Abstract
Purpose: To compare our renal and clinical outcomes for robot-assisted laparoscopic heminephrectomy (RAL-HN) in the pediatric population with duplicated systems with those of current contemporary open and laparoscopic series.
Patients and Methods: Sixteen children underwent RAL-HN from 2009 to 2014. Data were collected via retrospective chart review including demographics, preoperative and postoperative imaging, operative time, estimated blood loss (EBL), length of stay (LOS), complications, and renal outcomes.
Results: Mean age at surgery was 37.5±49.2 months. Mean operative time was 135±36 minutes with an EBL of 10±5 mL. Mean LOS was 2±0.8 days, and no major perioperative complications were observed. Mean follow-up was 22.1±17.2 months. Two patients needed secondary ureterectomy for recurrent urinary tract infection in the setting of a refluxing ureteral stump. One of these patients also underwent a ureteral reimplantation of the ipsilateral normal ureter. No patients lost their remaining healthy moiety. Asymptomatic cyst formation was seen in four (25%) patients, and self- limited postoperative urinoma was seen in 2 (13%) patients. Postoperative perinephric abscess did not develop in any patient. Mean change in renal function based on nuclear renography of the duplex kidney was −2.7%±4.6%.
Conclusions: Compared with previously published literature evaluating open and laparoscopic heminephrectomy, RAL-HN provides comparable outcomes in regard to complication rate and renal function of the remnant moiety.
Introduction
Heminephrectomy is one of the options for treatment of children with duplicated systems with symptomatic or poorly functioning moieties. We have previously reported our outcomes of laparoscopic heminephrectomy and found it to be comparable to those of open surgery.1 With our growing experience in robotic surgical procedures, we began to approach the management of duplicated systems using robotic technology with the added benefits of three-dimensional visualization, magnification, elimination of tremor, the ability to work with seven degrees of freedom, and movement scaling.2 To date, several reports have been published demonstrating the safety and feasibility of robot-assisted laparoscopic heminephrectomy (RAL-HN); however limited data on renal functional outcomes of the remnant moiety has been documented.3–8 Herein we present our technique with salient points and experience with RAL-HN. Our objective is to present our clinical and renal functional outcomes after RAL-HN with comparisons with contemporary open and laparoscopic series.
Patients and Methods
Institutional Review Board approval of the study was obtained. Retrospective data of 16 consecutive children undergoing RAL-HN at our institution by a single surgeon (MSG) from September 2009 to February 2014 were collected. Data were collected via chart review in regard to demographic information, preoperative and postoperative imaging (ultrasonography, dimercaptosuccinic acid [DMSA] scan and voiding cystourethrography), hospital stay, operative time, estimated blood loss, narcotic use, surgical approach, and conversion to open surgery. In addition, perioperative and long-term complications were recorded. Operative time was defined as time from incision to closure based on intraoperative records. Narcotic use was determined using medication administration records from the electronic medical record. The dosage and frequency varied per patient, and therefore was converted to morphine milligram per kilogram equivalents to allow for direct comparison between patients.
Renal function of the remnant moiety was determined by comparison of the preoperative with the postoperative nuclear renography; in those unwilling to have this imaging performed, the viability was determined by the presence of healthy parenchyma on postoperative Doppler ultrasonography and complications related to the remaining moiety. Nuclear renography was predominantly performed using DMSA renal scan; however, one patient received a preoperative mercaptoacetyltriglycine scan before being referred to our institution. Preoperatively, the relative function of each moiety was considered, but it was difficult to obtain because of anatomic configuration of the diseased moiety. Considerations for heminephrectomy included visually minimal radiotracer uptake and minimal to no cortex on ultrasonography in the diseased moiety. Postoperative renal scans and/or renal Doppler ultrasonographies were performed usually 6 to 12 weeks after surgery. All patients were suggested to have a postoperative renal scan based on previously published data suggesting that postoperative loss of renal function of the remaining moiety may be missed by Doppler renal ultrasonography alone.9 Given the relatively low risk, however, Doppler ultrasonography was used as an alternative indicator of renal outcome in certain circumstances. The presence of cyst, urinoma, or perinephric abscess was determined by evaluation of the postoperative renal Doppler ultrasonography.
