell M. Spleen lymphocyte function modulated by a cocoa-enriched diet. Clin Exp Immunol. 2007;149:535–42. doi: 10.1111/j.1365-2249.2007.03430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ramiro-Puig E. Urpi-Sarda M. Perez-Cano FJ. Franch A. Castellote C. Andres-Lacueva C. Izquierdo-Pulido M. Castell M. Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. J Agric Food Chem. 2007;55:6431–6438. doi: 10.1021/jf070487w. [DOI] [PubMed] [Google Scholar]
- 203.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 204.Rein D. Paglieroni TG. Pearson DA. Wun T. Schmitz HH. Gosselin R. Keen CL. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr. 2000;130:2120S–2126S. doi: 10.1093/jn/130.8.2120S. [DOI] [PubMed] [Google Scholar]
- 205.Rein D. Paglieroni TG. Wun T. Pearson DA. Schmitz HH. Gosselin R. Keen CL. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 206.Reunanen A. Knekt P. Marniemi J. Maki J. Maatela J. Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50:431–437. [PubMed] [Google Scholar]
- 207.Rios LY. Bennett RN. Lazarus SA. Remesy C. Scalbert A. Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–1110. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]
- 208.Rose N. Koperski S. Golomb BA. Mood food: chocolate and depressive symptoms in a cross-sectional analysis. Arch Intern Med. 2010;170:699–703. doi: 10.1001/archinternmed.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 210.Roura E. Andres-Lacueva C. Estruch R. Lourdes Mata Bilbao M. Izquierdo-Pulido M. Lamuela-Raventos RM. The effects of milk as a food matrix for polyphenols on the excretion profile of cocoa (-)-epicatechin metabolites in healthy human subjects. Br J Nutr. 2008;100:846–851. doi: 10.1017/S0007114508922534. [DOI] [PubMed] [Google Scholar]
- 211.Roura E. Andres-Lacueva C. Estruch R. Mata-Bilbao ML. Izquierdo-Pulido M. Waterhouse AL. Lamuela-Raventos RM. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann Nutr Metab. 2007;51:493–498. doi: 10.1159/000111473. [DOI] [PubMed] [Google Scholar]
- 212.Rozin P. Levine E. Stoess C. Chocolate craving and liking. Appetite. 1991;17:199–212. doi: 10.1016/0195-6663(91)90022-k. [DOI] [PubMed] [Google Scholar]
- 213.Ruzaidi A. Amin I. Nawalyah AG. Hamid M. Faizul HA. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J Ethnopharmacol. 2005;98:55–60. doi: 10.1016/j.jep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 214.Saari JT. Copper deficiency and cardiovascular disease: role of peroxidation, glycation, and nitration. Can J Physiol Pharmacol. 2000;78:848–855. doi: 10.1139/cjpp-78-10-848. [DOI] [PubMed] [Google Scholar]
- 215.Schachinger V. Britten MB. Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 216.Schewe T. Steffen Y. Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys. 2008;476:102–106. doi: 10.1016/j.abb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 217.Schmitt CA. Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 218.Scholey AB. French SJ. Morris PJ. Kennedy DO. Milne AL. Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24:1505–1514. doi: 10.1177/0269881109106923. [DOI] [PubMed] [Google Scholar]
- 219.Schramm DD. Karim M. Schrader HR. Holt RR. Kirkpatrick NJ. Polagruto JA. Ensunsa JL. Schmitz HH. Keen CL. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003;73:857–869. doi: 10.1016/s0024-3205(03)00373-4. [DOI] [PubMed] [Google Scholar]
- 220.Schramm DD. Wang JF. Holt RR. Ensunsa JL. Gonsalves JL. Lazarus SA. Schmitz HH. German JB. Keen CL. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am J Clin Nutr. 2001;73:36–40. doi: 10.1093/ajcn/73.1.36. [DOI] [PubMed] [Google Scholar]
- 221.Schroeter H. Bahia P. Spencer JP. Sheppard O. Rattray M. Cadenas E. Rice-Evans C. Williams RJ. (-)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J Neurochem. 2007;101:1596–1606. doi: 10.1111/j.1471-4159.2006.04434.x. [DOI] [PubMed] [Google Scholar]