Technique
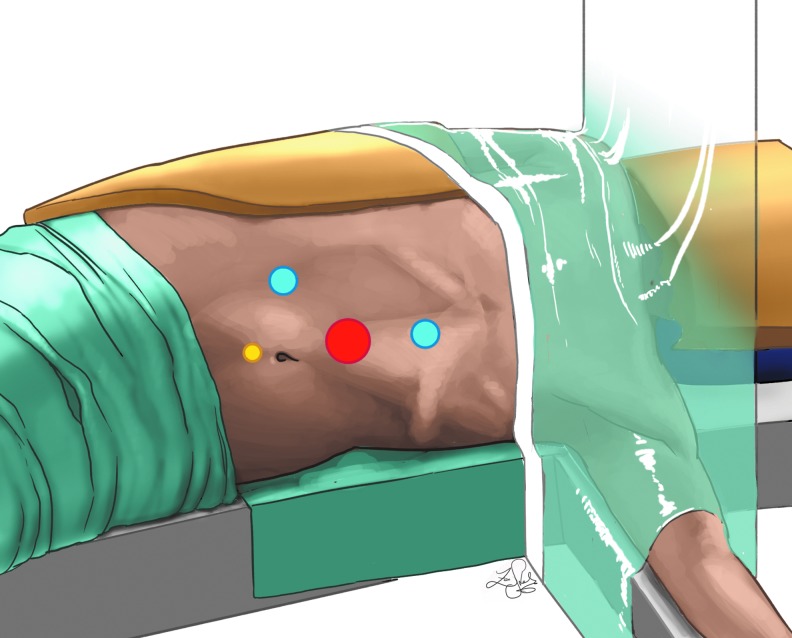
Patients are positioned in a 30 degree to 45 degree lateral decubitus position with the ipsilateral side raised using a sandbag. The ipsilateral arm is placed at the patient's side in the natural anatomic position, and the contralateral arm is outstretched supported by a bedside attachment limb support for older children. In young infants and toddlers, the contralateral arm is rested on the patient's side. The patient is sufficiently padded with foam at all pressure points to prevent injury, as well as with large foam padding on the head protecting the face.10 All patients receive preoperative antibiotics, and a Foley catheter is placed preoperatively. Neither cystoscopy nor ureteral stent placement is performed, as described by some authors.11 Ports are placed using a transperitoneal approach. Initially, an umbilical 12-mm port is placed under direct vision using the Hassan technique. This size camera port is preferred because of enhanced visualization with the 12-mm camera. Insufflation is set to a flow rate of 2 L/min with a maximum pressure of 10 to 12 mm Hg. For neonates, it is decreased to 1 L/min with a maximum pressure of 8 to 10 mm Hg. Two 8-mm ports are placed, one in the midline approximately 4 to 6 cm away from the umbilicus below the xyphoid process. The other is placed midway between the umbilicus and the anterior superior iliac spine. Finally, a 5-mm port is placed in the midline at the suprapubic level for bedside assistant use during the surgical procedure (Fig. 1). As previously described by our group, the use of 8-mm ports rather than the standard 5-mm ports is preferred to allow for greater functional operating space.12 The da Vinci robotic system is then docked on the patient's ipsilateral side.
FIG. 1.
Positioning and port placement for robot-assisted left renal procedures.
A monopolar scissor is used in the right-handed 8-mm port and a precise bipolar forceps in the left hand. To begin, the colon is reflected, and the Gerota fascia is incised. The ureters are then identified. The diseased moiety ureter is separated from the healthy moiety and transected at the lower pole, while being sure that the stump is not too long or short because this may not facilitate the optimal traction (Fig. 2). It is then passed posteriorly to the renal hilum cranially (a retrograde technique) once the adequate plane is created behind the vessels. This maneuver helps to identify the vasculature to the diseased moiety, which is then clipped. It also prevents traction on the hilum of the remaining moiety to not disturb the renal vasculature (Fig. 3). The diseased moiety is transected from the normal using electrocautery or harmonic scalpel and is removed intact. The cut margin of the normal moiety is left open with no approximating sutures or sealants. The remaining distal stump of the ureter is then mobilized down to the common sheath using sharp and blunt dissection. It is then typically transected sharply and closed using 4-0 polydiaxanone suture. If an associated ureterocele was noted that was not punctured previously, the ureteral stump was left open. The distal ureter is removed via the 5-mm assistant port, and the diseased moiety is removed via the 12-mm opening of the camera port. Postoperatively all patients' diets were advanced as tolerated, and Foley catheters were removed on postoperative day 1. Patients were discharged home when tolerating a diet and pain was well controlled.
FIG. 2.
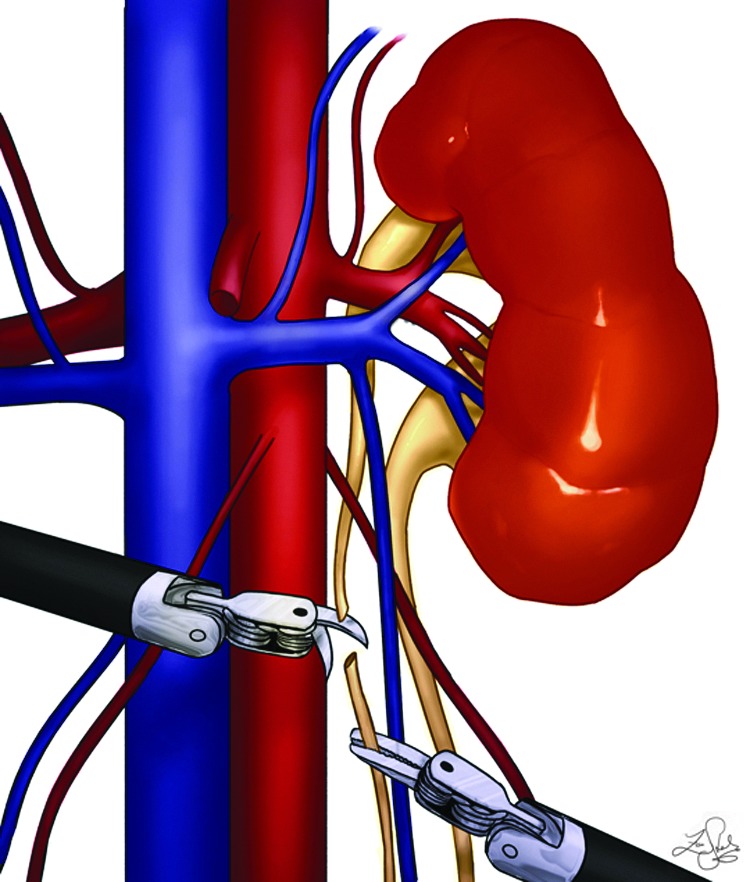
Robotic Heminephrectomy retrograde technique: Ureter transection at lower pole.
FIG. 3.
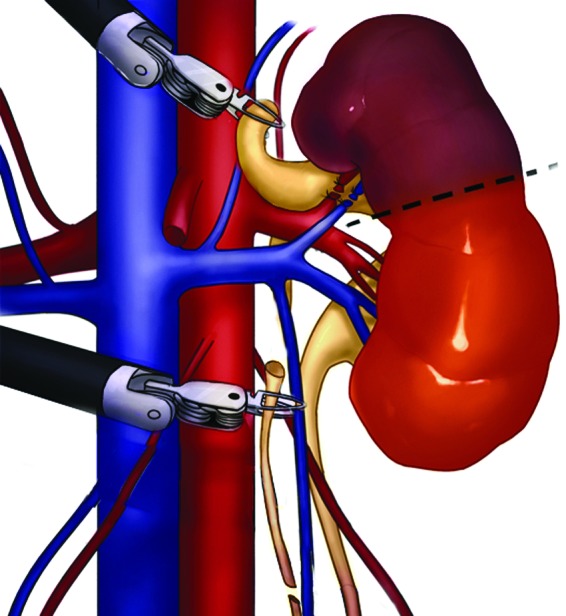
Robotic Heminephrectomy retrograde technique: Traction on ureter, vessels ligation, and removal of the distal stump.
Results
A total of 16 patients underwent RAL-HN for indications including a poorly functioning upper moiety, recurrent urinary tract infections (UTIs), incontinence, pain, and/or associated vesicoureteral reflux. Patient demographics, surgical indications, and outcomes are summarized in Tables 1 and 2.
Table 1.