- 222.Schroeter H. Heiss C. Balzer J. Kleinbongard P. Keen CL. Hollenberg NK. Sies H. Kwik-Uribe C. Schmitz HH. Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schwab US. Maliranta HM. Sarkkinen ES. Savolainen MJ. Kesaniemi YA. Uusitupa MI. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. 1996;45:143–149. doi: 10.1016/s0026-0495(96)90044-x. [DOI] [PubMed] [Google Scholar]
- 224.Seligson FH. Krummel DA. Apgar JL. Patterns of chocolate consumption. Am J Clin Nutr. 1994;60:1060S–1064S. doi: 10.1093/ajcn/60.6.1060S. [DOI] [PubMed] [Google Scholar]
- 225.Selmi C. Cocchi CA. Lanfredini M. Keen CL. Gershwin ME. Chocolate at heart: the anti-inflammatory impact of cocoa flavanols. Mol Nutr Food Res. 2008;52:1340–1348. doi: 10.1002/mnfr.200700435. [DOI] [PubMed] [Google Scholar]
- 226.Selmi C. Mao TK. Keen CL. Schmitz HH. Eric Gershwin M. The anti-inflammatory properties of cocoa flavanols. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S163–S171. doi: 10.1097/00005344-200606001-00010. ; discussion S172–S176. [DOI] [PubMed] [Google Scholar]
- 227.Serafini M. Bugianesi R. Maiani G. Valtuena S. De Santis S. Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- 228.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 229.Shapiro H. Lev S. Cohen J. Singer P. Polyphenols in the prevention and treatment of sepsis syndromes: rationale and pre-clinical evidence. Nutrition. 2009;25:981–997. doi: 10.1016/j.nut.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 230.Sharma SD. Meeran SM. Katiyar SK. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Mol Cancer Ther. 2010;9:569–580. doi: 10.1158/1535-7163.MCT-09-0638. [DOI] [PubMed] [Google Scholar]
- 231.Shiina Y. Funabashi N. Lee K. Murayama T. Nakamura K. Wakatsuki Y. Daimon M. Komuro I. Acute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. Int J Cardiol. 2009;131:424–429. doi: 10.1016/j.ijcard.2007.07.131. [DOI] [PubMed] [Google Scholar]
- 232.Shively CA. Tarka SM., Jr Methylxanthine composition and consumption patterns of cocoa and chocolate products. Prog Clin Biol Res. 1984;158:149–178. [PubMed] [Google Scholar]
- 233.Sies H. Schewe T. Heiss C. Kelm M. Cocoa polyphenols and inflammatory mediators. Am J Clin Nutr. 2005;81:304S–312S. doi: 10.1093/ajcn/81.1.304S. [DOI] [PubMed] [Google Scholar]
- 234.Singh M. Arseneault M. Sanderson T. Murthy V. Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem. 2008;56:4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- 235.Smit HJ. Blackburn RJ. Reinforcing effects of caffeine and theobromine as found in chocolate. Psychopharmacology (Berl) 2005;181:101–106. doi: 10.1007/s00213-005-2209-3. [DOI] [PubMed] [Google Scholar]
- 236.Smit HJ. Gaffan EA. Rogers PJ. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology (Berl) 2004;176:412–419. doi: 10.1007/s00213-004-1898-3. [DOI] [PubMed] [Google Scholar]
- 237.Song Y. Manson JE. Cook NR. Albert CM. Buring JE. Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96:1135–1141. doi: 10.1016/j.amjcard.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 238.Sorond FA. Lipsitz LA. Hollenberg NK. Fisher ND. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr Dis Treat. 2008;4:433–440. [PMC free article] [PubMed] [Google Scholar]
- 239.Spadafranca A. Martinez Conesa C. Sirini S. Testolin G. Effect of dark chocolate on plasma epicatechin levels, DNA resistance to oxidative stress and total antioxidant activity in healthy subjects. Br J Nutr. 2010;103:1008–1014. doi: 10.1017/S0007114509992698. [DOI] [PubMed] [Google Scholar]
- 240.Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–273. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Spencer JP. Flavonoids: modulators of brain function? Br J Nutr. 2008;99 E(Suppl 1):ES60–ES77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- 242.Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4:243–250. doi: 10.1007/s12263-009-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Spencer JP. Abd-el-Mohsen MM. Rice-Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch Biochem Biophys. 