Patient Demographics
| Total no. patients | 16 |
| Male | 4 (25%) |
| Female | 12 (75%) |
| Mean age (mos) | 37.5±49.2 (3–189) |
| Mean weight (kg) | 17.8±16.7 (8–73.5) |
| Mean follow-up (mos) | 22.1±17.2 (3–56) |
Table 2.
Surgical Indications and Postoperative Outcomes for Patients Undergoing Robot-Assisted Laparoscopic Heminephrectomy
| Age (months) | Sex | Weight (kg) | Indication for surgery | Pathology in normal moiety | Intervention for normal moiety | Ipsilateral preop renal functiona | Ipsilateral postop renal functiona | Postoperative cyst/urinoma |
|---|---|---|---|---|---|---|---|---|
| 3 | F | 8.0 | Poorly functioning moiety | None | None | 39% | 39% | Cyst |
| 4 | M | 8.4 | Poorly functioning moiety, UTI | None | None | 43% | 33% | Urinoma |
| 6 | M | 9.3 | Poorly function moiety, associated ureterocele | None | None | 41% | 40% | NR |
| 9 | M | 11.3 | Poorly functioning moiety, UTI, associated grade I VUR | Grade IV VUR | Delayed RAL ureteral reimplantation of the common sheath | 31% | 22% | NR |
| 10 | F | 9.1 | Poorly functioning moiety, previous ureterocele incision c/b UTI and high grade VUR | None | None | 43% | NR | NR |
| 11 | F | 11.2 | Poorly functioning moiety with ectopic ureter | None | None | NR | 40% | NR |
| 12 | F | 8.9 | Poorly functioning moiety with ectopic ureter | None | None | NR | 47% | Cyst |
| 12 | F | 10.0 | Poorly functioning moiety | None | None | 47% | 39% | NR |
| 13 | F | 10.9 | Poorly functioning moiety, UTI | None | None | 40% | NR | NR |
| 22 | F | 13.0 | Poorly functioning moiety, previous ureterocele incision c/b UTI and high grade VUR | None | None | NR | 46% | NR |
| 36 | F | 17.3 | Poorly functioning moiety, previous ureterocele incision c/b UTI | None | None | 42% | 37% | NR |
| 37 | F | 12.2 | Poorly functioning moiety, previous ureterocele incision c/b UTI & VUR | Grade III VUR | Concomitant RAL ureteral reimplantation of common sheath | 58% | NR | Cyst |
| 56 | F | 24.6 | Poorly functioning moiety, incontinence from ectopic ureter | None | None | 42% | NR | NR |
| 60 | F | 18.7 | Poorly functioning moiety, UTI, ectopic ureter | None | None | 51% | NR | NR |
| 108 | F | 37.7 | Poorly functioning moiety, UTI, cecoureterocele, urinary incontinence | None | None | 45% | 46% | Cyst |
| 189 | M | 73.5 | Poorly functioning moiety, pain | None | None | NR | NR | Urinoma |
On nuclear renography dimercaptosuccinic acid when available.
UTI=urinary tract infection,;VUR=vesicoureteral reflux; RAL=robotic-assisted laparoscopic; c/b=complicated b; NR=not reported.
Table 3 shows operative parameters, which revealed a mean operative time of 135±36 minutes. The maximum operative time of 201 minutes also included a concomitant ipsilateral common sheath reimplantation. Length of stay (LOS) was 2 days (1–3 days). Average narcotic use among all patients was low and ranged from 0 to 0.81mg/kg morphine with three (19%) patients needing no postoperative narcotics during their hospital stay. Intraoperatively, all patients received local bupivicaine before port placement. Postoperatively, pain was managed with scheduled ketorolac, acetaminophen, and ibuprofen with on-demand dosing of morphine as needed. All ureters were divided at the common sheath and ligated if refluxing. Patients were followed for an average of 22.1 months (3–56 mos). Two patients needed secondary ureterectomy for recurring febrile UTI secondary to refluxing ureteral stump. One of these patients also had vesicoureteral reflux of the remaining ipsilateral moiety and, as a result, also underwent ureteral reimplantation. Four (25%) patients had asymptomatic cyst formation (collection of clear fluid at the margin of resection). Aurinoma (fluid collection surrounding the margin of the remnant moiety) developed in two (13%) patients. No patients with urinoma needed any additional intervention, and urinoma was self-limited in nature. Evidence of a perinephric abscess did not develop in any patient.
Table 3.
Operative and Postoperative Arameters
| Mean OR time (min) | 135±36 (78–201) |
| EBL (mL) | 10±5 (5–20) |
| LOS (days) | 2±0.8 (1–3) |
| Mean morphine use (mg/kg) | 0.18±0.21 (0–0.82) |
OR=operating room; EBL=estimated blood loss; LOS=length of stay.
Of a total of 16 patients, 7 had both preoperative and postoperative renography studies available. The mean change in renal function was −2.7%±4.6%. Five patients decreased in renal function from 1% to 10.5%, while one increased renal function by 1% and one was unchanged. Of those who did not have postoperative renography because of parental preference, all nine patients had postoperative Doppler ultrasonography revealing a viable remnant moiety.
Discussion
The use of robotics in pediatric urology is becoming ever more prevalent with its ease of use and rapid learning curve. Limitations of robotics, however, include initial cost, space requirements, trained staff in the operating room, lack of tactile feedback, and limitations for teaching.2 Our report describes our technique using RAL-HN in the pediatric population using the retrograde technique. In addition, we add to the existing literature on pediatric outcomes using the robotic technology for heminephrectomy and provide data on renal functional outcomes in comparison with existing open and laparoscopic series. The retrograde technique calls for transection of the upper pole ureter at the lower pole allowing for mobilization of the upper pole moiety ureter posterior to the renal hilum. This provides traction and subsequently allows for optimal visualization of the vasculature to the diseased moiety. When comparing outcomes from recent studies from open and laparoscopic literature (Table 4), our postoperative length of stay and complication rate was similar to that of laparoscopic cases. Our operative time was lower than most reported times for laparoscopic cases. In addition, our LOS and complication rates were similar to that of the previously reported robotic heminephrectomy outcomes and lower than reported laparoscopic cases. Urinoma and postoperative cyst formation rates were similar to that reported in the literature. The LOSs for open series were variable ranging from a mean of 1.4 to 4.4 days. In comparing changes in renal function, our patients appear to have the similar mean change in renal function in comparison with those reported by previous authors with no loss of function of the remnant moiety.
Table 4.
Pediatric Heminephrectomy/Partial Nephrectomy Reports
| Author | Approach | N | Mean age (mo) | OR time (min) | LOS (days) | Reoperation rate | Cyst formation | Urinoma formation | Perinephric abscess | Clavien grade complications | Mean change in renal function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Robinson et al.,23 2003 | Open | 10 | 62 | 113.5 | 1.4 | 10% | 0% | 0% | 0% | IIIb (10%) | NR |
| Gundeti et al.,13 2005 | Open | 60 | 46 | NR | NR | NR | 18% | 0% | 0% | I (8%) | −6.8% |
| Piaggo et al.,24 2006 | Open | 20 | 7.5* | 115 | 3 | 20% | NR | 0% | 0% | I (5%)IIIa(5%) | NR |
| Garcia-Aparicio et al.,25 2010 | Open | 8 | 6.9 | 152 | 4.4 | NR | NR | 0% | 0% | 0% | NR |
| Hu et al.,22 2011 | Open | 25 | 26 | 137 | 1.5 | NR | 0% | 0% | 0% | II (8%) | NR |
| Robinson et al.,23 2003 | Laparoscopic | 8 | 13.4 | 200.4 | 1.1 | 0% | 25% | 0% | 0% | IIIb (12.5%) | NR |
| Piaggo et al.,24 2006 | Transperitoneal laparoscopic | 14 | 6.5* | 180 | 2 | 7% | NR | 7% | 0% | I (7%)IIIa (7%) | NR |
| You et al.,14 2010 | Laparoscopic | 17 | 28 | 167 | 17.6% | 35% | 0% | 0% | I (12%) | −2.8% | |
| Garcia-Aparicio et al.,25 2010 | Transperitoneal laparoscopic | 9 | 14 | 182 | 2.4 | NR | NR | 0% | 0% | 0% | NR |
| Jayram et al.,1 2011 | Laparoscopic | 142 | 11.4 | 120 | 2 | 2.8% | 27% | 5% | 0% | I (3.5%)IIIa (2.1%) | −4.9% |
| Olsen and Jørgensen,5 2005 | Retroperitoneal robotic | 14 | 58.8 | 176 | 1 | 7% | NR | 0% | 0% | III (7.1%) | NR |
| Lee et al.,3 2009 | Transperitoneal robotic | 9 | 86 | 275 | 2.9 | 0% | NR | 11% | 0% | II (11%)IIIa (11%) | NR |
| Mason et al.,4 2014 | Transperitoneal robotic | 21 | 49.2 | 301 | 3.2 | 4.7% | 29% | 0% | 0% | II (9.5%)III (4.7%) | NR |
| Current study | Transperitoneal robotic | 16 | 37.5 | 135 | 1.9 | 13% | 25% | 12.5% | 0% | IIIa (13%) | −4.7% |
median age.