2004;423:148–161. doi: 10.1016/j.abb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 244.Stahl L. Miller KB. Apgar J. Sweigart DS. Stuart DA. McHale N. Ou B. Kondo M. Hurst WJ. Preservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powder. J Food Sci. 2009;74:C456–C461. doi: 10.1111/j.1750-3841.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 245.Steffen Y. Jung T. Klotz LO. Schewe T. Grune T. Sies H. Protein modification elicited by oxidized low-density lipoprotein (LDL) in endothelial cells: protection by (-)-epicatechin. Free Radic Biol Med. 2007;42:955–970. doi: 10.1016/j.freeradbiomed.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 246.Steffen Y. Schewe T. Sies H. Myeloperoxidase-mediated LDL oxidation and endothelial cell toxicity of oxidized LDL: attenuation by (-)-epicatechin. Free Radic Res. 2006;40:1076–1085. doi: 10.1080/10715760600883247. [DOI] [PubMed] [Google Scholar]
- 247.Steffen Y. Schewe T. Sies H. (-)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 248.Steinberg FM. Bearden MM. Keen CL. Cocoa and chocolate flavonoids: implications for cardiovascular health. J Am Diet Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 249.Stipanuk M. Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: Saunders Company; 2000. [Google Scholar]
- 250.Stoclet JC. Chataigneau T. Ndiaye M. Oak MH. El Bedoui J. Chataigneau M. Schini-Kerth VB. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 251.Strandberg TE. Strandberg AY. Pitkala K. Salomaa VV. Tilvis RS. Miettinen TA. Chocolate, well-being and health among elderly men. Eur J Clin Nutr. 2008;62:247–253. doi: 10.1038/sj.ejcn.1602707. [DOI] [PubMed] [Google Scholar]
- 252.Suganuma M. Okabe S. Kai Y. Sueoka N. Sueoka E. Fujiki H. Synergistic effects of (—)-epigallocatechin gallate with (—)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- 253.Suwaidi JA. Hamasaki S. Higano ST. Nishimura RA. Holmes DR., Jr. Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 254.Sydow K. Mondon CE. Cooke JP. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med. 2005;10(Suppl 1):S35–S43. doi: 10.1177/1358836X0501000106. [DOI] [PubMed] [Google Scholar]
- 255.Taubert D. Berkels R. Roesen R. Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–1030. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 256.Taubert D. Roesen R. Lehmann C. Jung N. Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 257.Tschop M. Smiley DL. Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 258.U.S. Food and Drug Administration. CFR—Code of Federal Regulations Title 21. 2010. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=163&showFR=1. [Sep 20;2010 ]. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=163&showFR=1
- 259.Uauy R. Olivares M. Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr. 1998;67:952S–959S. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 260.Ueshima K. Magnesium and ischemic heart disease: a review of epidemiological, experimental, and clinical evidences. Magnes Res. 2005;18:275–284. [PubMed] [Google Scholar]
- 261.Umesawa M. Iso H. Date C. Yamamoto A. Toyoshima H. Watanabe Y. Kikuchi S. Koizumi A. Kondo T. Inaba Y. Tanabe N. Tamakoshi A. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am J Clin Nutr. 2008;88:195–202. doi: 10.1093/ajcn/88.1.195. [DOI] [PubMed] [Google Scholar]
- 262.United States Department of Agriculture. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. 2010.
- 263.Urpi-Sarda M. Llorach R. Khan N. Monagas M. Rotches-Ribalta M. Lamuela-Raventos R. Estruch R. Tinahones FJ. Andres-Lacueva C. Effect of milk on the urinary excretion of microbial phenolic acids after cocoa powder consumption in humans. J Agric Food Chem. 2010;58:4706–4711. doi: 10.1021/jf904440h. [DOI] [PubMed] [Google Scholar]
- 264.Urpi-Sarda M. Ramiro-Puig E. Khan N. Ramos-Romero S. Llorach R. Castell M. Gonzalez-Manzano S. Santos-Buelga C. Andres-Lacueva C. Distribution of epicatechin metabolites in lymphoid tissues and testes of young rats with a cocoa-enriched diet. Br J Nutr. 2010;103:1393–1397. doi: 10.1017/S0007114509993473. [DOI] [PubMed] [Google Scholar]
- 264a.USDA National Nutrient Database for Standard Reference. 2009. Release 22.