Postoperative function of the remnant moiety is an important long-term outcome of heminephrectomy. In a previous open series, Gundeti and associates13 noted a mean decrease of function in the remnant moiety of 6.8%. Several theories have been proposed to explain the cause, including ischemic injury or vasospasm resulting in decreased function of the remnant kidney.13,14 Moreover, the poorly functioning moiety is likely to contribute to some, albeit small, percentage of the total renal function of the preoperative kidney, and removal may result in a loss of kidney function. In addition, the area of interest on nuclear renography is often drawn arbitrarily with no distinct demarcation of the moieties. In our laparoscopic series, we noted there were 4.9% of patients who experienced renal atrophy or a significant loss of function of the remaining moiety. Other studies have noted similar loss rates ranging from 0% to 9.1%.9,15–17 The mechanism for this remains unclear. We noted in upper pole heminephrectomy, inadvertent injury to the renal hilum may be more likely when mobilizing and controlling the upper pole with subsequent greater risk of moiety loss. Also, additional traction on the hilum when obtaining vascular control may result in ischemia of the remnant moiety and hence loss of function. Wallis and colleagues9 postulated that infants may be at higher risk for residual ischemia in the remaining moiety, specifically with the retroperitoneoscopic approach, given the smaller working space with the hemodynamic effects of CO2 insufflation on renal blood flow.9
Asymptomatic cyst formation at the resection margin was seen in 25% of our patients. These have been reported in previous series with similar incidence without any clinical consequence.13,18 They may form because of accumulated blood or fluid from the secreting surface of the remaining urothelium. They may also be secondary to operative technique during moiety transection because the collecting system of the upper moiety is often transected to prevent the injury to the remaining moiety, leaving a portion of the diseased moiety intact. In addition, we do not close the raw edges of the resection margin; therefore, the secretory surface of the upper moiety is left open, which may contribute to this formation. Based on our experience, cysts at the resection margin appear to be of no clinical significance. In our open, laparoscopic or robotic series, no patients have become symptomatic or needed intervention for cyst formation.
The rate of secondary ureterectomy in our study was 13%. The reported incidence of secondary ureterectomy after heminephrectomy and subtotal ureterectomy ranges between 1% and 12%.19–21 Most have concluded that given the low incidence of symptomatic dilated ureteral stumps and the low morbidity of surgical removal that a subtotal ureterectomy at the time of heminephrectomy is an appropriate option to avoid injury to the healthy moiety ureter. In addition to surgical removal, De Caluwe and coworkers20 noted successful resolution of symptoms and reflux with subureteral endoscopic correction using polytetrafluoroethylene paste. Opponents of subtotal ureterectomy, however, argue the distal stump may act as a reservoir for infected urine and mimic pyelonephritis—hence, recommending removal of the entire distal ureter. Based on anecdotal experience, we have found that patients with grade 5 vesicoureteral reflux are more likely to need subtotal ureterectomy and, for those patients, one should seriously consider total ureterectomy at the time of heminephrectomy. Some consideration may be given to leaving a portion of the posterior wall attached to the normal ureter below the pelvic brim as a means to reduce complications associated with devascularization. Given that the rate of secondary ureterectomy in both ours and other literature is less than 15%, performing total ureterectomy in all patients undergoing heminephrectomy would represent an overtreatment of the vast majority.