- 265.Victor VM. Rocha M. Sola E. Banuls C. Garcia-Malpartida K. Hernandez-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 266.Vogel RA. Measurement of endothelial function by brachial artery flow-mediated vasodilation. Am J Cardiol. 2001;88:31E–34E. doi: 10.1016/s0002-9149(01)01764-7. [DOI] [PubMed] [Google Scholar]
- 267.Wan Y. Vinson JA. Etherton TD. Proch J. Lazarus SA. Kris-Etherton PM. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am J Clin Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 268.Wang JF. Schramm DD. Holt RR. Ensunsa JL. Fraga CG. Schmitz HH. Keen CL. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J Nutr. 2000;130:2115S–2119S. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- 269.Wang-Polagruto JF. Villablanca AC. Polagruto JA. Lee L. Holt RR. Schrader HR. Ensunsa JL. Steinberg FM. Schmitz HH. Keen CL. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S177–S186. doi: 10.1097/00005344-200606001-00013. ; discussion S206–S209. [DOI] [PubMed] [Google Scholar]
- 270.Weickert MO. Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138:439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 271.Weisburger JH. Antimutagenesis and anticarcinogenesis, from the past to the future. Mutat Res. 2001;480–481:23–35. doi: 10.1016/s0027-5107(01)00166-x. [DOI] [PubMed] [Google Scholar]
- 272.Weisburger JH. Chemopreventive effects of cocoa polyphenols on chronic diseases. Exp Biol Med (Maywood) 2001;226:891–897. doi: 10.1177/153537020122601003. [DOI] [PubMed] [Google Scholar]
- 273.Welsch CA. Lachance PA. Wasserman BP. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698–1704. doi: 10.1093/jn/119.11.1698. [DOI] [PubMed] [Google Scholar]
- 274.Widlansky ME. Gokce N. Keaney JF., Jr. Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 275.Williams MJ. Sutherland WH. McCormick MP. de Jong SA. Walker RJ. Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. J Am Coll Cardiol. 1999;33:1050–1055. doi: 10.1016/s0735-1097(98)00681-0. [DOI] [PubMed] [Google Scholar]
- 276.Williams S. Tamburic S. Lally C. Eating chocolate can significantly protect the skin from UV light. J Cosmet Dermatol. 2009;8:169–173. doi: 10.1111/j.1473-2165.2009.00448.x. [DOI] [PubMed] [Google Scholar]
- 277.Williamson G. Bioavailability and health effects of cocoa polyphenols. Inflammopharmacology. 2009;17:111. doi: 10.1007/s10787-008-8030-y. [DOI] [PubMed] [Google Scholar]
- 278.Willner P. Benton D. Brown E. Cheeta S. Davies G. Morgan J. Morgan M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl) 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- 279.Wober C. Holzhammer J. Zeitlhofer J. Wessely P. Wober-Bingol C. Trigger factors of migraine and tension-type headache: experience and knowledge of the patients. J Headache Pain. 2006;7:188–195. doi: 10.1007/s10194-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Woodside JV. McKinley MC. Young IS. Saturated and trans fatty acids and coronary heart disease. Curr Atheroscler Rep. 2008;10:460–466. doi: 10.1007/s11883-008-0072-5. [DOI] [PubMed] [Google Scholar]
- 281.World Cocoa Foundation. How chocolate is made. www.worldcocoafoundation.org/learn-about-cocoa/tree-to-table/how-chocolate-is-made.asp. [Jan 10;2010 ]. www.worldcocoafoundation.org/learn-about-cocoa/tree-to-table/how-chocolate-is-made.asp
- 282.Wright LE. Castell DO. The adverse effect of chocolate on lower esophageal sphincter pressure. Am J Dig Dis. 1975;20:703–707. doi: 10.1007/BF01070826. [DOI] [PubMed] [Google Scholar]
- 283.Yang CS. Wang X. Lu G. Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yeboah J. Crouse JR. Hsu FC. Burke GL. Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 285.Yeboah J. Folsom AR. Burke GL. Johnson C. Polak JF. Post W. Lima JA. Crouse JR. Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yusuf S. Hawken S. Ounpuu S. Dans T. Avezum A. Lanas F. McQueen M. Budaj A. Pais P. Varigos J. Lisheng L. Investigators IS. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 287.Zadak Z. Hyspler R. Ticha A. Hronek M. Fikrova P. Rathouska J. Hrnciarikova D. Stetina R. Antioxidants and vitamins in clinical conditions. Physiol Res. 2009;58(Suppl 1):S13–S17. doi: 10.33549/physiolres.931861. [DOI] [PubMed] [Google Scholar]
- 288.Zellner DA. Garriga-Trillo A. Centeno S. Wadsworth E. Chocolate craving and the menstrual cycle. Appetite. 2004;42:119–21. doi: 10.1016/j.appet.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 289.Zellner DA. Garriga-Trillo A. Rohm E. Centeno S. Parker S. Food liking and craving: A cross-cultural approach. Appetite. 1999;33:61–70. doi: 10.1006/appe.1999.0234. [DOI] [PubMed] [Google Scholar]