RAL-HN affords several advantages. Specifically, in our experience, we have found that it allows easy and efficient identification of the ureters. Therefore, we do not require the placement of a retrograde ureteral catheter preoperatively, avoiding additional instrumentation. While some advocate the use of retrograde ureteral catheters to exclude urinary leakage from the normal moiety before closure, we do not think this is necessary because we routinely resect into the collecting system of the diseased moiety to avoid resection of any normal moiety tissue. In addition, the use of ureter passed posteriorly to the renal hilum avoids traction on the renal hilum and allows clear visualization of the vessels to the upper moiety. Given the risk of vascular damage to the normal moiety, we find the robotic approach specifically useful in limiting traction on vasculature and reducing the risk of resultant ischemic damage. We did not find space to be a limitation in our smaller (<10 kg) patients (n=5), and improved dexterity and magnification allows for ease of completion of the operation. The final clinical outcomes as noted above are similar to that of open and laparoscopic series, and there was no postoperative loss of renal moiety in cases assessed using nuclear renography, specifically in our vulnerable infant population. Proponents of open heminephrectomy describe excellent outcomes with surgeon experience. A recent study by Hu and colleagues22 reviewed the outcomes of 25 patients who underwent open partial nephrectomy using a small supracostal-12 incision (mean 3.7 cm in length) and found that patients had minimal pain with a mean morphine use of 0.36 mg/kg and short LOS, a mean of 1.5 days. They propose that using a supracostal approach affords the ability to have direct visualization of the upper pole with a small incision and minimizes traction. In addition, they note the outcomes, specifically operative time and LOS, improved 6% and 10%, respectively, over each successive year, likely because of surgeon experience.
Limitations of our study include the retrospective nature, small sample size, and short average follow-up. Our preliminary results suggest that RAL-HN is a safe and feasible approach for management of a duplex system in the pediatric population with relatively few complications, little blood loss, reasonable operative times, and short postoperative LOS in comparison with contemporary series. However, delayed loss of function, residual cysts, and ureteral stump problems may be identified with longer follow-up.
Conclusions
RAL-HN is a safe surgical treatment for children with duplicated systems. Patients appear to have appropriate surgical and renal outcomes with no loss of the remnant moiety in comparison with contemporary open and laparoscopic series.
Abbreviations Used
- DMSA
dimercaptosuccinic acid
- LOS
length of stay
- RAL-HN
robot-assisted laparoscopic heminephrectomy
- UTI
urinary tract infection
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jayram G, Roberts J, Hernandez A, et al. Outcomes and fate of the remnant moiety following laparoscopic heminephrectomy for duplex kidney: A multicenter review. J Pediatr Urol 2011;7:272–275 [DOI] [PubMed] [Google Scholar]
- 2.Harrell WB, Snow BW. Minimally invasive pediatric nephrectomy. Curr Opin Urol 2005;15:277–281 [DOI] [PubMed] [Google Scholar]
- 3.Lee RS, Sethi AS, Passerotti CC, et al. Robot assisted laparoscopic partial nephrectomy: A viable and safe option in children. J Urol 2009;181:823–829 [DOI] [PubMed] [Google Scholar]
- 4.Mason MD, Anthony Herndon CD, Smith-Harrison LI, et al. Robotic-assisted partial nephrectomy in duplicated collecting systems in the pediatric population: Techniques and outcomes. J Pediatr Urol 2014;10:374–379 [DOI] [PubMed] [Google Scholar]
- 5.Olsen LH, Jørgensen TM. Robotically assisted retroperitoneoscopic heminephrectomy in children: Initial clinical results. J Pediatr Urol 2005;1:101–104 [DOI] [PubMed] [Google Scholar]
- 6.Pedraza R, Palmer L, Moss V, Franco I. Bilateral robotic assisted laparoscopic heminephroureterectomy. J Urol 2004;171:2394–2395 [DOI] [PubMed] [Google Scholar]
- 7.Freilich DA, Nguyen HT. Robotic-assisted laparoscopic heminephrectomy. In: Pediatric Robotic Urology. Springer: New York, NY: 2009; pp 137–172. Available at: http://link.springer.com./chapter/10.1007/978-1-60327-422-7_10 Accessed: April28, 2014 [Google Scholar]
- 8.Cannon GM, Lee RS. Pediatric laparoscopic and robotic upper pole nephrectomy for nonfunctioning moieties. In: Robotic and Laparoscopic Reconstructive Surgery in Children and Adults. Ost MC, ed. Current Clinical Urology. Humana Press; 2011; pp 73–81. Available at: http://link.springer.com./chapter/10.1007/978-1-60327-914-7_6 Accessed: April28, 2014 [Google Scholar]
- 9.Wallis MC, Khoury AE, Lorenzo AJ, et al. Outcome analysis of retroperitoneal laparoscopic heminephrectomy in children. J Urol 2006;175:2277–2282 [DOI] [PubMed] [Google Scholar]
- 10.Chang C, Steinberg Z, Shah A, Gundeti MS. Patient positioning and port placement for robot-assisted surgery. J Endourol 2014;28:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DS, Bird VG, Cooper CS, et al. Laparoscopic upper-pole heminephrectomy for ectopic ureter: Surgical technique. J Endourol 2003;17:469–473 [DOI] [PubMed] [Google Scholar]
- 12.Dangle PP, Kearns J, Anderson B, Gundeti MS. Outcomes of infants undergoing robot-assisted laparoscopic pyeloplasty compared to open repair. J Urol 2013;190:2221–2226 [DOI] [PubMed] [Google Scholar]
- 13.Gundeti MS, Ransley PG, Duffy PG, et al. Renal outcome following heminephrectomy for duplex kidney. J Urol 2005;173:1743–1744 [DOI] [PubMed] [Google Scholar]
- 14.You D, Bang JK, Shim M, et al. Analysis of the late outcome of laparoscopic heminephrectomy in children with duplex kidneys. BJU Int 2010;106:250–254 [DOI] [PubMed] [Google Scholar]
- 15.Singh RR, Wagener S, Chandran H. Laparoscopic management and outcomes in non-functioning moieties of duplex kidneys in children. J Pediatr Urol 2010;6:66–69 [DOI] [PubMed] [Google Scholar]
- 16.Castellan M, Gosalbez R, Carmack AJ, et al. Transperitoneal and retroperitoneal laparoscopic heminephrectomy—what approach for which patient? J Urol 2006;176:2636–2639 [DOI] [PubMed] [Google Scholar]
- 17.Goyal A, Hennayake S. Prone retroperitoneoscopic approach for heminephrectomy: Specific advantages relating to access to vascular pedicle. J Pediatr Urol 2010;6:153–156 [DOI] [PubMed] [Google Scholar]
- 18.Mushtaq I, Haleblian G. Laparoscopic heminephrectomy in infants and children: First 54 cases. J Pediatr Urol 2007;3:100–103 [DOI] [PubMed] [Google Scholar]
- 19.Ade-Ajayi N, Wilcox DT, Duffy PG, Ransley PG. Upper pole heminephrectomy: Is complete ureterectomy necessary? BJU Int 2001;88:77–79 [DOI] [PubMed] [Google Scholar]
- 20.De Caluwé D, Chertin B, Puri P. Long-term outcome of the retained ureteral stump after lower pole heminephrectomy in duplex kidneys. Eur Urol 2002;42:63–66 [DOI] [PubMed] [Google Scholar]
- 21.Androulakakis PA, Stephanidis A, Antoniou A, Christophoridis C. Outcome of the distal ureteric stump after (hemi) nephrectomy and subtotal ureterectomy for reflux or obstruction. BJU Int 2001;88:586–589 [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Henrichon S, Durbin-Johnson B, et al. Pediatric open partial nephrectomy: Analysis of contemporary outcomes with a supracostal-12 approach. J Pediatr Urol 2011. Available at: http://www.sciencedirect.com/science/article/pii/S1477513111002361 Accessed: February17, 2013 [DOI] [PubMed]
- 23.Robinson BC, Snow BW, Cartwright PC, et al. Comparison of laparoscopic versus open partial nephrectomy in a pediatric series. J Urol 2003;169:638–640 [DOI] [PubMed] [Google Scholar]
- 24.Piaggio L, Franc-Guimond J, Figueroa TE, et al. Comparison of laparoscopic and open partial nephrectomy for duplication anomalies in children. Urol 2006;175:2269–2273 [DOI] [PubMed] [Google Scholar]
- 25.García-Aparicio L, Krauel L, Tarrado X, et al. Heminephroureterectomy for duplex kidney: Laparoscopy versus open surgery. J Pediatr Urol 2010;6:157–160 [DOI] [PubMed] [Google Scholar]